Effects of Janus Kinase Inhibitors on Cardio-Vascular Risk in Rheumatic Diseases: A Prospective Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
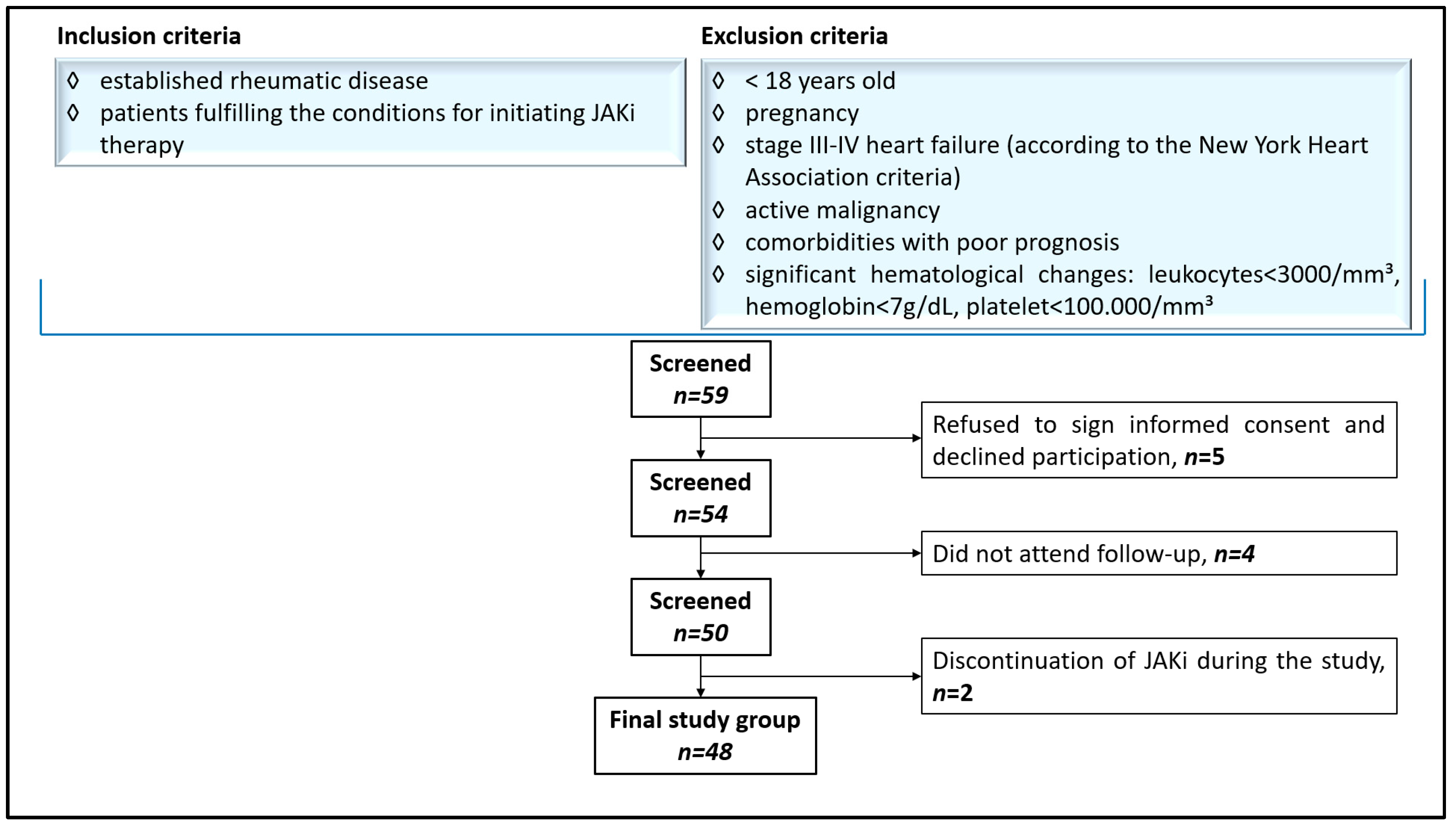
2.2. Patient Selection and Protocol
2.3. Study Procedures
2.4. Statistical Analysis
3. Results
3.1. Study Characteristics, Clinical and Biological Assessment
3.2. Non-Invasive Vascular Assessment
3.3. Correlations Between Vascular, Clinical, and Biological Parameters
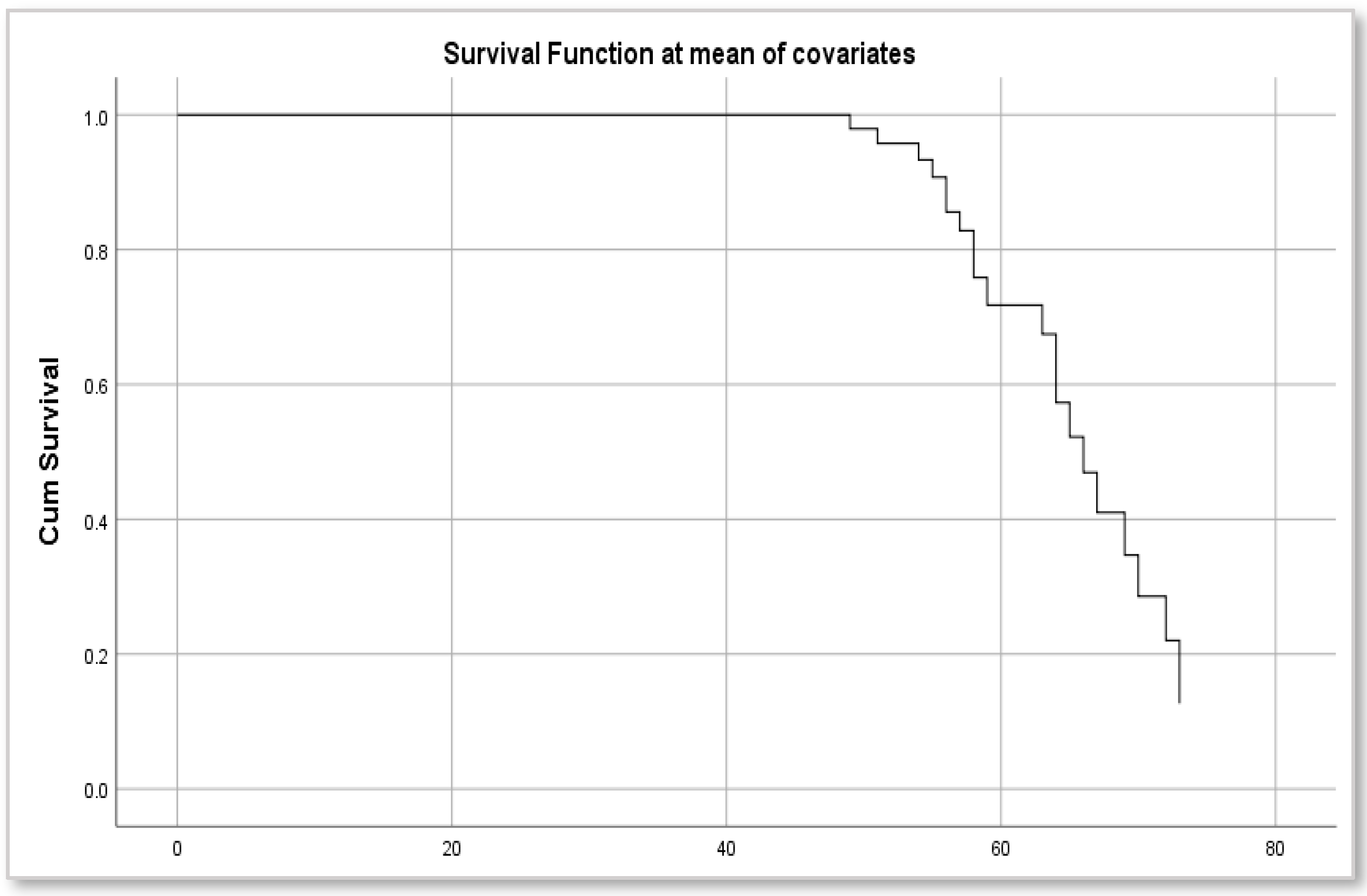
3.4. Risk of Acute Cardiovascular Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerola, A.M.; Rollefstad, S.; Semb, A.G. Atherosclerotic cardiovascular disease in rheumatoid arthritis: Impact of inflammation and antirheumatic treatment. Eur. Cardiol. 2021, 16, e18. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhang, C.; Zhang, G.; Guo, Q.; Ma, D.; Wang, X.; Wang, H.; Zhang, L. Related risk factors and treatment management of psoriatic arthritis complicated with cardiovascular disease. Front. Cardiovasc. Med. 2022, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Drosos, G.C.; Vedder, D.; Houben, E.; Boekel, L.; Atzeni, F.; Badreh, S.; Boumpas, D.T.; Brodin, N.; Bruce, I.N.; Gonzalez-Gay, M.A.; et al. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systematic lupus erythematosus and antiphospholipid syndrome. Ann. Rheum. Dis. 2022, 81, 768–779. [Google Scholar] [CrossRef]
- Crowson, C.S.; Rollefstad, S.; Ikdahl, E.; Kitas, G.D.; van Riel, P.L.C.M.; Gabriel, S.E.; Matteson, E.L.; Kvien, T.K.; Douglas, K.; Sandoo, A.; et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2018, 77, 48–54. [Google Scholar] [CrossRef]
- Restivo, V.; Candiloro, S.; Daidone, M.; Norrito, R.; Cataldi, M.; Minutolo, G.; Carraci, F.; Fasano, S.; Ciccia, F.; Casuccio, A.; et al. Systematic review and meta-analysis of cardiovascular risk in rheumatological disease: Symptomatic and non-symptomatic events in rheumatoid arthritis and systemic lupus erythematosus. Autoimmun. Rev. 2022, 21, 102925. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Malik, R.; Gill, D.; Franceschini, N.; Sudlow, C.L.M.; Dichgans, M. Interleukin-6 signaling effects on ischemic stroke and other cardiovascular outcomes: A Mendelian randomization study. Circ. Genom. Precis. Med. 2020, 13, e002872. [Google Scholar] [CrossRef]
- Hannawi, S.; Hannawi, H.; Alokaily, F.; Salmi, I. Variables associated with subclinical atherosclerosis among rheumatoid arthritis patients of Gulf Cooperative Council Countries. Saudi Med. J. 2020, 41, 128–137. [Google Scholar] [CrossRef]
- Hedar, A.M.; Stradner, M.H.; Roessler, A.; Goswami, N. Autoimmune rheumatic diseases and vascular function: The concept of autoimmune atherosclerosis. J. Clin. Med. 2021, 10, 4427. [Google Scholar] [CrossRef]
- Cem, O.; Askin, A.; Yasar, K.; Ozgul Ucar, E.; Izzet Selcuk, P.; Fulya, D.; Kubilay, S.; Huseyin, T. Clinical significance of aortic stiffness, carotid intima-media thickness and serum osteoprotegerin level in rheumatoid arthritis patients. Egypt Rheumatol. 2019, 41, 111–115. [Google Scholar] [CrossRef]
- Yang, C.W.; Guo, Y.C.; Li, C.I.; Liu, C.S.; Lin, C.H.; Liu, C.H.; Wang, M.C.; Yang, S.Y.; Li, T.C.; Lin, C.C. Subclinical atherosclerosis markers of carotid intima-media thickness, carotid plaques, carotid stenosis, and mortality in community-dwelling adults. Int. J. Environ. Res. Public Health 2020, 17, 4745. [Google Scholar] [CrossRef]
- Anjum, M.A.; Iqbal, M.J.; Joher, I.; Usman, M.; Hussain, A.; Rabbani, A. The incidence of peripheral artery disease in patients with rheumatoid arthritis. PMJHS 2021, 15, 1511. [Google Scholar] [CrossRef]
- Van, N.H.T.; Trai, P.M.; Linh, P.T.N.; Tien, H.A. Brachial-ankle pulse velocity in patients with rheumatoid arthritis. Ro. J. Rheumatol. 2024, 33, 100–104. [Google Scholar] [CrossRef]
- Bilim, S.; Icagasioglu, A.; Akbal, A.; Kasapoglu, E.; Gursel, S. Assessment of subclinical atherosclerosis with ankle-brachial index in psoriatic arthritis: A case-control study. Arch. Rheumatol. 2021, 36, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Luo, Y.; O’Shea, J.J.; Nakayamada, S. Janus kinase-targeting therapies in rheumatology: A mechanism-based approach. Nat. Rev. Rheumatol. 2022, 18, 133–135. [Google Scholar] [CrossRef]
- Clarke, B.; Yates, M.; Adas, M.; Bechman, K.; Galloway, J. The safety of JAK-1 inhibitors. Rheumatology 2021, 60 (Suppl. S2), ii24–ii30. [Google Scholar] [CrossRef]
- Harrington, R.; Harkins, P.; Conway, R. Janus kinase inhibitors in rheumatoid arthritis: An update on the efficacy and safety of tofacitinib, baricitinib and upadacitinib. J. Clin. Med. 2023, 12, 6690. [Google Scholar] [CrossRef]
- Caporali, R.; Taylor, P.C.; Aletaha, D.; Sanmarti, R.; Takeuchi, T.; Mo, D.; Haladyj, E.; Bello, N.; Zaremba-Pechmann, L.; Fang, Y.; et al. Efficacy of baricitinib in patients with moderate-to-severe rheumatoid arthritis up to 6.5 years of treatment: Results of a long-term study. Rheumatology 2024, 63, 2799–2809. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.; Buch, D.H.; Dougados, M.; Bhatt, D.L.; Giles, J.T.; Ytterberg, S.R.; Koch, G.G.; Vranic, I.; Wu, J.; Wang, C.; et al. Risk of major adverse cardiovascular events with tofacitinib versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis with or without a history of atherosclerotic cardiovascular disease: A post hoc analysis from ORAL Surveillance. Ann. Rheum. Dis. 2022, 81, 119–129. [Google Scholar] [CrossRef]
- Benucci, M.; Bernardini, P.; Coccia, C.; De Luca, R.; Levani, J.; Economou, A.; Damiani, A.; Russo, E.; Amedei, A.; Guidacci, S.; et al. JAK inhibitors and autoimmune rheumatic diseases. Autoimmun. Rev. 2023, 22, 103276. [Google Scholar] [CrossRef]
- Toth, L.; Juhasz, M.F.; Szabo, L.; Abada, A.; Kiss, F.; Hegyi, P.; Farkas, N.; Nagy, G.; Helyes, Z. Janus kinase inhibitors improve disease activity and patient-reported outcomes in rheumatoid arthritis: A systematic review and meta-analysis of 24,135 patients. Int. J. Mol. Sci. 2022, 23, 1246. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension: Developed by the task force on the management of elevated blood pressure and hypertension of the European Society of Cardiology (ESC) and endorsed by the European Society of Endocrinology (ESR) and the European Stroke Organization (ESO). Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/AphA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef] [PubMed]
- Ziembicka, A.K.; Przewlocki, T. Clinical significance of carotid intima-media complex and carotid plaque assessment by ultrasound for the prediction of adverse cardiovascular events in primary and secondary care patients. J. Clin. Med. 2021, 10, 4628. [Google Scholar] [CrossRef]
- Raj, R.; Thomas, S.; Gorantla, V. Accelerated atherosclerosis in rheumatoid arthritis: A systematic review. F1000Res 2023, 11, 466. [Google Scholar] [CrossRef]
- Dijkshoorn, B.; Raadsen, R.; Nurmohamed, M.T. Cardiovascular disease risk in rheumatoid arthritis Anno 2022. J. Clin. Med. 2022, 11, 2704. [Google Scholar] [CrossRef]
- Anghel, D.; Sirbu, C.; Hoinoiu, E.M.; Petrache, O.G.; Plesa, C.F.; Negru, M.; Ionita-Radu, F. Influence of anti-TNF therapy and homocystein level on carotid intima-media thickness in rheumatoid arthritis patients. Exp. Ther. Med. 2021, 23, 59. [Google Scholar] [CrossRef]
- Rojas-Gimenez, M.; Lopez-Medina, C.; Calvo-Gutierrez, J.; Puche-Larrubia, M.A.; Gomez-Garcia, I.; Segui-Azpilcueta, P.; Abalos-Aguilera, M.C.; Ruiz, D.; Collantes-Estevez, E.; Escudero-Contreras, A. Association between carotid intima-media thickness and the use of biological or small molecule therapies in patients with rheumatoid arthritis. Diagnostics 2022, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Anyfanti, P.; Angeloudi, E.; Dara, A.; Pagkopoulou, E.; Moysidou, G.S.; Deuteraiou, K.; Boutel, M.; Bekiari, E.; Doumas, M.; Kitas, G.D.; et al. Non-invasive assessment of micro- and macrovascular function after initiation of JAK inhibitors in patients with rheumatoid arthritis. Diagnostics 2024, 14, 834. [Google Scholar] [CrossRef]
- Semb, A.G.; Rollfstad, S.; Provan, S.A.; Kvien, T.K.; Stranden, E.; Olsen, I.C.; Hisdal, J. Carotid plaque characteristics and disease activity in rheumatoid arthritis. J. Rheumatol. 2013, 40, 359–368. [Google Scholar] [CrossRef]
- Czókolyová, M.; Hamar, A.; Pusztai, A.; Tajti, G.; Végh, E.; Pethõ, Z.; Bodnár, N.; Horváth, A.; Soõs, B.; Szamosi, S.; et al. Effects of one-year tofacitinib therapy on lipids and adipokines in association with vascular pathophysiology in rheumatoid arthritis. Biomolecules 2022, 12, 1483. [Google Scholar] [CrossRef]
- Anyfanti, P.; Angeloudi, E.; Pagkopoulou, E.; Bekiari, E. JAK inhibition in patients with rheumatoid arthritis: Haemodynamic effects and impact on micro- and macrovascular function. Study design and rationale. Mediterr. J. Rheumatol. 2022, 33, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Patrick, D.M.; Van Beusecum, J.P.; Kirabo, A. The role of inflammation in hypertension: Novel concepts. Curr. Opin. Physiol. 2021, 19, 92–98. [Google Scholar] [CrossRef]
- Pereira Filho, A.J.G.; Sartipy, F.; Lundin, F.; Qahlberg, E.; Sigvant, B. Impact of ankle-brachial index calculations on peripheral arterial disease prevalence and as a predictor of cardiovascular risk. Eur. J. Vasc. Endovasc. Surg. 2022, 62, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Poredos, P.; Stanek, A.; Catalano, M.; Boc, V. Ankle-brachial index: Diagnostic tool of peripheral arterial disease and predictor of cardiovascular risk—An update of current knowledge. Angiology 2024, 0, 00033197241226512. [Google Scholar] [CrossRef]
- Lin, C.C.; Li, C.I.; Liu, C.S.; Lin, C.H.; Yang, S.Y.; Li, T.C. Prediction of all-cause and cardiovascular mortality using ankle-brachial index and brachial-ankle pulse velocity in patients with type 2 diabetes. Sci. Rep. 2022, 12, 11053. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef]
- Aymon, R.; Mongin, D.; Guemara, R.; Salis, Z.; Askling, J.; Choquette, D.; Codreanu, C.; Di Giuseppe, D.; Flouri, I.; Huschek, D.; et al. Incidence of major adverse cardiovascular events in patients with rheumatoid arthritis treated with JAK inhibitors compared with biologic disease-modifying antirheumatic drugs: Data from an international collaboration of registries. Arthritis Rheumatol. 2025, 0, 1–11. [Google Scholar] [CrossRef]
- Partalidou, S.; Patoulias, D.; Deuteraiou, K.; Avgerou, P.; Kitas, G.; Tzitiridou-Chatzopoulou, M.; Dimitroulas, T. Risk of major adverse cardiovascular events and venous thromboembolism with JAK inhibitors versus TNF inhibitors in rheumatoid arthritis patients: A systematic review and meta-analysis. Mediterr. J. Rheumatol. 2024, 35 (Suppl. S1), 10–19. [Google Scholar] [CrossRef]
- Anyfanti, P.; Angeloudi, E.; Pagkopoulou, E.; Boutel, M.; Moysidou, G.S.; Deuteraiou, K.; Bekiari, E.; Doumas, M.; Kitas, G.D.; Dimitroulas, T. Effects of treatment with Janus kinase inhibitors on coronary microvascular perfusion in patients with rheumatoid arthritis: An observational prospective cohort study. Rheumatol. Int. 2025, 45, 111. [Google Scholar] [CrossRef]

| Variable | n = 48 |
|---|---|
| Age (Years) | 56.83 |
| Female/male ratio | 4.3:1 |
| Current smoking, n (%) | 8 (17%) |
| Raised BMI (kg/m2), n (%) | 36 (75%) |
| Arterial hypertension, n (%) | 28 (58%) |
| Dyslipidemia, n (%) | 17 (35.4%) |
| DM, n (%) | 9 (19%) |
| PAD, n (%) | 0 |
| History of stroke, n (%) | 1 (2%) |
| History of MI, n (%) | 0 |
| Positive RF, n (%) | 35 (76%) |
| Positive ACPA, n (%) | 36 (78%) |
| JAKi therapy | |
| Baricitinib, n (%) | 20 (42%) |
| Upadacitinib, n (%) | 15 (31%) |
| Tofacitinib, n (%) | 13 (27%) |
| Concomitant csDMARDs | |
| Methotrexate, n (%) | 21 (44%) |
| Leflunomide, n (%) | 16 (33%) |
| Sulfasalazine, n (%) | 1 (2%) |
| Predictor | Coefficient (T0) | Coefficient (T1) | p (T0) | p (T1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| TC | −0.0014 | −0.0010 | 0.790 | 0.570 | |||||
| LDL-C | 0.0026 | −0.0022 | 0.648 | 0.318 | |||||
| Triglyceride | −0.0019 | 0.0006 | 0.279 | 0.653 | |||||
| Lp(a) | 0.0002 | 0.0005 | 0.883 | 0.581 | |||||
| CRP | −0.0052 | −0.1029 | 0.875 | 0.105 | |||||
| IL-6 | −0.0004 | 0.0043 | 0.763 | 0.369 | |||||
| IL-1β | 0.00418 | −0.0226 | 0.476 | 0.690 | |||||
| R | R2 | Adjusted R2 | Standard Error of the Estimate | Durbin-Watson | |||||
| T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 |
| −0.441 | −0.569 | 0.194 | 0.324 | 0.003 | 0.164 | 0.459 | 0.306 | 2.07 | 2.17 |
| cIMT | |||||||||
| Predictor | Coefficient (T0) | Coefficient (T1) | p (T0) | p (T1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| TC | 0.0016 | −0.0010 | 0.673 | 0.409 | |||||
| LDL-C | −0.0014 | −0.00004 | 0.728 | 0.976 | |||||
| Triglyceride | −0.0008 | −0.0001 | 0.523 | 0.873 | |||||
| Lp(a) | 0.0002 | −0.00004 | 0.836 | 0.939 | |||||
| CRP | 0.0221 | −0.0265 | 0.346 | 0.520 | |||||
| IL-6 | −0.0001 | −0.0014 | 0.887 | 0.669 | |||||
| IL-1β | 0.0783 | −0.0039 | 0.061 | 0.917 | |||||
| R | R2 | Adjusted R2 | Standard Error of the Estimate | Durbin-Watson | |||||
| T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 |
| 0.469 | −0.362 | 0.220 | 0.131 | 0.035 | −0.075 | 0.328 | 0.209 | 1.85 | 2.22 |
| ABI | |||||||||
| Predictor | Coefficient (T0) | Coefficient (T1) | p (T0) | p (T1) |
|---|---|---|---|---|
| TC | −0.0027 | −0.0015 | 0.557 | 0.575 |
| LDL-C | 0.0016 | −0.0017 | 0.755 | 0.593 |
| Triglyceride | 0.0002 | 0.0009 | 0.910 | 0.653 |
| Lp(a) | 0.0030 | 0.0037 | 0.011 | 0.005 |
| CRP | −0.0356 | −0.0691 | 0.221 | 0.459 |
| IL-6 | −0.0019 | −0.0048 | 0.116 | 0.485 |
| IL-1β | 0.0240 | 0.0471 | 0.657 | 0.564 |
| Smoking | 0.3218 | 0.1675 | 0.081 | 0.370 |
| Hypertension | 0.3463 | 0.1486 | 0.014 | 0.335 |
| Pseudo-R2 (McFadden) | Standard Error | |||
| T0 | T1 | T0 | T1 | |
| 0.444 | 0.325 | 0.971 | 1.087 | |
| Atheromatous carotide plaque presence | ||||
| Parameter | Mean at T0 | Mean at T1 | T1-T0 | p |
|---|---|---|---|---|
| cIMT | 0.29 | 0.125 | −0.165 | 0.019 |
| ABI | 0.125 | 0.04 | −0.085 | 0.103 |
| Carotid plaque | 0.39 | 0.47 | 0.08 | 0.159 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, D.; Badescu, M.C.; Rezus, E.; Tanase, D.M.; Ouatu, A.; Dima, N.; Buliga-Finis, O.-N.; Gosav, E.M.; Rezus, C. Effects of Janus Kinase Inhibitors on Cardio-Vascular Risk in Rheumatic Diseases: A Prospective Pilot Study. J. Clin. Med. 2025, 14, 4676. https://doi.org/10.3390/jcm14134676
Popescu D, Badescu MC, Rezus E, Tanase DM, Ouatu A, Dima N, Buliga-Finis O-N, Gosav EM, Rezus C. Effects of Janus Kinase Inhibitors on Cardio-Vascular Risk in Rheumatic Diseases: A Prospective Pilot Study. Journal of Clinical Medicine. 2025; 14(13):4676. https://doi.org/10.3390/jcm14134676
Chicago/Turabian StylePopescu, Diana, Minerva Codruta Badescu, Elena Rezus, Daniela Maria Tanase, Anca Ouatu, Nicoleta Dima, Oana-Nicoleta Buliga-Finis, Evelina Maria Gosav, and Ciprian Rezus. 2025. "Effects of Janus Kinase Inhibitors on Cardio-Vascular Risk in Rheumatic Diseases: A Prospective Pilot Study" Journal of Clinical Medicine 14, no. 13: 4676. https://doi.org/10.3390/jcm14134676
APA StylePopescu, D., Badescu, M. C., Rezus, E., Tanase, D. M., Ouatu, A., Dima, N., Buliga-Finis, O.-N., Gosav, E. M., & Rezus, C. (2025). Effects of Janus Kinase Inhibitors on Cardio-Vascular Risk in Rheumatic Diseases: A Prospective Pilot Study. Journal of Clinical Medicine, 14(13), 4676. https://doi.org/10.3390/jcm14134676











