Abstract
Background/Objectives: Natural oils are widely promoted and used around the world as part of skincare. Among them, extra virgin olive oil (EVOO) stands out for its broad range of organic compositions and well-known moisturizing properties. This study aimed to evaluate the effects of topically applied EVOO compared to petrolatum on skin barrier function (SBF) and microtopography. Methods: A within-person randomized clinical trial was conducted in healthy adult volunteers. EVOO and petrolatum were applied to defined areas on the volar forearm. Parameters related to the SBF, including stratum corneum hydration (SCH), transepidermal water loss (TEWL), temperature, and erythema, were assessed. The skin microtopography was evaluated through two approaches: (1) topographic parameters—surface roughness, desquamation, smoothness, and wrinkles; and (2) stratum corneum (SC) composition—corneocytes subtypes and the desquamation index (DI). The participants completed a tolerability questionnaire for each product. Results: A total of 54 participants (50% female; mean age: 28.57 ± 11.02 years) completed the study. Both EVOO and petrolatum significantly improved the SBF by increasing SCH and reducing erythema and skin temperature. Petrolatum additionally reduced TEWL. Regarding the skin microtopography, both products decreased the desquamation index and reduced the prevalence of mature corneocyte types (types 2–5). These effects were more pronounced with petrolatum. Notably, EVOO significantly increased the proportion of early-stage corneocytes (type 1). Conclusions: Both EVOO and petrolatum effectively enhanced the SBF and improved the microtopographic features of the skin. While petrolatum exerted a stronger occlusive effect by reducing TEWL and desquamation, EVOO uniquely promoted epidermal renewal by increasing epidermal turnover.
1. Introduction
Plant oils have long been used in dermatology and cosmetology due to their wide-ranging physiological benefits for the skin []. The topical application of plant oils can provide a protective barrier by exerting an occlusive effect, thereby enhancing skin hydration through a reduction in transepidermal water loss (TEWL) []. Unlike systemic treatments, topical formulations have the advantages of higher local bioavailability and reduced systemic exposure, making them ideal for targeted skin therapy [,].
Several plant-derived oils have been studied for their ability to support skin health []. In particular, olive oil (OO) is rich in monounsaturated fatty acids (especially oleic acid) and contains a complex profile of bioactive compounds, such as polyphenols, including phenolic alcohols, acids, flavonoids, lignans, and secoiridoids []. These phenolic compounds exhibit strong antioxidant, anti-inflammatory, and skin-regenerative properties, which could provide enhanced support to the skin barrier function (SBF) and reduce cutaneous inflammation [,]. Extra virgin OO (EVOO) obtained by cold-pressing retains a higher concentration of these bioactive molecules compared to refined oils, due to the absence of heat or chemical processing []. EVOO could potentially offer superior benefits for maintaining and restoring skin barrier integrity [,,]. In clinical practice, especially in primary care [], EVOO is commonly used for procedures, such as softening earwax prior to cerumen removal, managing chronic wounds, and preventing xerosis in geriatric patients [,]. Due to its anti-inflammatory properties, it is also occasionally used in conditions such as eczema, rosacea, and psoriasis []. Similarly, petrolatum is widely used for skin disorders, including postoperative wounds [], irritant contact dermatitis [], and chronic hand eczema []. The use of both substances is often guided by their accessibility and low cost, particularly in resource-limited settings [,]. However, despite their widespread traditional use and favorable compositions, there remains limited robust scientific evidence supporting their effects on the biophysical parameters of the SBF.
Therefore, the aim of this study is to investigate the effects of topically applied EVOO and petrolatum on the SBF and microtopography in healthy adult volunteers. By assessing the key indicators, such as TEWL, hydration, and surface texture, this research seeks to elucidate the dermatological potential of EVOO and support its evidence-based use in skin care and therapy.
2. Materials and Methods
2.1. Study Design
A controlled within-subject trial was conducted to evaluate changes in the SBF and microtopography following the topical application of the study products in healthy adult volunteers. This within-subject design was chosen to minimize interindividual variability by allowing each participant to serve as their own control.
2.2. Participants
2.2.1. Recruitment
Participants were recruited from the Dermatology Department of Virgen de las Nieves University Hospital in Granada, Spain, between December 2023 and February 2024.
2.2.2. Inclusion Criteria
- Age ≥ 18 years.
- No skin diseases.
- Signed informed consent provided.
2.2.3. Exclusion Criteria
- Failure to meet any of the inclusion criteria.
- Use of systemic or topical treatments that could interfere with the SBF.
- Pregnancy or breastfeeding.
- Refusal or inability to sign the informed consent form.
2.2.4. Sample Size Calculation
Assuming an alpha risk of 0.05 and a beta risk of 0.2 in a two-sided test, a total of 52 subjects were required to recognize a difference equal to or greater than 5 units in the main variable (TEWL), assuming a common standard deviation of 6 subjects.
2.3. Test Products and Application Procedure
The study area was located on the right volar forearm of each participant. Three adjacent 9 cm2 test sites (3 cm × 3 cm) were demarcated, with a 2 cm separation between them to prevent cross-contamination or interaction between products. Each site received one of the following treatments:
- Control: Clean skin.
- EVOO: 0.2 mL of cold-pressed organic EVOO (Rodríguez del Pozo®, Jaén, Spain).
- Comparator: 0.2 mL of pharmaceutical-grade white petrolatum (Vaseline Orravan®, Barcelona, Spain).
The topical products were gently applied to the designated areas using a standardized protocol, ensuring that exactly 0.2 mL of each product was applied per site to maintain consistency. After application, a 60 min exposure period was observed. At the end of this period, any remaining product was gently blotted with a sterile gauze pad using a soft dabbing motion before biophysical measurements were taken (Figure 1).
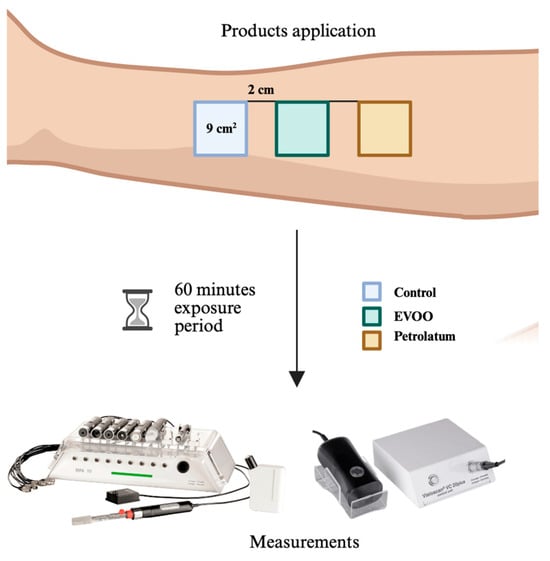
Figure 1.
Data collection method.
2.4. Variables
The sociodemographic variables, such as age, sex, occupation, and skin care habits, were collected during a clinical interview. The SBF and microtopography measurements were performed in the same room with an average ambient humidity of 45.00% and a temperature of 22.00 ± 1.00 °C.
2.4.1. Skin Barrier Function
- SCH (Corneometer® CM 825, Microcaya®, Bilbao, Spain).
- TEWL (Tewameter® TM 300, Microcaya®, Bilbao, Spain).
- pH (SkinpH-Meter® PH 905, Microcaya®, Bilbao, Spain).
- Erythema and melanin index (Mexameter® MX 18, Microcaya®, Bilbao, Spain).
- Skin temperature (Skin-Thermometer® ST 500, Microcaya®, Bilbao, Spain).
- Skin surface lipids (Sebumeter® SM 815, Microcaya®, Bilbao, Spain).
- Elasticity (Cutometer® Dual MPA 580, Microcaya®, Bilbao, Spain):
- ▪
- R0 or Uf (skin distensibility). The maximum displacement reached in a measurement.
- ▪
- R2 or Ua/Uf (gross elasticity). The ratio of final retraction to maximal deformation. This value lies between 0 and 1; values closer to 1 are characteristic of more elastic skin.
- ▪
- R7 or Ur/Uf (biological elasticity). The ratio between immediate retraction and total deformation. This parameter measures how much of the skin’s retraction happens right after the force is removed, compared to the total retraction observed throughout the entire measurement.
These parameters were measured with a multiprobe adapter (MPA®, Courage + Khazaka electronic GmbH, Köln, Germany). The measurements were taken 10 times on the volar forearm of each participant.
2.4.2. Microtopography
Topography
- Smoothness (the evenness and texture of the skin’s surface);
- Roughness (the unevenness or irregularity of the skin’s surface);
- Desquamation (the process of shedding dead skin cells from the surface of the skin);
- Wrinkles (the presence and severity of fine lines and wrinkles on the skin);
- Surface (the size of the “wavy” surface is compared to the fully stretched flat (“ironed”) surface (x:1));
- Volume (the three-dimensional size or fullness of certain skin features, such as wrinkles or blemishes);
- Contrast (the difference in brightness, color, or texture between different areas of the skin);
- Entropy (the measurement of randomness or disorder in the skin’s texture or features) and variance (the degree of variation or difference in the skin’s texture or features);
- Energy (the intensity or strength of certain skin characteristics, such as pigmentation or hydration);
- Homogeneity (the uniformity or consistency of certain skin features across different areas);
- Anisotropy index (the measure of the directionality or orientation dependence of skin features, such as wrinkles or texture);
- Total cell count (the total number of cells present within the area of skin being analyzed).
These parameters were measured using a Visioscan® VC 20plus (Courage + Khazaka electronic GmbH, Bilbao, Spain) and the SELS® (Surface Evaluation of the Living Skin) method (Institute for Experimental Dermatology, Prof. Tronnier, University of Witten-Herdecke, Colonia, Germany).
Stratum Corneum Composition
- Desquamation index (quantifies the rate and extent of skin cell shedding: a high desquamation index indicates the presence of many large and thick corneocytes, which typically signifies dehydrated or damaged skin).
- Cell (corneocytes) types (both in % and mm2):
- -
- Class 1 cells (C1C): These are the newest cells, with minimal keratinization, appearing round and with intact structures.
- -
- Class 2 cells (C2C): Slightly older, these cells have increased keratinization and begin to show some structural changes.
- -
- Class 3 cells (C3C): More advanced in the keratinization process, these cells are flatter and display signs of lipid loss.
- -
- Class 4 cells (C4C): These mature cells are highly flattened with significant structural changes and have lost most of their intracellular components.
- -
- Class 5 cells (C5C): The oldest and most keratinized, these cells are extremely flat, often fragmented, and show extensive cellular breakdown.
These parameters were measured using Corneofix® foil (Courage + Khazaka electronic GmbH, Bilbao, Spain) and Visioscan® VC 20 plus equipment (Courage + Khazaka electronic GmbH, Bilbao, Spain).
2.5. Statistical Analyses
Descriptive analyses were conducted for all variables. Qualitative variables were expressed as proportions, while quantitative variables were presented as means with standard deviations. Normality of data was assessed using Kolmogorov–Smirnov and Shapiro–Wilk tests. Depending on variance homogeneity, evaluated via Levene’s test, Student’s t-test or Welch’s test was applied for comparing continuous variables between groups. Pearson’s correlation coefficient was used to examine relationships between normally distributed continuous variables. Statistical significance was set at p < 0.05. SPSS (Version 24.0) facilitated data analysis. G*Power 3.1.9.2, Heinrich-Heine-Universität Düsseldorf, was used to calculate sample size.
2.6. Ethics
This study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Virgen de las Nieves University Hospital on 31 January 2024 (1877-N-23). The nature of the study was explained to all the participants, who agreed to participate by verbal and written consent. All measurements were noninvasive and participant data were kept confidential.
3. Results
3.1. Population Socio-Demographics
The study included 54 individuals, comprising 50.00% female (27/54), with a mean age of 28.57 years (±11.02). Among them, 24.10% (13/54) had a smoking habit and 66.70% (36/54) had an alcohol habit (Table 1).

Table 1.
Socio-demographic characteristics.
3.2. Skin Barrier Function After Application of Extra Virgin Olive Oil and Petrolatum
The differences observed in the parameters measured with the MPA system are presented in Figure 2. The skin temperature and erythema significantly decreased following the application of both EVOO (31.22 °C vs. 30.77 °C, p < 0.01; 231.79 AU vs. 196.03 AU, p < 0.001) and petrolatum (31.22 °C vs. 30.64 °C, p = 0.014; 231.79 AU vs. 192.13 AU, p < 0.01). The SCH increased after the application of EVOO (40.23 AU vs. 48.66 AU, p < 0.001) and petrolatum (40.23 AU vs. 49.15 AU, p < 0.01). The TEWL showed a decreasing trend following treatment with EVOO (9.56 g·m−2·h−1 vs. 8.52 g·m−2·h−1, p = 0.06) and a significant decrease with petrolatum (9.56 g·m−2·h−1 vs. 8.18 g·m−2·h−1, p = 0.016). Furthermore, petrolatum produced a significant change in the TEWL compared to that of EVOO (8.52 g·m−2·h−1 vs. 8.18 g·m−2·h−1, p < 0.001). The R7 elasticity increased slightly after petrolatum application in comparison to EVOO (0.73% vs. 0.76%, p = 0.045). No other significant differences were found for the remaining parameters (Table 2).
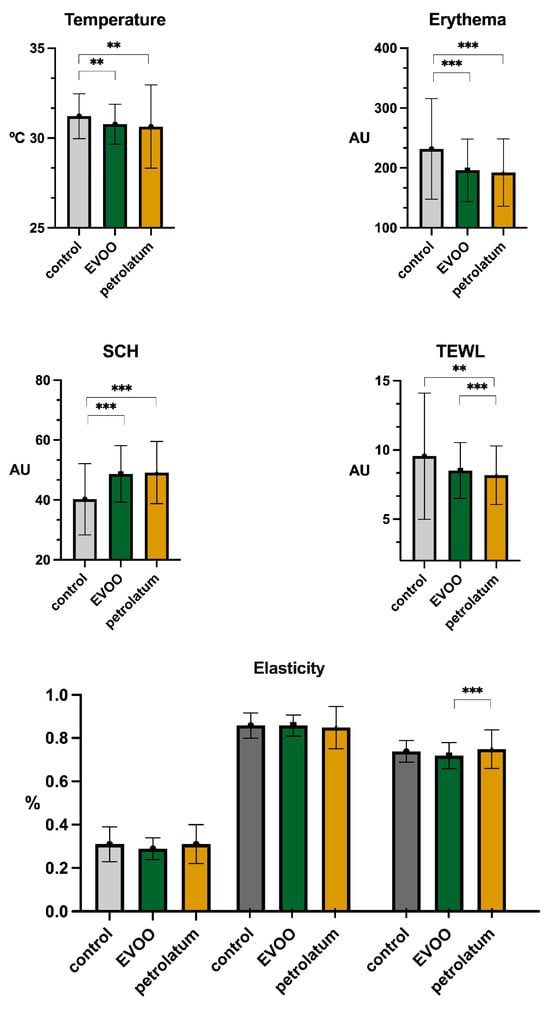
Figure 2.
Biophysical parameters of skin measured at control, EVOO, and petrolatum sites. Elasticity is represented as R0 (left), R2 (middle), and R7 (right). “**” = p < 0.01; “***” = p < 0.001. Abbreviations: AU: arbitrary units; SCH: skin corneum hydration; TEWL: transepidermal water loss; EVOO: extra virgin olive oil.

Table 2.
Biophysical parameters of skin barrier function.
3.3. Microtopography After Application of Extra Virgin Olive Oil and Petrolatum
3.3.1. Surface Evaluation of Living Skin
The SELS parameters are shown in Table 3. The skin surface area increased by 72.38% after EVOO application (759.59% vs. 831.97%, p = 0.002) and by 95.15% after petrolatum use (759.59% vs. 854.75%, p < 0.01). The contrast also increased following both EVOO and petrolatum (1.57 AU vs. 1.83 AU, p = 0.02 and 1.57 AU vs. 1.91 AU, p < 0.001, respectively). The other variables, such as the variance, energy, and anisotropy index, changed significantly only after petrolatum application (5.45 AU vs. 5.79 AU, p = 0.2; 0.02 AU vs. 0.01 AU, p = 0.033; 28.85 AU vs. 22.95 AU, p < 0.001). The homogeneity decreased after both EVOO and petrolatum application (1.31 AU vs. 1.28 AU, p = 0.001; 1.31 AU vs. 1.27 AU, p < 0.001). The total number of corneocytes decreased significantly after EVOO application (130.74 AU vs. 106.92 AU, p < 0.001) and after petrolatum use (130.74 AU vs. 105.66 AU, p < 0.001). No other significant changes were observed in the remaining parameters.

Table 3.
Surface evaluation of living skin (SELS®).
3.3.2. Desquamation
The desquamation index and the cellular composition of the SC were both significantly affected by the application of EVOO and petrolatum, as shown in Figure 3.
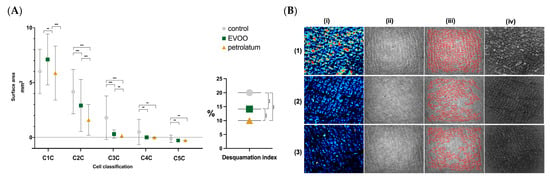
Figure 3.
(A) The cellular composition of the stratum corneum and desquamation index in the control, EVOO-treated, and petrolatum-treated areas. “**” = p < 0.01; “***” = p < 0.001. C1C = class 1 cells, C2C = class 2 cells, C3C = class 3 cells, C4C = class 4 cells, C5C = class 5 cells. (B) Images captured with Visioscan® VC 20 plus (Courage + Khazaka electronic GmbH, Bilbao, Spain). (1) Control skin, (2) skin after EVOO application, (3) skin after petrolatum application. (i) The desquamation was measured using Corneofix® (Courage + Khazaka electronic GmbH, Bilbao, Spain). The corneocytes were analyzed by their thickness across five layers: very thick (red), still bright (orange), medium thick (green), less thick (light and dark blue), and the bottom layer (black). (ii) SELS evaluation. The images display a range of gray levels: the darker areas represent lines and wrinkles, while the extremely bright areas correspond to plateaus in the skin microrelief. (iii) This section shows the distribution of lines in the image structure, their directions, and the cells surrounded by lines (polygons). The distribution of red lines was taken into account for the anisotropy index calculation using Visioscan® VC 20 plus software (Institute for Experimental Dermatology, Prof. Tronnier, University of Witten-Herdecke, Colonia, Germany). The higher the anisotropy index, the more directional the skin. Young skin should have a lower anisotropy index (many different directions) than older skin (more prominent directions). (iv) Irregular skin surface photographs were taken into account for the calculation of the texture parameters (contrast, entropy, variance, energy, and homogeneity) with Visioscan® VC 20 plus software (Institute for Experimental Dermatology, Prof. Tronnier, University of Witten-Herdecke, Colonia, Germany).
The area of C1C on the SC was significantly higher after EVOO application compared to both the control and petrolatum-treated sites (7.11 mm2 vs 6.02 mm2, p = 0.012; 7.11 mm2 vs. 5.87 mm2, p < 0.001). The C2C and C3C decreased after both treatments compared to those of the control (4.19 mm2 vs. 2.92mm2, p = 0.001; 4.19mm2 vs. 1.61mm2, p < 0.001; 1.80 mm2 vs. 0.31 mm2, p < 0.001; 1.80 mm2 vs. 0.15 mm2, p < 0.001). The reductions in the C2C and C3C were significantly greater with petrolatum than with EVOO (2.92mm2 vs. 1.61mm2, p < 0.001; 0.31mm2 vs. 0.15mm2, p = 0.003). The C4C and C5C also decreased significantly after both EVOO and petrolatum use (0.51mm2 vs. 0.02mm2, p = 0.003; 0.51mm2 vs. 0.17mm2, p = 0.003; 0.15 mm2 vs. 0.004 mm2, p = 0.002; 0.15 mm2 vs. 0.005 mm2, p = 0.003). The desquamation index decreased significantly after EVOO application (20.03AU vs 14.13 AU, p < 0.001), and even more markedly with petrolatum, both compared to the control and to the EVOO site (20.03 AU vs. 10.03 AU, p < 0.001; 14.13 AU vs. 10.03 AU, p < 0.001) (Table 4).

Table 4.
Stratum corneum cell counts and desquamation.
3.4. Tolerability
Color scored higher after the use of EVOO compared to petrolatum, with an increase of 1.09 points (8.14 points vs. 7.05 points, p = 0.02). The rest of the tolerability parameters did not show significant differences (Table 5).

Table 5.
Tolerability parameters’ scores after application of EVOO and petrolatum.
4. Discussion
The results obtained in this study demonstrate that both EVOO and petrolatum enhance the SBF, but with notable differences in their observed effects and potential mechanisms of action.
When the SBF is compromised, as seen in conditions like atopic dermatitis or psoriasis, the skin temperature, erythema, and TEWL increase [,,], while the SCH decreases []. Both actives effectively reduced the temperature and erythema and improved the SCH. Petrolatum also produced a significant reduction in the TEWL []. In fact, petrolatum is widely recognized for its superior occlusive properties, which form a semi-permeable film. This occlusive ability of petrolatum also resulted in a slight improvement in skin elasticity, which may be related to the increased water retention []. Its barrier-forming capacity makes petrolatum especially beneficial in clinical contexts involving fissures, xerosis, and chronic wounds, where protection from external irritants and TEWL is crucial [,,]. These findings align with previous studies on skin barrier excipients [].
In our study, petrolatum induced more significant changes in the anisotropy and homogeneity, suggesting that its use leads to more uniform skin []. Furthermore, petrolatum reduced the number of corneocytes in the superficial layers, particularly those involved in desquamation (C2C, C3C, C4C, and C5C), resulting in a significantly lower desquamation index. Its hydrophobic nature created a physical barrier that prevented TEWL, thereby maintaining SCH and improving barrier integrity [].
In contrast, EVOO exhibited a different effect. While it also improved the SBF parameters, EVOO showed a distinct pattern in the cellular composition of the SC. Unlike petrolatum, EVOO favored an increase in C1C, which are primarily involved in cell turnover and skin recovery. This suggests that EVOO may stimulate cellular regeneration. The observed increase in C1C following EVOO application may be attributed to its unique biochemical composition, particularly its phenolic compounds, unsaturated fatty acids, and antioxidant capacity []. EVOO is rich in bioactive components, such as hydroxytyrosol, oleuropein, and tyrosol, which have been shown to exert anti-inflammatory, antioxidant, and cytoprotective effects on skin cells [].
These phenolic antioxidants may play a key role in supporting keratinocyte health and proliferation, protecting basal and suprabasal cells from oxidative damage, and promoting more effective epidermal turnover. In addition, oleic acid, the predominant fatty acid in EVOO, is known to enhance skin permeability and can facilitate the penetration of active compounds into the epidermis, potentially triggering biological responses at the level of keratinocyte differentiation and maturation [].
The increase in C1C, which represent early-stage corneocytes, suggests that EVOO may promote a faster transition from viable keratinocytes to corneocytes, thereby accelerating desquamation and cellular renewal. This regenerative effect could be particularly advantageous in conditions requiring the stimulation of skin repair or turnover, such as post-inflammatory skin or aging skin [,]. The larger area of C1C observed after EVOO application could indicate an acceleration of the desquamation process and skin renewal, which may be beneficial in contexts where promoting skin regeneration is desired. This regenerative potential is supported by the clinical use of EVOO in the treatment of pressure ulcers [], where it has been shown to promote wound healing, improve skin integrity, and reduce the incidence and severity of ulcers, particularly in immobilized or elderly patients [].
The tolerance to both products was similar, with no significant differences in the evaluated characteristics, except for color, for which the participants reported a higher tolerance for the EVOO.
While both treatments improved the SBF, the effect of petrolatum appeared more pronounced in preventing TEWL, whereas EVOO seemed to have a more dynamic effect by promoting cell turnover.
The main limitation of this study is its cross-sectional design, which does not reflect the effects of continuous or long-term use of the moisturizing agents. This limits the ability to comprehensively evaluate the outcomes of daily use. Additionally, the assessment of the treatment effects was limited to a single time point (60 min post-application). Although this time frame is widely used in dermatological assessments and is considered appropriate for assessing immediate topical responses [,,,], it does not provide insight into the persistence of these effects over time. Nevertheless, a major strength of this study is the objective evaluation of the immediate effects on the skin barrier function and microtopography, using measurement techniques not previously applied in this context. Assessing the acute effects is particularly relevant for identifying rapid improvements, as well as potential irritation or early signs of sensitivity, which are key for the short-term safety and tolerability of topical formulations. This approach could help tailor the use of emollients to address the most affected parameters of barrier function, which may be particularly useful in treating skin conditions where these parameters are compromised. Furthermore, these findings can aid in the formulation of clinically relevant products adapted to specific skin needs. Future studies should include longer follow-up periods and repeated applications to better understand the long-term and cumulative impact of these formulations on skin barrier function and structure.
5. Conclusions
Both products enhanced the SBF and skin microtopography through different mechanisms. The biophysical parameters, such as temperature, erythema, SCH, and SC composition, improved with both actives. However, petrolatum demonstrated additional benefits, significantly improving TEWL and the desquamation index compared to EVOO. On the other hand, EVOO was particularly effective at enhancing C1C corneocytes, which are involved in cellular renewal and early-stage desquamation, suggesting its role in promoting skin regeneration.
Author Contributions
Conceptualization, R.S.-d.l.T., T.M.-V. and S.A.-S.; methodology, A.R.-S. and R.S.-d.l.T.; software, R.S.-d.l.T. and T.M.-V.; validation, S.A.-S., M.S.G.-P. and R.S.-d.l.T.; formal analysis, A.R.-S., R.S.-d.l.T. and A.G.-F.; investigation, A.R.-S. and R.S.-d.l.T.; resources, R.S.-d.l.T., A.G.-F. and S.A.-S.; data curation, R.S.-d.l.T. and A.R.-S.; writing—original draft preparation, R.S.-d.l.T. and A.R.-S.; writing—review and editing, R.S.-d.l.T., T.M.-V., A.R.-S., M.S.G.-P. and S.A.-S.; visualization, R.S.-d.l.T., T.M.-V., A.R.-S., M.S.G.-P. and S.A.-S.; supervision, T.M.-V. and S.A.-S.; project administration, R.S.-d.l.T. and A.G.-F.; funding acquisition, R.S.-d.l.T., A.G.-F. and S.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Programa de Proyectos de Investigación Precompetitivos para Jóvenes Investigadores (P20.b.) of Vicerrectorado de Investigacion y Transferencia, University of Granada (PPJIB-2024-39).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Virgen de las Nieves University Hospital (protocol code 1877-N-23 and date of approval, 31 January 2024).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study. Written informed consent was obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available from the corresponding author on reasonable request.
Conflicts of Interest
R.S.-d.l.T. is an employee of the University of Granada; T.M.-V. and S.A.-S. are employees of the Virgen de las Nieves University Hospital; M.S.G.-P. is an employee of the Andalusian Service of Health; A.G.-F. is an employee of the Institute for Biotechnological and Pharmaceutical Investigation and Orphan Drugs, Ltd. The remaining authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AU | Arbitrary units |
| C1C | Class 1 cells |
| C2C | Class 2 cells |
| C3C | Class 3 cells |
| C4C | Class 4 cells |
| C5C | Class 5 cells |
| EVOO | Extra virgin olive oil |
| TEWL | Transepidermal water loss |
| OO | Olive oil |
| SBF | Skin barrier function |
| SC | Stratum corneum |
| SCH | Stratum corneum hydration |
References
- Vaughn, A.R.; Clark, A.K.; Sivamani, R.K.; Shi, V.Y. Natural Oils for Skin-Barrier Repair: Ancient Compounds Now Backed by Modern Science. Am. J. Clin. Dermatol. 2018, 19, 103–117. [Google Scholar] [CrossRef]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef]
- Poljšak, N.; Kočevar Glavač, N. Vegetable Butters and Oils as Therapeutically and Cosmetically Active Ingredients for Dermal Use: A Review of Clinical Studies. Front. Pharmacol. 2022, 13, 868461. [Google Scholar] [CrossRef] [PubMed]
- Melguizo-Rodríguez, L.; González-Acedo, A.; Illescas-Montes, R.; García-Recio, E.; Ramos-Torrecillas, J.; Costela-Ruiz, V.J.; García-Martínez, O. Biological Effects of the Olive Tree and Its Derivatives on the Skin. Food Funct. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Donato-Trancoso, A.; de Carvalho Faria, R.V.; de Souza Ribeiro, B.C.; Nogueira, J.S.; Atella, G.C.; Chen, L.; Romana-Souza, B. Dual Effects of Extra Virgin Olive Oil in Acute Wounds. Wound Repair. Regen. 2023, 31, 338–348. [Google Scholar] [CrossRef]
- Hueso-Montoro, C.; Moya-Muñoz, N.; Martín-Cebrián, J.; Huertas-Fernández, R.; Sánchez-Crisol, I.; García-Fernández, F.P.; Capilla-Díaz, C. Efficacy of Gel Containing Organic Extra Virgin Olive Oil for Peristomal Skin Hygiene: A Pilot Randomised Controlled Trial. J. Tissue Viability 2023, 32, 188–193. [Google Scholar] [CrossRef]
- Danby, S.G.; Alenezi, T.; Sultan, A.; Lavender, T.; Chittock, J.; Brown, K.; Cork, M.J. Effect of Olive and Sunflower Seed Oil on the Adult Skin Barrier: Implications for Neonatal Skin Care. Pediatr. Dermatol. 2013, 30, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Avilés-Izquierdo, J.A.; Izquierdo Del Monte, M.G.; Martín-Madruga, M.E.; Ardiaca-Burgues, L.; Pulido-Fernández, S.; Lázaro-Ochaita, P. Enfermedades Dermatológicas Como Motivo de Consulta En Atención Primaria. Piel. Form. Contin. En. Dermatol. 2006, 21, 176–179. [Google Scholar] [CrossRef]
- Piel. Formación Continuada En Dermatología, Volume 39, Issue 10—Clinical Key. Available online: https://www.clinicalkey.com/#!/browse/toc/1-s2.0-S0213925124X00106/null/journalIssue (accessed on 30 April 2025).
- Chattopadhyay, A.; Chang, K.; Nguyen, K.; Galvez, M.G.; Legrand, A.; Davis, C.; McGoldrick, R.; Long, C.; Pham, H.; Chang, J. An Inexpensive Bismuth-Petrolatum Dressing for Treatment of Burns. Plast. Reconstr. Surg. Glob. Open 2016, 4, e737. [Google Scholar] [CrossRef]
- Alonso, C.; Larburu, I.; Bon, E.; González, M.M.; Iglesias, M.T.; Urreta, I.; Emparanza, J.I. Efficacy of Petrolatum Jelly for the Prevention of Diaper Rash: A Randomized Clinical Trial. J. Spec. Pediatr. Nurs. 2013, 18, 123–132. [Google Scholar] [CrossRef]
- English, J.; Aldridge, R.; Gawkrodger, D.J.; Kownacki, S.; Statham, B.; White, J.M.L.; Williams, J. Consensus Statement on the Management of Chronic Hand Eczema. Clin. Exp. Dermatol. 2009, 34, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Montero-Vilchez, T.; Cuenca-Barrales, C.; Rodriguez-Pozo, J.A.; Diaz-Calvillo, P.; Tercedor-Sanchez, J.; Martinez-Lopez, A.; Molina-Leyva, A.; Arias-Santiago, S. Epidermal Barrier Function and Skin Homeostasis in Atopic Dermatitis: The Impact of Age. Life 2022, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Montero-Vilchez, T.; Segura-Fernández-Nogueras, M.V.; Pérez-Rodríguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernández-González, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin Barrier Function in Psoriasis and Atopic Dermatitis: Transepidermal Water Loss and Temperature as Useful Tools to Assess Disease Severity. J. Clin. Med. 2021, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Pretel-Lara, C.; Sanabria-de la Torre, R.; Arias-Santiago, S.; Montero-Vilchez, T. Skin Barrier Function and Microtopography in Patients with Atopic Dermatitis. J. Clin. Med. 2024, 13, 5861. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Rodriguez-Pozo, J.A.; Cuenca-Barrales, C.; Sanabria-De-la-Torre, R.; Torres-De-Pinedo, J.M.; Arias-Santiago, S. Stratum Corneum Hydration As a Potential Marker of Response to Dupilumab in Atopic Dermatitis®: A Prospective Observational Study. Dermatitis 2024, 35, 250–257. [Google Scholar] [CrossRef]
- Kang, S.Y.; Um, J.Y.; Chung, B.Y.; Lee, S.Y.; Park, J.S.; Kim, J.C.; Park, C.W.; Kim, H.O. Moisturizer in Patients with Inflammatory Skin Diseases. Medicina 2022, 58, 888. [Google Scholar] [CrossRef]
- Torres, A.; Rego, L.; Martins, M.S.; Ferreira, M.S.; Cruz, M.T.; Sousa, E.; Almeida, I.F. How to Promote Skin Repair? In-Depth Look at Pharmaceutical and Cosmetic Strategies. Pharmaceuticals 2023, 16, 573. [Google Scholar] [CrossRef]
- Ordoñez-Toro, A.; Montero-Vilchez, T.; Muñoz-Baeza, J.; Sanabria-De-la-Torre, R.; Buendia-Eisman, A.; Arias-Santiago, S. The Assessment of Skin Homeostasis Changes after Using Different Types of Excipients in Healthy Individuals. Int. J. Environ. Res. Public Health 2022, 19, 16678. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Muguet, V.; Christen-Zaech, S. Restoring Skin Hydration and Barrier Function: Mechanistic Insights Into Basic Emollients for Xerosis Cutis. Int. J. Dermatol. 2025, 64, 5–12. [Google Scholar] [CrossRef]
- Lodén, M. Role of Topical Emollients and Moisturizers in the Treatment of Dry Skin Barrier Disorders. Am. J. Clin. Dermatol. 2003, 4, 771–788. [Google Scholar] [CrossRef]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Di Maio, I.; Selvaggini, R.; Taticchi, A. Biological Activities of Phenolic Compounds of Extra Virgin Olive Oil. Antioxidants 2013, 3, 1–23. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Ruiz, C.; Milia, E.; Lorenzo, M.L.; Jimenez, B.; Sánchez-Ortiz, A.; Rivas, A. Phenolic Compounds in Extra Virgin Olive Oil Stimulate Human Osteoblastic Cell Proliferation. PLoS ONE 2016, 11, e0150045. [Google Scholar] [CrossRef]
- Gutierrez, J.; Komarnytsky, S. Cottonseed Oil Composition and Its Application to Skin Health and Personal Care. Front. Pharmacol. 2025, 16, 1559139. [Google Scholar] [CrossRef]
- Morkovin, E.; Litvinov, R.; Koushner, A.; Babkov, D. Resveratrol and Extra Virgin Olive Oil: Protective Agents Against Age-Related Disease. Nutrients 2024, 16, 4258. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, S.P.; Greco, M.E.; Amato, S.; Bennardo, L.; Zappia, E.; Pignataro, E.; Pellacani, G. Evaluating the Impact of Oleocanthal and Oleacein on Skin Aging: Results of a Randomized Clinical Study. Medicina 2024, 60, 947. [Google Scholar] [CrossRef]
- Lupiáñez-Pérez, I.; Gómez-González, A.J.; Morilla-Herrera, J.C.; Marfil-Gómez, R.; León-Campos, Á.; Caro-Bautista, J.; Villa-Estrada, F.; Aranda-Gallardo, M.; Moya-Suárez, A.B.; Morales-Asencio, J.M. A Protocol for a Randomized Trial Measuring Flowmetry in Risk Areas for Pressure Ulcer: Hyperoxygenated Fatty Acids vs Olive Oil. J. Tissue Viability 2022, 31, 501–505. [Google Scholar] [CrossRef]
- Hernández-Vásquez, A.; Visconti-Lopez, F.J.; Cabanillas-Ramirez, C.; Díaz-Seijas, D.; Meléndez-Escalante, J.; Comandé, D.; Santero, M. Efficacy and Safety of Topical Application of Olive Oil for Preventing Pressure Ulcers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 14921. [Google Scholar] [CrossRef] [PubMed]
- Heuer, K.; Hoffmanns, M.A.; Demir, E.; Baldus, S.; Volkmar, C.M.; Röhle, M.; Fuchs, P.C.; Awakowicz, P.; Suschek, C.V.; Opländer, C. The Topical Use of Non-Thermal Dielectric Barrier Discharge (DBD): Nitric Oxide Related Effects on Human Skin. Nitric Oxide 2015, 44, 52–60. [Google Scholar] [CrossRef]
- Fujii, M.; Nabe, T.; Tomozawa, J.; Kohno, S. Involvement of Skin Barrier Dysfunction in Itch-Related Scratching in Special Diet-Fed Hairless Mice. Eur. J. Pharmacol. 2006, 530, 152–156. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Pham, L.D.; Nguyen, T.T.T.; Chu, H.; Nguyen, T.D.; Ngo, D.K.; Nguyen, Q.C.; Le Thai Van, T. Moisturizing Effectiveness of Immediate Compared with Delayed Moisturization. J. Cosmet. Dermatol. 2022, 21, 5134–5140. [Google Scholar] [CrossRef]
- Dobrev, H. Use of Cutometer to Assess Epidermal Hydration. Skin. Res. Technol. 2000, 6, 239–244. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).