Autograft vs. Xenograft Duraplasty Using the Onlay Technique in Pediatric Posterior Fossa Tumor Surgery: A Comparative Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
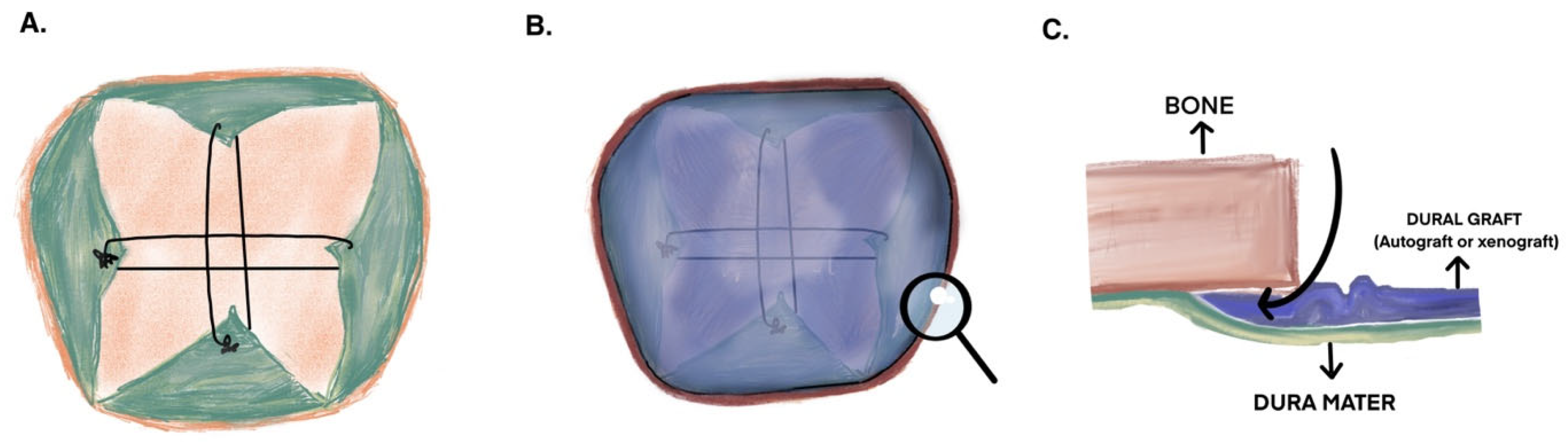
2.3. Surgical Technique
Autograft Preparation and the Duraplasty Technique
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Between-Group Comparisons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSF | Cerebrospinal Fluid |
| GCS | Glascow Coma Score |
| EVD | External Ventricular Drain |
| ICU | Intensive Care Unit |
| VP | Ventriculoperitoneal |
References
- Mukhtar Khan, M. Complications Following Posterior Fossa Tumour Surgery in Children: Experience from a Tertiary Care Neurosurgical Facility in a Developing Country. Pak. J. Neurol. Surg. 2018, 22, 177–182. [Google Scholar]
- Altaf, I.; Vohra, A.H.; Shams, S. Management of cerebrospinal fluid leak following posterior cranial fossa surgery. Pak. J. Med. Sci. 2016, 32, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Gecici, N.N.; Gurses, M.E.; Isikay, A.I.; Bilginer, B.; Hanalioglu, S. Duraplasty with autologous cervical fascia in pediatric posterior fossa tumor surgery: A single-center experience with 214 cases. Child’s Nerv. Syst. 2024, 40, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Khurana, D.; Suresh, A.; Nayak, R.; Shetty, M.; Sarda, R.K.; Knowles, J.C.; Kim, H.-W.; Singh, R.K.; Singh, B.N. Biosubstitutes for dural closure: Unveiling research, application, and future prospects of dura mater alternatives. J. Tissue Eng. 2024, 15, 20417314241228118. [Google Scholar] [CrossRef]
- Dong, R.P.; Zhang, Q.; Yang, L.L.; Cheng, X.L.; Zhao, J.W. Clinical management of dural defects: A review. World J. Clin. Cases 2023, 11, 2903–2915. [Google Scholar] [CrossRef]
- Cosgrove, G.R.; Delashaw, J.B.; Grotenhuis, J.A.; Tew, J.M.; van Loveren, H.; Spetzler, R.F.; Payner, T.; Rosseau, G.; Shaffrey, M.E.; Hopkins, L.N.; et al. Safety and efficacy of a novel polyethylene glycol hydrogel sealant for watertight dural repair. J. Neurosurg. 2007, 106, 52–58. [Google Scholar] [CrossRef]
- Steinbok, P.; Singhal, A.; Mills, J.; Cochrane, D.D.; Price, A.V. Cerebrospinal fluid (CSF) leak and pseudomeningocele formation after posterior fossa tumor resection in children: A retrospective analysis. Child’s Nerv. Syst. 2007, 23, 171–174. [Google Scholar] [CrossRef]
- Abla, A.A.; Link, T.; Fusco, D.; Wilson, D.A.; Sonntag, V.K.H. Comparison of dural grafts in Chiari decompression surgery: Review of the literature. J. Craniovertebr. Junction Spine 2010, 1, 29–37. [Google Scholar] [CrossRef]
- Hale, A.T.; Gannon, S.R.; Zhao, S.; Dewan, M.C.; Bhatia, R.; Bezzerides, M.; Stanton, A.N.; Naftel, R.P.; Shannon, C.N.; Pruthi, S.; et al. Graft dural closure is associated with a reduction in CSF leak and hydrocephalus in pediatric patients undergoing posterior fossa brain tumor resection. J. Neurosurg. Pediatr. 2020, 25, 228–234. [Google Scholar] [CrossRef]
- Wang, B.; Shi, W.; Zhang, Y.; Wang, Y.; Yang, C.; Huang, T.; Tian, Q.L.; Qu, Y.; Wang, J.L. Duraplasty with autologous nuchal ligament fascia to reduce postoperative complications in pediatric patients undergoing neoplasia resection with a suboccipital midline approach. J. Neurosurg. Pediatr. 2022, 30, 538–546. [Google Scholar] [CrossRef]
- Perrini, P.; Lorenzini, D.; Vercelli, A.; Perrone, A.; Di Carlo, D.T. Post-Operative Complications after Foramen Magnum Decompression with Duraplasty Using Different Graft Materials in Adults Patients with Chiari I Malformation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3382. [Google Scholar] [CrossRef] [PubMed]
- Azzam, D.; Romiyo, P.; Nguyen, T.; Sheppard, J.P.; Alkhalid, Y.; Lagman, C.; Prashant, G.N.; Yang, I. Dural Repair in Cranial Surgery Is Associated with Moderate Rates of Complications with Both Autologous and Nonautologous Dural Substitutes. World Neurosurg. 2018, 113, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Rahmatullah, M.I.; Al Fauzi, A.; Suroto, N.S.; Parenrengi, M.A.; Suryaningtyas, W.; Fauziah, D.; Utomo, B.; Wahid, B.D.J.; Wisnawa, I. Comparison of Complication between Autograft and Xenograft Duraplasty: A Systematic Review and Meta-Analysis. J. Med. Chem. Sci. 2023, 6, 2793–2803. [Google Scholar] [CrossRef]
- Zhang, Z.-D.; Zhao, L.-Y.; Liu, Y.-R.; Zhang, J.-Y.; Xie, S.-H.; Lin, Y.-Q.; Tang, Z.-N.; Fang, H.-Y.; Yang, Y.; Li, S.-Z.; et al. Absorbable Artificial Dura Versus Nonabsorbable Artificial Dura in Decompressive Craniectomy for Severe Traumatic Brain Injury: A Retrospective Cohort Study in Two Centers. Front. Surg. 2022, 9, 877038. [Google Scholar] [CrossRef]
- Berjano, R.; Vinas, F.C.; Dujovny, M. A review of dural substitutes used in neurosurgery. Crit. Rev. Neurosurg. 1999, 9, 217–222. [Google Scholar] [CrossRef]
- Danish, S.F.; Samdani, A.; Hanna, A.; Storm, P.; Sutton, L. Experience with acellular human dura and bovine collagen matrix for duraplasty after posterior fossa decompression for Chiari malformations. J. Neurosurg. Pediatr. 2006, 104, 16–20. [Google Scholar] [CrossRef]
- Mekonnen, M.; Hovis, G.; Mahgerefteh, N.; Chandla, A.; Malkhasyan, Y.; Zhang, A.B.; Yang, I. A Case Series of DuraMatrix-Onlay ® Plus in Cranial Surgery Is Associated With a Low Complication Profile. Brain. Tumor. Res. Treat. 2023, 11, 232. [Google Scholar] [CrossRef]
- Kshettry, V.R.; Lobo, B.; Lim, J.; Sade, B.; Oya, S.; Lee, J.H. Evaluation of non-watertight dural reconstruction with collagen matrix onlay graft in posterior fossa surgery. J. Korean Neurosurg. Soc. 2016, 59, 52–57. [Google Scholar] [CrossRef]
- Williams, L.E.; Vannemreddy, P.S.; Watson, K.S.; Slavin, K.V. The need in dural graft suturing in Chiari i malformation decompression: A prospective, single-blind, randomized trial comparing sutured and sutureless duraplasty materials. Surg. Neurol. Int. 2013, 4, 5419. [Google Scholar] [CrossRef]
- Aboelkhir, M.; Elmaghraby, M.; Rewehy, A. Outcome of Posterior Fossa Decompression with Duraplasty by Different Types of Graft in Patients with Chiari Malformation Type I. Al-Azhar Int. Med. J. 2022, 3, 185–190. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, L.; Zhang, J.; You, N.; Liu, Y.; Yao, A.; Zhao, K.; Zhang, J.; Xu, B. Duraplasty with Cervical Fascia Autograft to Reduce Postoperative Complications of Posterior Fossa Tumor Surgery with Suboccipital Midline Approach. World Neurosurg. 2020, 134, e1115–e1120. [Google Scholar] [CrossRef] [PubMed]
- Zaben, M.; Richards, A.; Merola, J.; Patel, C.; Leach, P. Surgical site infection in paediatric posterior fossa surgery: Does pathology matter? Child’s Nerv. Syst. 2021, 37, 1859–1861. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, P.; Liang, B.; Ding, X.; Gao, H.; Feng, E. The comparison of the watertight and non-watertight dural closure in supratentorial craniotomy: A single-institute 10-year experience with 698 patients. Medicine 2023, 102, e35199. [Google Scholar] [CrossRef] [PubMed]
- Chotai, S.; Tang, A.R.; McDermott, J.R.; Guidry, B.S.; Grisham, C.J.; Yengo-Kahn, A.M.; Morone, P.J.; Thompson, R.C.; Chambless, L.B. Comparison of supratentorial meningioma resection outcomes by dural reconstruction technique. J. Neurosurg. 2023, 138, 70–77. [Google Scholar] [CrossRef]
- Dafford, E.E.; Anderson, P.A. Comparison of dural repair techniques. Spine J. 2015, 15, 1099–1105. [Google Scholar] [CrossRef]
- Dustur, S.; Parenrengi, M.A.; Suryaningtyas, W. Management of pseudomeningocele following posterior fossa tumor surgery with absence of hydrocephalus: A case report. Int. J. Surg. Case. Rep. 2022, 98, 107552. [Google Scholar] [CrossRef]
- Moskowitz, S.I.; Liu, J.; Krishnaney, A.A. Postoperative complications associated with dural substitutes in suboccipital craniotomies. Neurosurgery 2009, 64 (Suppl. 3), ons28–ons34. [Google Scholar] [CrossRef]
- Legnani, F.G.; Saladino, A.; Casali, C.; Vetrano, I.G.; Varisco, M.; Mattei, L.; Prada, F.; Perin, A.; Mangraviti, A.; Solero, C.L.; et al. Craniotomy vs. craniectomy for posterior fossa tumors: A prospective study to evaluate complications after surgery. Acta Neurochir. 2013, 155, 2281–2286. [Google Scholar] [CrossRef]
- Dubey, A.; Sung, W.-S.; Shaya, M.; Patwardhan, R.; Willis, B.; Smith, D.; Nanda, A. Complications of posterior cranial fossa surgery—An institutional experience of 500 patients. Surg. Neurol. 2009, 72, 369–375. [Google Scholar] [CrossRef]
| Autograft (n = 13) | Xenograft (n = 37) | ||
|---|---|---|---|
| Age (year) | 7.6 ± 3.6 | 7.4 ± 5.5 | |
| Gender (M:F) | 8:5 | 22:15 | |
| Tumor location | |||
| Intraventricular | 8 (61.5) | 23 (62.2) | |
| Intraparenchymal | 5 (38.5) | 12 (32.4) | |
| Extra-axial | 0 (0.0) | 2 (5.4) | |
| Amount of resection | |||
| Subtotal | 2 (15.4) | 6 (16.2) | |
| Near total | 3 (23.1) | 6 (16.2) | |
| Gross total | 8 (61.5) | 25 (67.6) | |
| Perioperative EVD | 1 (7.7) | 7 (18.9) | |
| Pathology | |||
| Medulloblastoma | 2 (15.4) | 12 (32.4) | |
| Pilocytic astrocytoma | 7 (53.8) | 11 (29.7) | |
| Ependymoma | 2 (15.4) | 7 (18.9) | |
| Epidermoid | 0 (0.0) | 2 (5.4) | |
| Embryonal tumor (WHO IV) | 1 (7.7) | 2 (5.4) | |
| Diffuse astrocytoma (IDH mutant) (WHO III) | 1 (7.7) | 1 (2.7) | |
| Low-grade glial tumor | 0 (0.0) | 1 (2.7) | |
| Autograft (n = 13) | Xenograft (n = 37) | p | ||
|---|---|---|---|---|
| Duration of surgery (min.) | 245.4 ± 55.4 | 244.2 ± 58.1 | 0.921 | |
| Postoperative ICU admission (day) | 2.8 ± 2.4 | 8.4 ± 17.0 | 0.158 | |
| Postoperative meningitis | 1 (7.7) | 11 (29.7) | 0.110 | |
| Postoperative CSF fistula | 0 (0.0) | 8 (21.6) | 0.067 | |
| Postoperative pseudo-meningocele | 1 (7.7) | 12 (32.4) | 0.080 | |
| Postoperative hydrocephalus | 1 (7.7) | 9 (24.3) | 0.197 | |
| V/P shunt | 1 (7.7) | 8 (21.6) | 0.261 | |
| Additional surgical complications | 0.549 | |||
| Air embolism | 0 (0.0) | 1 (2.7) | ||
| Nonsurgical complications | 0.238 | |||
| Hyponatremia | 1 (7.7) | 0 (0.0) | ||
| Urinary tract infection | 1 (7.7) | 0 (0.0) | ||
| Keratitis | 1 (7.7) | 0 (0.0) | ||
| Blepharitis | 0 (0.0) | 1 (2.7) | ||
| Catheter infection | 0 (0.0) | 1 (2.7) | ||
| Seizure | 0 (0.0) | 1 (2.7) | ||
| Perioperative acidosis | 0 (0.0) | 1 (2.7) | ||
| Septicemia | 0 (0.0) | 1 (2.7) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Türk, Ç.; Sevgi, U.T.; Bahadır, S.; Çamlar, M.; Özer, F. Autograft vs. Xenograft Duraplasty Using the Onlay Technique in Pediatric Posterior Fossa Tumor Surgery: A Comparative Analysis. J. Clin. Med. 2025, 14, 4674. https://doi.org/10.3390/jcm14134674
Türk Ç, Sevgi UT, Bahadır S, Çamlar M, Özer F. Autograft vs. Xenograft Duraplasty Using the Onlay Technique in Pediatric Posterior Fossa Tumor Surgery: A Comparative Analysis. Journal of Clinical Medicine. 2025; 14(13):4674. https://doi.org/10.3390/jcm14134674
Chicago/Turabian StyleTürk, Çağlar, Umut Tan Sevgi, Sinan Bahadır, Mahmut Çamlar, and Füsun Özer. 2025. "Autograft vs. Xenograft Duraplasty Using the Onlay Technique in Pediatric Posterior Fossa Tumor Surgery: A Comparative Analysis" Journal of Clinical Medicine 14, no. 13: 4674. https://doi.org/10.3390/jcm14134674
APA StyleTürk, Ç., Sevgi, U. T., Bahadır, S., Çamlar, M., & Özer, F. (2025). Autograft vs. Xenograft Duraplasty Using the Onlay Technique in Pediatric Posterior Fossa Tumor Surgery: A Comparative Analysis. Journal of Clinical Medicine, 14(13), 4674. https://doi.org/10.3390/jcm14134674






