Minimally Invasive Techniques in Posterior Atlanto-Axial Fixation: State of the Art and Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Risk of Bias and Quality of Studies
2.2. Data Collection
2.3. Statistical Analysis
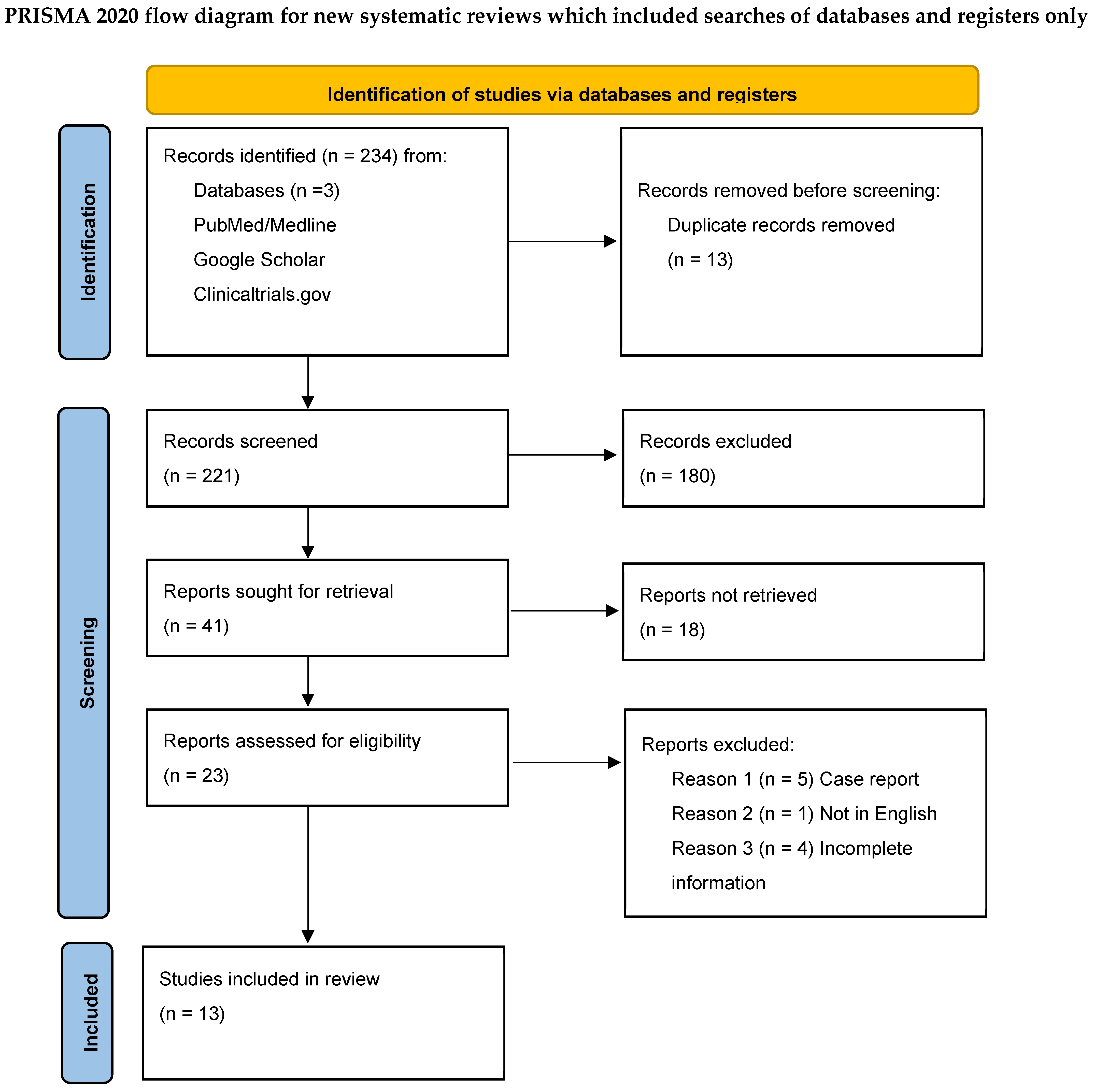
3. Results
4. Discussion
4.1. Safety and Feasibility of MIS C1–C2 Posterior Fixation
4.2. Technical Aspects of MIS C1–C2 Posterior Fixation
4.3. Image Guidance and Navigation in MIS C1–C2 Posterior Fixation
4.4. Effects of MIS C1–C2 Fixation on Intraoperative Metrics and Postoperative Hospitalization
4.5. Fusion Rate
4.6. Future Perspectives
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MIS | Minimally Invasive Surgery |
| VA | Vertebral Artery |
| H-LoS | Hospital Length-of-Stay |
| SD | Standard Deviation |
| VAS | Visual Analog Scale |
| NDI | Neck Disability Index |
| CT | Computed Tomography |
| NA | Not available/Not applicable |
| ERAS | Enhanced Recovery After Surgery |
References
- Signorelli, F.; Visocchi, M. Biomechanics of the CVJ. In Surgery of the Cranio-Vertebral Junction; Tessitore, E., Dehdashti, A.R., Schonauer, C., Thomé, C., Eds.; Springer: Cham, Switzerland, 2020; pp. 87–90. [Google Scholar]
- Dagtekin, A.; Avci, E.; Hamzaoglu, V.; Ozalp, H.; Karatas, D.; Esen, K.; Bagdatoglu, C.; Baskaya, M.K. Management of occipitocervical junction and upper cervical trauma. J. Craniovertebral Junction Spine 2018, 9, 148–155. [Google Scholar] [CrossRef] [PubMed]
- d’Avella, E.; Cavallo, L.M.; De Notaris, M.; Pineda, J.; Di Somma, A.; Cappabianca, P.; Prats-Galino, A. Relevant anatomy of the Cranio-Vertebral Junction. In Surgery of the Cranio-Vertebral Junction; Tessitore, E., Dehdashti, A.R., Schonauer, C., Thomé, C., Eds.; Springer: Cham, Switzerland, 2020; pp. 3–7. [Google Scholar]
- Goel, A. Craniovertebral junction instability: A review of facts about facets. Asian Spine J. 2015, 9, 636–644. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jannelli, G.; Moiraghi, A.; Paun, L.; Cuvinciuc, V.; Bartoli, A.; Tessitore, E. Atlantoaxial posterior screw fixation using intra-operative spinal navigation with three-dimensional isocentric C-arm fluoroscopy. Int. Orthop. 2022, 46, 321–329. [Google Scholar] [CrossRef]
- Ferrante, A.; Ciccia, F.; Giammalva, G.R.; Iacopino, D.G.; Visocchi, M.; Macaluso, F.; Maugeri, R. The Craniovertebral Junction in Rheumatoid Arthritis: State of the Art. Acta Neurochir. Suppl. 2019, 125, 79–86. [Google Scholar] [CrossRef]
- Guilpain, P.; Kettaneh, A.; Chamouard, J.-M.; Stirnemann, J.; Thomas, M.; Fain, O. Compression of the spinal cord revealing a seronegative rheumatoid arthritis. Rev. Méd. Intern. 2003, 24, 59–62. [Google Scholar] [CrossRef]
- Rathod, A.; Kadam, A.; Dhamangaonkar, A. Giant cell tumor with pathological fracture of C2 with C1-C2 instability: A rare case with review of literature. J. Craniovertebral Junction Spine 2018, 9, 205–208. [Google Scholar] [CrossRef]
- Molliqaj, G.; Dammann, P.; Schaller, K.; Sure, U.; Tessitore, E. Management of craniovertebral junction tuberculosis presenting with atlantoaxial dislocation. In New Trends in Craniovertebral Junction Surgery; (Acta Neurochirurgica Supplement); Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Moulding, H.D.; Bilsky, M.H. Metastases to the craniovertebral junction. Neurosurgery 2010, 66 (Suppl. 3), 113–118. [Google Scholar] [CrossRef] [PubMed]
- Bourdillon, P.; Perrin, G.; Lucas, F.; Debarge, R.; Barrey, C. C1-C2 stabilization by Harms arthrodesis: Indications, technique, complications and outcomes in a prospective 26-case series. Orthop. Traumatol. Surg. Res. 2014, 100, 221–227. [Google Scholar] [CrossRef]
- Jeanneret, B.; Magerl, F. Primary posterior fusion C1/2 in odontoid fractures: Indications, technique, and results of transarticular screw fixation. J. Spinal Disord. 1992, 5, 464–475. [Google Scholar] [CrossRef]
- Harms, J.; Melcher, R.P. Posterior C1-C2 fusion with polyaxial screw and rod fixation. Spine 2001, 26, 2467–2471. [Google Scholar] [CrossRef]
- Tian, F.; Tu, L.Y.; Gu, W.F.; Zhang, E.F.; Wang, Z.B.; Chu, G.; Ka, H.; Zhao, J. Percutaneous versus open pedicle screw instrumentation in treatment of thoracic and lumbar spine fractures: A systematic review and meta-analysis. Medicine 2018, 97, e12535. [Google Scholar] [CrossRef] [PubMed]
- Kocis, J.; Kelbl, M.; Kocis, T.; Návrat, T. Percutaneous versus open pedicle screw fixation for treatment of type A thoracolumbar fractures. Eur. J. Trauma Emerg. Surg. 2020, 46, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Rao, P.J.; Mobbs, R.J. Percutaneous versus open pedicle screw fixation for treatment of thoracolumbar fractures: Systematic review and meta-analysis of comparative studies. Clin. Neurol. Neurosurg. 2015, 135, 85–92. [Google Scholar] [CrossRef]
- Panero, I.; Lagares, A.; Alén, J.A.; García-Perez, D.; Eiriz, C.; Castaño-Leon, A.M.; Cepeda, S.; Moreno-Gómez, L.M.; Sinovas, O.E.; Paredes, I. Efficacy of percutaneous pedicle screws for thoracic and lumbar spine fractures compared with open technique. J. Neurosurg. Sci. 2023, 67, 462–470. [Google Scholar] [CrossRef]
- Li, H.; Yang, L.; Xie, H.; Yu, L.; Wei, H.; Cao, X. Surgical outcomes of mini-open Wiltse approach and conventional open approach in patients with single-segment thoracolumbar fractures without neurologic injury. J. Biomed. Res. 2015, 29, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Junhui, L.; Zhengbao, P.; Wenbin, X.; Lu, H.; Shengyun, L.; Shunwu, F.; Fengdong, Z. Comparison of pedicle fixation by the Wiltse approach and the conventional posterior open approach for thoracolumbar fractures, using MRI, histological and electrophysiological analyses of the multifidus muscle. Eur. Spine J. 2017, 26, 1506–1514. [Google Scholar] [CrossRef]
- Gelinne, A.; Piazza, M.; Bhowmick, D.A. Minimally invasive modification of the Goel-Harms atlantoaxial fusion technique: A case series and illustrative guide. Neurosurg. Focus 2023, 54, E14. [Google Scholar] [CrossRef]
- Koepke, L.G.; Heuer, A.; Stangenberg, M.; Dreimann, M.; Beyerlein, J.; Schaefer, C.; Viezens, L. The limitations of fully threaded screws in isolated percutaneous transarticular screw fixation of C1/C2. Sci. Rep. 2022, 12, 6484. [Google Scholar] [CrossRef]
- Kaminski, A.; Gstrein, A.; Kälicke, T.; Muhr, G.; Müller, E.J. Mini-open percutaneous transarticular screw fixation for acute and late atlantoaxial instability. Acta Orthopædica Belg. 2008, 74, 102–108. [Google Scholar]
- Schmidt, R.; Richter, M.; Gleichsner, F.; Geiger, P.; Puhl, W.; Cakir, B. Posterior atlantoaxial three-point fixation: Comparison of intraoperative performance between open and percutaneous techniques. Arch. Orthop. Trauma. Surg. 2006, 126, 150–156. [Google Scholar] [CrossRef]
- ElSaghir, H.; Boehm, H.; Greiner-Perth, R. Mini-open approach combined with percutaneous transarticular screw fixation for C1-C2 fusion. Neurosurg. Rev. 2005, 28, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Deng, R.; Qing-Yan, L.; Chu, L.; Ke-Xiao, Y.; Zhou, X.; Zhong-Liang, D. Endoscopically-Assisted Percutaneous Unilateral Atlantoaxial Screw-Rod Nonfusion Fixation Treatment for Type II Odontoid Fractures in Geriatric Patients: Case Series and Technical Note. Pain Physician 2020, 23, E241–E250. [Google Scholar]
- Meyer, M.; Farah, K.; Graillon, T.; Dufour, H.; Blondel, B.; Fuentes, S. Minimally Invasive Percutaneous C1-C2 Fixation Using an Intraoperative Three-Dimensional Imaging-Based Navigation System for Management of Odontoid Fractures. World Neurosurg. 2020, 137, 266–271. [Google Scholar] [CrossRef]
- Lvov, I.; Grin, A.; Kordonskiy, A.; Sytnik, A.; Smirnov, V.; Khushnazarov, U.; Krylov, V. Minimally Invasive Posterior Transarticular Stand-Alone Screw Instrumentation of C1-C2 Using a Transmuscular Approach: Description of Technique, Results and Comparison with Posterior Midline Exposure. World Neurosurg. 2019, 128, e796–e805. [Google Scholar] [CrossRef] [PubMed]
- Dusad, T.; Kundnani, V.; Dutta, S.; Patel, A.; Mehta, G.; Singh, M. Minimally Invasive Microscope-Assisted Stand-Alone Transarticular Screw Fixation without Gallie Supplementation in the Management of Mobile Atlantoaxial Instability. Asian Spine J. 2018, 12, 710–719. [Google Scholar] [CrossRef]
- Alhashash, M.; Shousha, M.; Gendy, H.; Barakat, A.S.; Boehm, H. Percutaneous Posterior Transarticular Atlantoaxial Fixation for the Treatment of Odontoid Fractures in the Elderly: A Prospective Study. Spine 2018, 43, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Srikantha, U.; Khanapure, K.S.; Jagannatha, A.T.; Joshi, K.C.; Varma, R.G.; Hegde, A.S. Minimally invasive atlantoaxial fusion: Cadaveric study and report of 5 clinical cases. J. Neurosurg. Spine 2016, 25, 675–680. [Google Scholar] [CrossRef]
- Díaz, R.; Berbeo, M.E.; Villalobos, L.M.; Vergara, M.F.; Osorio, E. Minimally Invasive Posterior Trans-muscular C1-C2 Screw Fixation Through an Anatomical Corridor to Preserve Occipitocervical Tension Band: Surgical Anatomy and Clinical Experience. In Pediatric Craniovertebral Junction Diseases; (Advances and Technical Standards in Neurosurgery); Springer: Cham, Switzerland, 2014; Volume 40, pp. 261–271. [Google Scholar] [CrossRef]
- Holly, L.T.; Isaacs, R.E.; Frempong-Boadu, A.K. Minimally invasive atlantoaxial fusion. Neurosurgery 2010, 66 (Suppl. 3), 193–197. [Google Scholar] [CrossRef]
- Dietz, N.; Sharma, M.; Adams, S.; Alhourani, A.; Ugiliweneza, B.; Wang, D.; Nuño, M.; Drazin, D.; Boakye, M. Enhanced Recovery After Surgery (ERAS) for Spine Surgery: A Systematic Review. World Neurosurg. 2019, 130, 415–426. [Google Scholar] [CrossRef]
- Debono, B.; Wainwright, T.W.; Wang, M.Y.; Sigmundsson, F.G.; Yang, M.M.; Smid-Nanninga, H.; Bonnal, A.; Le Huec, J.C.; Fawcett, W.J.; Ljungqvist, O.; et al. Consensus statement for perioperative care in lumbar spinal fusion: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Spine J. 2021, 21, 729–752. [Google Scholar] [CrossRef]
- Debono, B.; Corniola, M.V.; Pietton, R.; Sabatier, P.; Hamel, O.; Tessitore, E. Benefits of Enhanced Recovery After Surgery for fusion in degenerative spine surgery: Impact on outcome, length of stay, and patient satisfaction. Neurosurg. Focus 2019, 46, E6. [Google Scholar] [CrossRef] [PubMed]
- Lvov, I.; Grin, A.; Talypov, A.; Smirnov, V.; Kordonskiy, A.; Barbakadze, Z.; Abdrafiev, R.; Krylov, V. Efficacy and Safety of Goel-Harms Technique in Upper Cervical Spine Surgery: A Systematic Review and Meta-Analysis. World Neurosurg. 2022, 167, e1169–e1184. [Google Scholar] [CrossRef] [PubMed]
- Neo, M.; Fujibayashi, S.; Miyata, M.; Takemoto, M.; Nakamura, T. Vertebral artery injury during cervical spine surgery: A survey of more than 5600 operations. Spine 2008, 33, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Gluf, W.M.; Schmidt, M.H.; Apfelbaum, R.I. Atlantoaxial transarticular screw fixation: A review of surgical indications, fusion rate, complications, and lessons learned in 191 adult patients. J. Neurosurg. Spine 2005, 2, 155–163. [Google Scholar] [CrossRef]
- Yoshida, M.; Neo, M.; Fujibayashi, S.; Nakamura, T. Comparison of the anatomical risk for vertebral artery injury associated with the C2-pedicle screw and atlantoaxial transarticular screw. Spine 2006, 31, E513–E517. [Google Scholar] [CrossRef]
- Wright, N.M.; Lauryssen, C. Vertebral artery injury in C1-2 transarticular screw fixation: Results of a survey of the AANS/CNS section on disorders of the spine and peripheral nerves. American Association of Neurological Surgeons/Congress of Neurological Surgeons. J. Neurosurg. 1998, 88, 634–640. [Google Scholar] [CrossRef]
- Vergara, P.; Bal, J.S.; Hickman Casey, A.T.; Crockard, H.A.; Choi, D. C1-C2 posterior fixation: Are 4 screws better than 2? Neurosurgery 2012, 71 (Suppl. 1), 86–95. [Google Scholar] [CrossRef]
- Rezvani, M.; Sourani, A.; Nikzad, H. Postoperative complications of Goel-Harms C1-C2 screw-rod fixation technique for C1-C2 instability after C2 nerve sacrifice, a prospective study over two years follow up. J. Clin. Neurosci. 2021, 88, 52–56. [Google Scholar] [CrossRef]
- Yeom, J.S.; Buchowski, J.M.; Kim, H.-J.; Chang, B.-S.; Lee, C.-K.; Riew, K.D. Postoperative occipital neuralgia with and without C2 nerve root transection during atlantoaxial screw fixation: A post-hoc comparative outcome study of prospectively collected data. Spine J. 2013, 13, 786–795. [Google Scholar] [CrossRef]
- Salunke, P.; Karthigeyan, M.; Futane, S. Pros and Cons of C2 Nerve Sectioning/Preservation in Posterior Fusion for Congenital Atlantoaxial Dislocation. World Neurosurg. 2018, 118, e925–e932. [Google Scholar] [CrossRef]
- Jing, L.; Sun, Z.; Zhang, P.; Wang, J.; Wang, G. Accuracy of Screw Placement and Clinical Outcomes After O-Arm-Navigated Occipitocervical Fusion. World Neurosurg. 2018, 117, e653–e659. [Google Scholar] [CrossRef] [PubMed]
- Kantelhardt, S.R.; Keric, N.; Giese, A. Management of C2 fractures using Iso-C(3D) guidance: A single institution’s experience. Acta Neurochir. 2012, 154, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Jack, M.M.; Harn, N.R.; Bertsch, J.R.; Arnold, P.M. Screw placement accuracy and outcomes following o-Arm-navigated atlantoaxial fusion:a feasibility study. Glob. Spine J. 2015, 6, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, E.; Mastantuoni, C.; Cabrilo, I.; Schonauer, C. Novelties for increased safety in cranio-vertebral surgery: A review. Acta Neurochir. 2023, 165, 3027–3038. [Google Scholar] [CrossRef]
- Yu, X.; Li, L.; Wang, P.; Yin, Y.; Bu, B.; Zhou, D. Intraoperative computed tomography with an integrated navigation system in stabilization surgery for complex craniovertebral junction malformation. J. Spinal Disord. Tech. 2014, 27, 245–252. [Google Scholar] [CrossRef]
- Nottmeier, E.W.; Young, P.M. Image-guided placement of occipitocervical instrumentation using a reference arc attached to the headholder. Neurosurgery 2010, 66 (Suppl. 3), 138–142. [Google Scholar] [CrossRef]
- Motov, S.; Butenschoen, V.M.; Krauss, P.E.; Veeravagu, A.; Yoo, K.H.; Stengel, F.C.; Hejrati, N.; Stienen, M.N. Current state and future perspectives of spinal navigation and robotics-an AO spine survey. Brain Spine 2025, 5, 104165. [Google Scholar] [CrossRef] [PubMed]
- Choo, A.D.; Regev, G.; Garfin, S.R.; Kim, C.W. Surgeons’ perceptions of spinal navigation: Analysis of key factors affecting the lack of adoption of spinal navigation technology. Int. J. Spine Surg. 2008, 2, 189–194. [Google Scholar] [CrossRef]
- Gierse, J.; Mandelka, E.; Medrow, A.; Bullert, B.; Gruetzner, P.A.; Franke, J.; Vetter, S.Y. Comparison of iCT-based navigation and fluoroscopic-guidance for atlantoaxial screw placement in 78 patients with traumatic cervical spine injuries. Eur. Spine J. 2024, 33, 2304–2313. [Google Scholar] [CrossRef]
- Oearsakul, T.; Tunthanathip, T.; Kaewborisutsakul, A. Accuracy of atlantoaxial screw placement using computed tomography-based navigation system-assisted surgery: The single-level vertebral registration. Interdiscip. Neurosurg. 2023, 32, 101740. [Google Scholar] [CrossRef]
- Tanenbaum, J.E.; Lubelski, D.; Rosenbaum, B.P.; Thompson, N.R.; Benzel, E.C.; Mroz, T.E. Predictors of outcomes and hospital charges following atlantoaxial fusion. Spine J. 2016, 16, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Ploumis, A.; Mehbod, A.; Garvey, T.; Gilbert, T.; Transfeldt, E.; Wood, K. Prospective assessment of cervical fusion status: Plain radiographs versus CT-scan. Acta Orthopædica Belg. 2006, 72, 342–346. [Google Scholar]
- Rhee, J.M.; Chapman, J.R.; Norvell, D.C.; Smith, J.; Sherry, N.A.; Riew, K.D. Radiological Determination of Postoperative Cervical Fusion: A Systematic Review. Spine 2015, 40, 974–991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Shen, L.; Shao, J.; Chou, D.; Song, J.; Zhang, J. Structural Allograft versus Autograft for Instrumented Atlantoaxial Fusions in Pediatric Patients: Radiologic and Clinical Outcomes in Series of 32 Patients. World Neurosurg. 2017, 105, 549–556. [Google Scholar] [CrossRef]
- Godzik, J.; Ravindra, V.M.; Ray, W.Z.; Schmidt, M.H.; Bisson, E.F.; Dailey, A.T. Comparison of structural allograft and traditional autograft technique in occipitocervical fusion: Radiological and clinical outcomes from a single institution. J. Neurosurg. Spine 2015, 23, 144–152. [Google Scholar] [CrossRef]
- Iyer, R.R.; Tuite, G.F.; Meoded, A.; Carey, C.C.; Rodriguez, L.F. A Modified Technique for Occipitocervical Fusion Using Compressed Iliac Crest Allograft Results in a High Rate of Fusion in the Pediatric Population. World Neurosurg. 2017, 107, 342–350. [Google Scholar] [CrossRef]
- Kalanjiyam, G.P.; Chandramohan, T.; Raman, M.; Kalyanasundaram, H. Artificial intelligence: A new cutting-edge tool in spine surgery. Asian Spine J. 2024, 18, 458–471. [Google Scholar] [CrossRef]
- Krakowski, P.; Jonak, J.; Karpiński, R.; Jaworski, Ł. Usefulness of rapid prototyping in planning complex trauma surgeries. Appl. Comput. Sci. 2019, 15, 65–72. [Google Scholar] [CrossRef]
- Wei, F.; Li, Z.; Liu, Z.; Liu, X.; Jiang, L.; Yu, M.; Xu, N.; Wu, F.; Dang, L.; Zhou, H.; et al. Upper cervical spine reconstruction using customized 3D-printed vertebral body in 9 patients with primary tumors involving C2. Ann. Transl. Med. 2020, 8, 332. [Google Scholar] [CrossRef]
- Brachet, A.; Bełżek, A.; Furtak, D.; Geworgjan, Z.; Tulej, D.; Kulczycka, K.; Karpiński, R.; Maciejewski, M.; Baj, J. Application of 3D Printing in Bone Grafts. Cells 2023, 12, 859. [Google Scholar] [CrossRef]
- Zhou, L.P.; Zhang, Z.G.; Li, D.; Fang, S.; Sheng, R.; Zhang, R.J.; Shen, C.L. Robotics in Cervical Spine Surgery: Feasibility and Safety of Posterior Screw Placement. Neurospine 2023, 20, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Ghaednia, H.; Fourman, M.S.; Lans, A.; Detels, K.; Dijkstra, H.; Lloyd, S.; Sweeney, A.; Oosterhoff, J.H.; Schwab, J.H. Augmented and virtual reality in spine surgery, current applications and future potentials. Spine J. 2021, 21, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cardozo, M.A.; Barot, K.; Brehm, S.; Bui, T.; Joseph, K.; Kann, M.R.; Trevino, G.; Olufawo, M.; Singh, S.; Yahanda, A.T.; et al. Pedicle screw placement in the cervical vertebrae using augmented reality-head mounted displays: A cadaveric proof-of-concept study. Spine J. 2024, 24, 2417–2427. [Google Scholar] [CrossRef] [PubMed]
- Cabrilo, I.; Bijlenga, P.; Schaller, K. Augmented Reality as an Aid in Neurosurgery. In Youmans and Winn Neurological Surgery, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 236–246, Chapter 30. [Google Scholar]
- Porche, K.; Yan, S.C.; Mehkri, Y.; Sriram, S.; MacNeil, A.; Melnick, K.; Garvan, C.; Vaziri, S.; Seubert, C.; Murad, G.; et al. The Enhanced Recovery After Surgery pathway for posterior cervical surgery: A retrospective propensity-matched cohort study. J. Neurosurg. Spine 2023, 39, 216–227. [Google Scholar] [CrossRef]
- Jannelli, G.; Fabrizio, M.; Paun, L.; Tessitore, E.; Cabrilo, I. Minimally invasive techniques in posterior atlantoaxial fixation: A systematic review. Brain Spine 2024, 4, 102901. [Google Scholar] [CrossRef]
| Study | Selection (0–4) | Comparability (0–2) | Outcome (0–3) | Total (0–9) |
|---|---|---|---|---|
| Gelinne et al., 2023 [20] | 4 | 2 | 3 | 9 |
| Koepke et al., 2022 [21] | 3 | 1 | 2 | 6 |
| Kaminski et al., 2008 [22] | 3 | 2 | 3 | 8 |
| Schmidt et al., 2006 [23] | 3 | 2 | 3 | 8 |
| ElSaghir et al., 2005 [24] | 3 | 1 | 2 | 6 |
| Shi et al., 2020 [25] | 3 | 2 | 2 | 7 |
| Meyer et al., 2020 [26] | 4 | 2 | 3 | 9 |
| Lvov et al., 2019 [27] | 4 | 2 | 3 | 9 |
| Dusad et al., 2018 [28] | 4 | 2 | 3 | 9 |
| Alhashash et al., 2018 [29] | 4 | 2 | 2 | 8 |
| Srikantha et al., 2016 [30] | 3 | 1 | 2 | 6 |
| Díaz et al., 2014 [31] | 3 | 1 | 2 | 6 |
| Holly et al., 2010 [32] | 3 | 2 | 2 | 7 |
| Year, Author | Sample Size (n) | Gender (n, M/F) | Mean Age (Years) | Diagnosis (Type, n) | Screw (n) | Technique | Approach Type | Screw Misplacement (n) | Operative Time (Min ± SD) | Rate of Fusion (%) | Fusion | Blood Loss (mL ± SD) | H-LoS (Days ± SD) | Post Operative Follow-Up (Months) | Postoperative Pain (n ± SD, Scale) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2023, Gelinne et al. [20] | 5 | 3/2 | 70 | Trauma, 5 | 20 | Harms | Percutaneous | 0 | 234 | NA | Interarticular cages with allograft | 30 | 2 | NA | 7 (VAS) |
| 2022, Koepke et al. [21] | 23 | 17/6 | 73 | Trauma, 19 Malignancy, 3 Autoimmune, 1 | 46 | Magerl | Percutaneous | 2 | NA | 5 | No | NA | 10 ± 5.68 | 6 | 2.6 ± 2.5 (VAS) |
| 2020, Shi et al. [25] | 7 | 5/2 | 73 | Trauma, 7 | 14 | Magerl | Endoscopic assisted percutaneous unilateral | 0 | 131.1 | 100 | No | <50 | NA | 16.9 | 16.9 (NDI) |
| 2020, Meyer et al. [26] | 5 | NA | NA | Trauma, 5 | 20 | Harms | Percutaneous | 0 | NA | 80 | No | NA | 4 | 11.2 | NA |
| 2019, Lvov et al. [27] | 15 | 12/3 | 44 | Trauma, 15 | 30 | Magerl | Endoscopic assisted Transmuscular | 0 | 90 | 90 | No | 50 | NA | 58 | 1 (VAS) |
| 2018, Dusad et al. [28] | 82 | NA | 36.26 | Trauma, 54 Autoimmune, 9 Infectious, 8 Hypoplastic, 7 Osteoarthritis, 5 Syndromic, 4 | 163 | Magerl | Percutaneous | 0 | 120.11 ± 15.82 | 97.5 | Allograft (interlaminar and interarticular) | 104.84 ± 21.75 | 7 | 24 | 3.3 ± 1.12 (VAS) |
| 2018, Alhashash et al. [29] | 20 | 11/9 | 81 | Trauma, 20 | 40 | Magerl | Percutaneous | 0 | 51.75 ± 13.7 | 88 | No | 41.7 ± 31.57 | 14.15 ± 4.48 | 22.28 | 2.4 (VAS) |
| 2016, Srikantha et al. [30] | 5 | 3/2 | 45 | Instability, 3 Trauma, 2 | 20 | Harms | Transmuscolar Tubular assisted | 1 | 192 | 80 | 3 Autograft 2 Allograft (interarticular) | 260 | 7.4 | 19 | NA |
| 2014, Diaz et al. [31] | 16 | NA | 57.5 | Trauma, 8 Autoimmune, 8 | 64 | Harms | Transmuscular Tubular assisted | 0 | 193.7 | NA | Allograft (interarticular) | 404 | 2 | NA | NA |
| 2010, Holly et al. [32] | 6 | 5/1 | 51 | Trauma, 5 Os Odontoideum, 1 | 24 | Harms | Transmuscolar Tubular assisted | 0 | NA | 100 | Allograft (inter articular) | 100 | NA | 32 | NA |
| 2008, Kaminski et al. [22] | 47 | 19/28 | 74.9 | Trauma, 28 | 94 | Magerl | Percutaneous | 3 | 98 | 100 | Autograft (Gallie) | NA | NA | 42 | NA |
| 2006, Schmidt et al. [23] | 17 | 12/5 | 53.4 | Trauma, 9 Autoimmune, 8 | 34 | Magerl | Percutaneous | 0 | 110.6 ± 23.7 | NA | Autograft (Gallie) | 382.6 ± 406.2 | NA | NA | NA |
| 2005, Elsaghir et al. [24] | 57 | 3/54 | 57 | Autoimmune, 57 | 114 | Magerl | Percutaneous | 1 | NA | 98 | Autograft (Gallie) | NA | NA | 30.4 | NA |
| Patients (n) | 305 |
|---|---|
| Age (years) | 59.7 ± 14.4 |
| Technique | |
| Harms–Goel | 37 (12.1) |
| Magerl | 268 (87.9) |
| Fusion rate | 84% |
| Blood loss (ml) | 158.1 ± 150.2 |
| VA injury (n, %) | 3, 0.9 |
| Length of surgery (minutes) | 135.7 ± 58.8 |
| H-LoS (days) | 6.7 ± 4.4 |
| Follow-up (months) | 26.2 ± 15.3 |
| Magerl (N = 268) | Harms–Goel (N = 37) | p-Value | |
|---|---|---|---|
| Age (years) | 61.6 ± 16.3 | 55.9 ± 10.7 | 0.039 |
| Operative time (min) | 100.3 ± 28 | 206.6 ± 23.8 | <0.001 |
| Fusion rate (%) | 82.6 ± 34.6 | 86.7 ± 11.6 | 0.475 |
| H-LoS (days) | 9.1 ± 4.4 | 3.9 ± 2.6 | <0.001 |
| Blood loss (ml) | 125.8 ± 145.7 | 198.50 ± 167.4 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jannelli, G.; Paun, L.; Barrey, C.Y.; Borrelli, P.; Schaller, K.; Tessitore, E.; Cabrilo, I. Minimally Invasive Techniques in Posterior Atlanto-Axial Fixation: State of the Art and Systematic Review. J. Clin. Med. 2025, 14, 4657. https://doi.org/10.3390/jcm14134657
Jannelli G, Paun L, Barrey CY, Borrelli P, Schaller K, Tessitore E, Cabrilo I. Minimally Invasive Techniques in Posterior Atlanto-Axial Fixation: State of the Art and Systematic Review. Journal of Clinical Medicine. 2025; 14(13):4657. https://doi.org/10.3390/jcm14134657
Chicago/Turabian StyleJannelli, Gianpaolo, Luca Paun, Cédric Y. Barrey, Paola Borrelli, Karl Schaller, Enrico Tessitore, and Ivan Cabrilo. 2025. "Minimally Invasive Techniques in Posterior Atlanto-Axial Fixation: State of the Art and Systematic Review" Journal of Clinical Medicine 14, no. 13: 4657. https://doi.org/10.3390/jcm14134657
APA StyleJannelli, G., Paun, L., Barrey, C. Y., Borrelli, P., Schaller, K., Tessitore, E., & Cabrilo, I. (2025). Minimally Invasive Techniques in Posterior Atlanto-Axial Fixation: State of the Art and Systematic Review. Journal of Clinical Medicine, 14(13), 4657. https://doi.org/10.3390/jcm14134657








