An Updated Systematic Review and Meta-Analysis of Diagnostic Accuracy of Dynamic Contrast Enhancement and Diffusion-Weighted MRI in Differentiating Benign and Malignant Non-Mass Enhancement Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
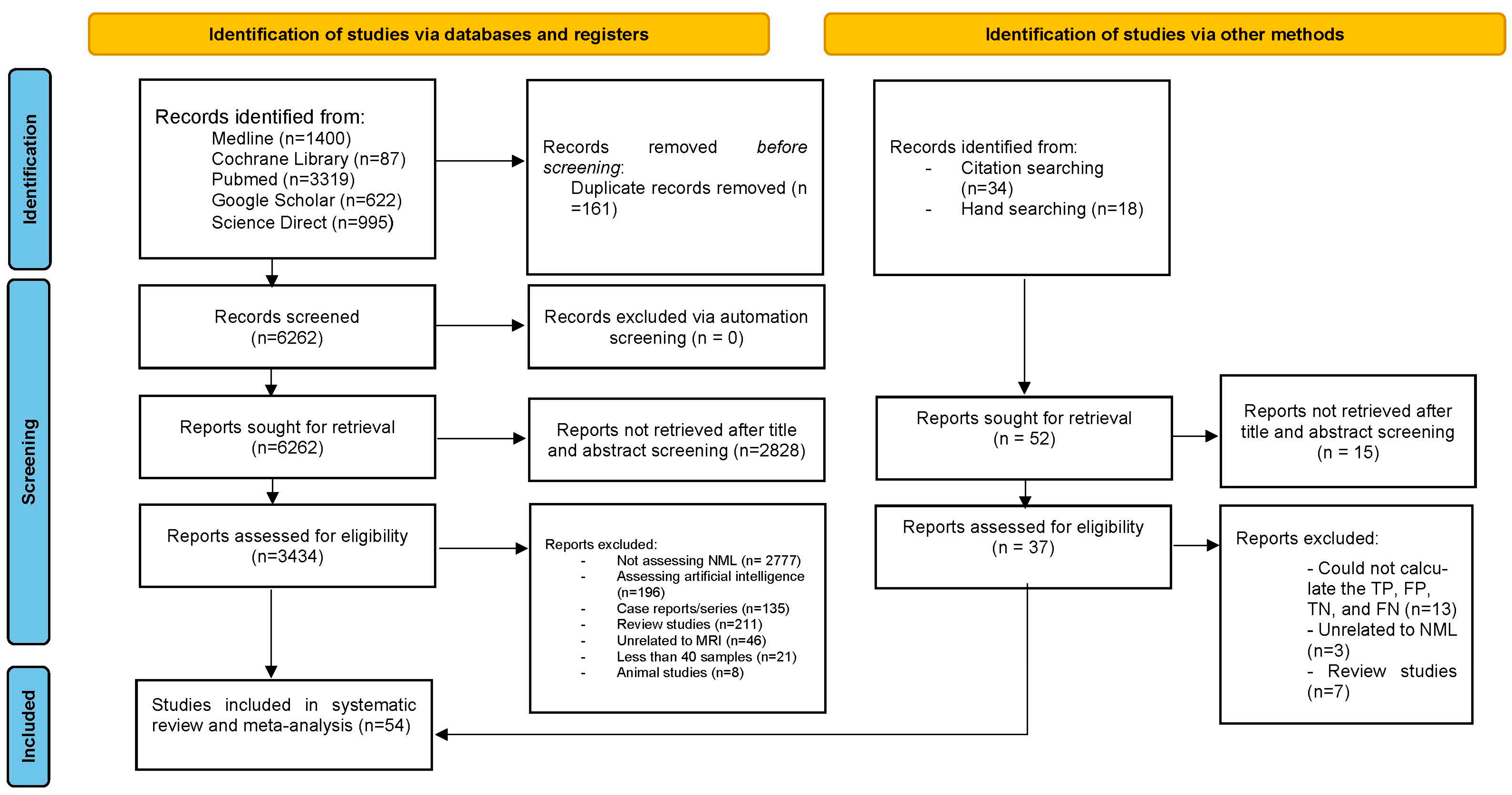
2.2. Search Strategy and Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis
3. Results
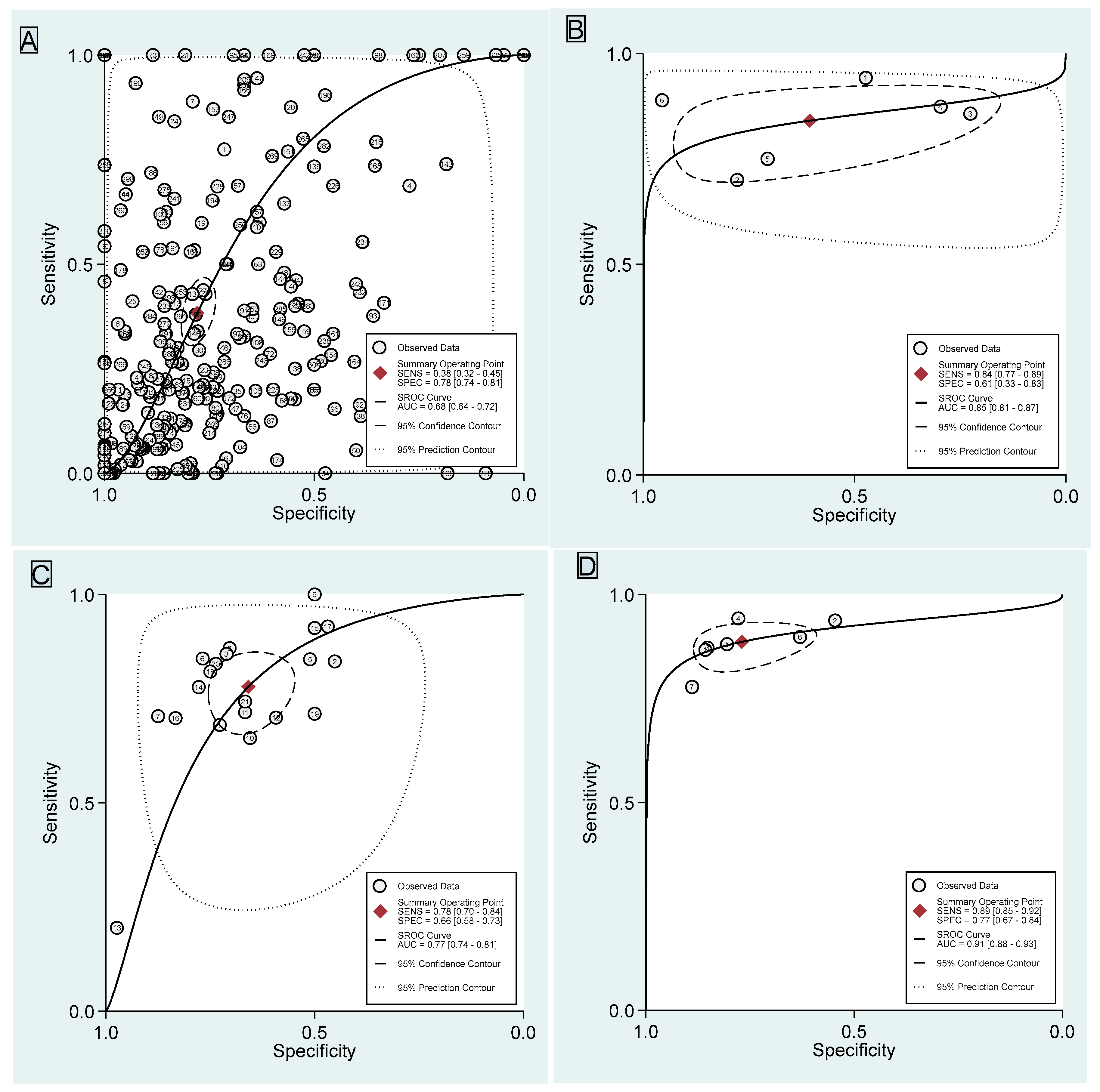
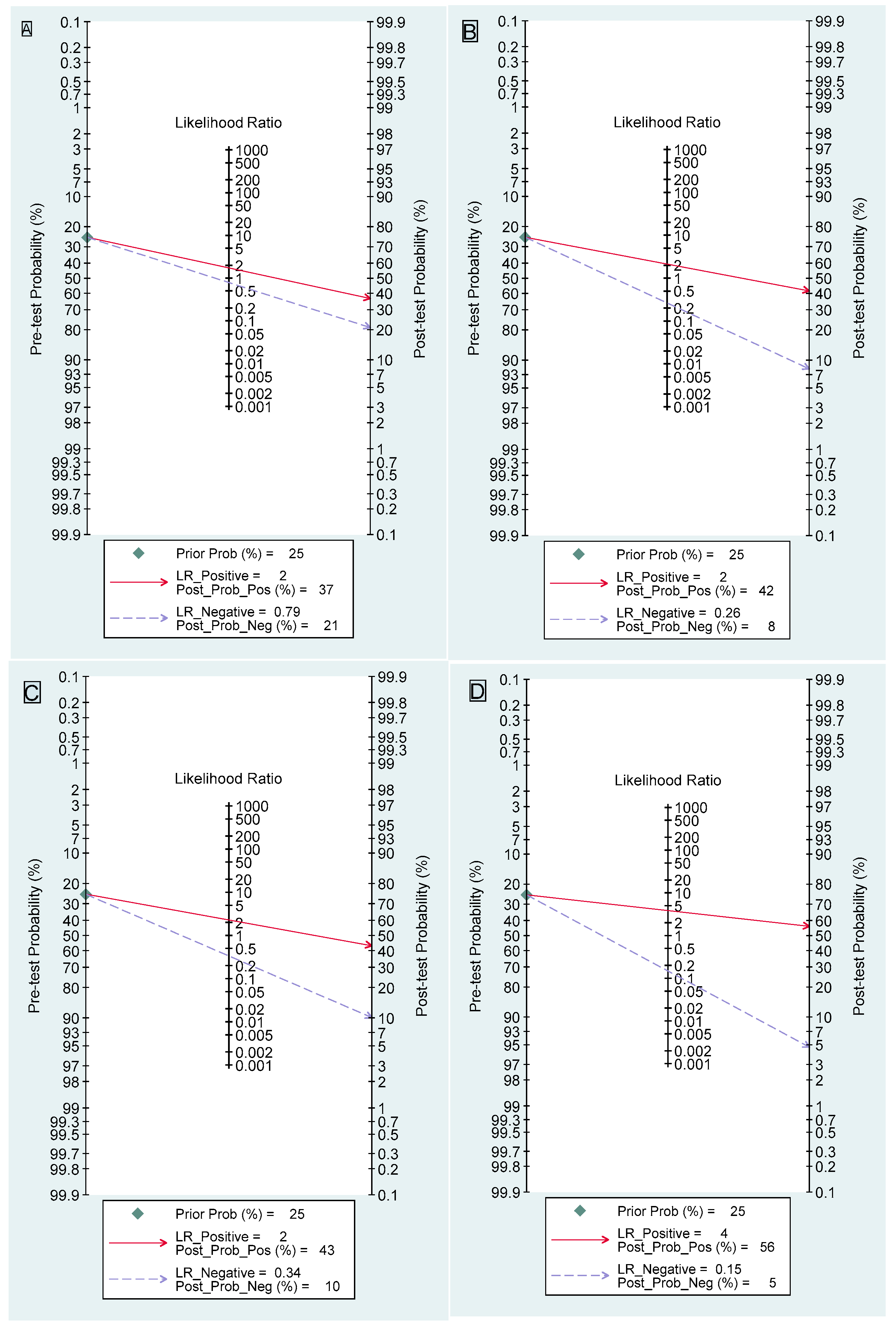
3.1. Dynamic Contrast-Enhanced Magnetic Resonance Imaging
3.2. Diffusion-Weighted Imaging
3.3. Apparent Diffusion Coefficient
3.4. Dynamic Contrast-Enhanced Magnetic Resonance Imaging Combined with Diffusion-Weighted Imaging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | Apparent Diffusion Coefficient |
| AUC | Area Under the Curve |
| BI-RADS | Breast Imaging Reporting and Data System |
| CI | Confidence Interval |
| DCIS | Ductal Carcinoma In Situ |
| DCE-MRI | Dynamic Contrast-Enhanced Magnetic Resonance Imaging |
| DWI | Diffusion-Weighted Imaging |
| FN | False Negative |
| FP | False Positive |
| HSROC | Hierarchical Summary Receiver Operating Characteristic |
| IEP | Internal Enhancement Pattern |
| LR+ | Positive Likelihood Ratio |
| LR− | Negative Likelihood Ratio |
| Mp-MRI | Multiparametric Magnetic Resonance Imaging |
| MRI | Magnetic Resonance Imaging |
| NME | Non-Mass Enhancement |
| NPV | Negative Predictive Value |
| PPV | Positive Predictive Value |
| PRISMA-DTA | Preferred Reporting Items for Systematic Review and Meta-Analysis of Diagnostic Test Accuracy |
| PROSPERO | International Prospective Register of Systematic Reviews |
| QUADAS-2 | Quality Assessment of Diagnostic Accuracy Studies-2 |
| ROI | Region of Interest |
| SROC | Summary Receiver Operating Characteristic |
| TE | Echo Time |
| TN | True Negative |
| TP | True Positive |
| TR | Repetition Time |
References
- de Faria Castro Fleury, E.; Castro, C.; do Amaral, M.S.C.; Roveda Junior, D. Management of Non-Mass Enhancement at Breast Magnetic Resonance in Screening Settings Referred for Magnetic Resonance-Guided Biopsy. Breast Cancer Basic Clin. Res. 2022, 16, 11782234221095897. [Google Scholar] [CrossRef] [PubMed]
- Scaranelo, A.M. What’s Hot in Breast MRI. Can. Assoc. Radiol. J. 2022, 73, 125–140. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A. ACR BI-RADS Atlas: Breast Imaging Re-porting and Data System. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads (accessed on 21 December 2024).
- Lee, S.M.; Nam, K.J.; Choo, K.S.; Kim, J.Y.; Jeong, D.W.; Kim, H.Y.; Kim, J.Y. Patterns of malignant non-mass enhancement on 3-T breast MRI help predict invasiveness: Using the BI-RADS lexicon fifth edition. Acta Radiol. 2018, 59, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Bayoumi, D.; Shokeir, F.A.; Karam, R.; Elboghdady, A. Validity of dynamic contrast-enhanced magnetic resonance imaging of the breast versus diffusion-weighted imaging and magnetic resonance spectroscopy in predicting the malignant nature of non-mass enhancement lesions. Egypt. J. Radiol. Nucl. Med. 2024, 55, 94. [Google Scholar] [CrossRef]
- Kataoka, M.; Iima, M.; Miyake, K.K.; Matsumoto, Y. Multiparametric imaging of breast cancer: An update of current applications. Diagn. Interv. Imaging 2022, 103, 574–583. [Google Scholar] [CrossRef]
- Avendano, D.; Marino, M.A.; Leithner, D.; Thakus, S.; Bernard-Davila, B.; Martinez, D.; Helbich, T.; Morris, E.; Jochelson, M.; Baltzer, P.; et al. Limited role of DWI with apparent diffusion coefficient mapping in breast lesions presenting as non-mass enhancement on dynamic contrast-enhanced MRI. Breast Cancer Res. 2019, 21, 136. [Google Scholar] [CrossRef]
- Baltzer, P.; Mann, R.M.; Iima, M.; Sigmund, E.; Clauser, P.; Gilbert, F.; Martincich, L.; Partridge, S.; Patterson, A.; Pinker, K.; et al. Diffusion-weighted imaging of the breast-a consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. Eur. Radiol. 2020, 30, 1436–1450. [Google Scholar] [CrossRef]
- Parsian, S.; Rahbar, H.; Allison, K.H.; Demartini, W.; Olson, M.; Lehman, C.; Partridge, S. Nonmalignant breast lesions: ADCs of benign and high-risk subtypes assessed as false-positive at dynamic enhanced MR imaging. Radiology 2012, 265, 696–706. [Google Scholar] [CrossRef]
- Partridge, S.C.; Mullins, C.D.; Kurland, B.F.; Allain, M.; DeMartini, W.; Eby, P.; Lehman, C. Apparent diffusion coefficient values for discriminating benign and malignant breast MRI lesions: Effects of lesion type and size. AJR Am. J. Roentgenol. 2010, 194, 1664–1673. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, H.; Li, X.; Liu, P.; Zhang, S.; Cao, S. Morphological Distribution and Internal Enhancement Architecture of Contrast-Enhanced Magnetic Resonance Imaging in the Diagnosis of Non-Mass-Like Breast Lesions: A Meta-Analysis. Breast J. 2013, 19, 259–268. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Zhang, L.; Zhe, X.; Tang, M.; Lei, X.; Zhang, X. Meta-analysis of dynamic contrast enhancement and diffusion-weighted MRI for differentiation of benign from malignant non-mass enhancement breast lesions. Front. Oncol. 2024, 14, 1332783. [Google Scholar] [CrossRef]
- Salameh, J.P.; Bossuyt, P.M.; McGrath, T.A.; McGrath, T.; Thombs, B.; Hyde, C.; Macaskill, P.; Deeks, J.; Leeflang, M.; Korevaar, D.; et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): Explanation, elaboration, and checklist. BMJ 2020, 370, m2632. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.A.; Comstock, C.E.; Lee, C.H. ACR BI-RADS® Magnetic Resonance Imaging. In ACR BI-RADS® Atlas; Breast Imaging Reporting and Data System: Reston, VA, USA, 2024; Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads (accessed on 21 December 2024).
- Muljadi, R.; Koesbandono Octavius, G.S. A systematic review and meta-analysis of diagnostic test accuracy of chest ultrasound in diagnosing pediatric pulmonary tuberculosis. Pediatr. Pulmonol. 2024, 59, 2381–2391. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.; Westwood, M.; Beynon, R.; Burke, M.; Sterne, J.A.; Glanville, J. Inclusion of methodological filters in searches for diagnostic test accuracy studies misses relevant studies. J. Clin. Epidemiol. 2011, 64, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 1–10. [Google Scholar] [CrossRef]
- Carol, H.L.; Jordana, P.; Janice, S.S.; John, M.L.; Mary, S.N. Contrast-Enhanced Mammography (CEM)—(A Supplement to ACR BI-RADS® Mammography 2013); American College of Radiology (ACR): Reston, VA, USA, 2025; Available online: https://www.acr.org/Clinical-Resources/Clinical-Tools-and-Reference/Reporting-and-Data-Systems/BI-RADS (accessed on 21 December 2024).
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.; Reitsma, J.; Leeflang, M.; Sterne, J.; Bossuyt, P. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.; Scholten, R.J.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef]
- Rutter, C.M.; Gatsonis, C.A. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat. Med. 2001, 20, 2865–2884. [Google Scholar] [CrossRef]
- Young Ho, L. Overview of the Process of Conducting Meta-analyses of the Diagnostic Test Accuracy. J. Rheum. Dis. 2018, 25, 3–10. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Stengel, D.; Bauwens, K.; Sehouli, J.; Ekkernkamp, A.; Porzsolt, F. A likelihood ratio approach to meta-analysis of diagnostic studies. J. Med. Screen. 2003, 10, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fine, J.P.; Safdar, N. Prevalence-dependent diagnostic accuracy measures. Stat. Med. 2007, 26, 3258–3273. [Google Scholar] [CrossRef] [PubMed]
- Dwamena, B.; Sylvester, R.; Carlos, R.C. MIDAS: Stata module for meta-analytical integration of diagnostic test accuracy studies. Stata. J. 2007, 12, 605–622. [Google Scholar]
- Yabuuchi, H.; Matsuo, Y.; Kamitani, T.; Setoguchi, T.; Okafuji, T.; Soeda, H.; Sakai, S.; Hatakenaka, M.; Kubo, M.; Tokunaga, E.; et al. Non-mass-like enhancement on contrast-enhanced breast MR imaging: Lesion characterization using combination of dynamic contrast-enhanced and diffusion-weighted MR images. Eur. J. Radiol. 2010, 75, e126–e132. [Google Scholar] [CrossRef]
- Imamura, T.; Isomoto, I.; Sueyoshi, E.; Yano, H.; Uga, T.; Abe, K.; Hayashi, T.; Honda, S.; Yamaguchi, T.; Uetani, M. Diagnostic performance of ADC for Non-mass-like breast lesions on MR imaging. Magn. Reson. Med. Sci. 2010, 9, 217–225. [Google Scholar] [CrossRef]
- Sakamoto, N.; Tozaki, M.; Higa, K.; Tsunoda, Y.; Ogawa, T.; Abe, S.; Ozaki, S.; Sakamoto, M.; Tsuruhara, T.; Kawano, N.; et al. Categorization of non-mass-like breast lesions detected by MRI. Breast Cancer 2008, 15, 241–246. [Google Scholar] [CrossRef]
- Sotome, K.; Yamamoto, Y.; Hirano, A.; Takahara, T.; Hasegawa, S.; Nakamaru, M.; Furukawa, A.; Miyazaki, H.; Morozumi, K.; Onishi, T.; et al. The role of contrast enhanced MRI in the diagnosis of non-mass image-forming lesions on breast ultrasonography. Breast Cancer 2007, 14, 371–380. [Google Scholar] [CrossRef]
- Thomassin-Naggara, I.; Trop, I.; Chopier, J.; David, J.; Lalonde, L.; Darai, E.; Rouzier, R.; Uzan, S. Nonmasslike enhancement at breast MR imaging: The added value of mammography and US for lesion categorization. Radiology 2011, 261, 69–79. [Google Scholar] [CrossRef]
- Di Ninno, A.A.M.; Mello, G.G.N.; Torres, U.S.; Shimizu, C.; Tucunduva, T.; Reis, F.; Ananias, M.; Lederman, H. MRI as a complementary tool for the assessment of suspicious mammographic calcifications: Does it have a role? Clin. Imaging 2021, 74, 76–83. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Chen, S.L.; Chen, Q. Non-mass enhancement breast lesions: MRI findings and associations with malignancy. Ann. Transl. Med. 2022, 10, 357. [Google Scholar] [CrossRef]
- Aydin, H. The MRI characteristics of non-mass enhancement lesions of the breast: Associations with malignancy. Br. J. Radiol. 2019, 92, 20180464. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Y.L.; Zhang, Y.; Chen, J.; Combs, F.; Parajuli, R.; Mehta, R.; Liu, H.; Chen, Z.; Zhao, Y.; et al. BI-RADS Reading of Non-Mass Lesions on DCE-MRI and Differential Diagnosis Performed by Radiomics and Deep Learning. Front. Oncol. 2021, 11, 728224. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Bai, Y.; Zhang, J.; Liu, M.; Li, X.; Zhang, A.; Zhang, X.; Ma, L. Optimization of apparent diffusion coefficient measured by diffusion-weighted MRI for diagnosis of breast lesions presenting as mass and non-mass-like enhancement. Tumour. Biol. 2013, 34, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.A.; Avendano, D.; Sevilimedu, V.; Thakur, S.; Martinez, D.; Gullo, R.; Horvat, J.; Helbich, T.; Baltzer, P.; Pinker, K. Limited value of multiparametric MRI with dynamic contrast-enhanced and diffusion-weighted imaging in non-mass enhancing breast tumors. Eur. J. Radiol. 2022, 156, 110523. [Google Scholar] [CrossRef]
- Kul, S.; Eyuboglu, I.; Cansu, A.; Alhan, E. Diagnostic efficacy of the diffusion weighted imaging in the characterization of different types of breast lesions. J. Magn. Reson. Imaging 2014, 40, 1158–1164. [Google Scholar] [CrossRef]
- Yang, X.; Dong, M.; Li, S.; Chai, R.; Zhang, Z.; Li, N.; Zhang, L. Diffusion-weighted imaging or dynamic contrast-enhanced curve: A retrospective analysis of contrast-enhanced magnetic resonance imaging-based differential diagnoses of benign and malignant breast lesions. Eur. Radiol. 2020, 30, 4795–4805. [Google Scholar] [CrossRef]
- Liu, D.; Ba, Z.; Gao, Y.; Wang, L. Subcategorization of suspicious non-mass-like enhancement lesions(BI-RADS-MRI Category4). BMC Med. Imaging 2023, 23, 182. [Google Scholar] [CrossRef]
- Zang, H.; Liu, H.L.; Zhu, L.Y.; Wang, X.; Wei, L.; Lou, J.; Zou, Q.; Wang, S.; Wang, S.; Jiang, Y. Diagnostic performance of DCE-MRI, multiparametric MRI and multimodality imaging for discrimination of breast non-mass-like enhancement lesions. Br. J. Radiol. 2022, 95, 20220211. [Google Scholar] [CrossRef]
- Coskun Bilge, A.; Demir, P.I.; Aydin, H.; Bostanci, I.E. Dynamic contrast-enhanced breast magnetic resonance imaging findings that affect the magnetic resonance-directed ultrasound correlation of non-mass enhancement lesions: A single-center retrospective study. Br. J. Radiol. 2022, 95, 20210832. [Google Scholar] [CrossRef]
- Kwon, B.R.; Chang, J.M.; Kim, S.Y.; Lee, S.H.; Shin, S.U.; Yi, A.; Cho, N.; Moon, W.K. Utility and Diagnostic Performance of Automated Breast Ultrasound System in Evaluating Pure Non-Mass Enhancement on Breast Magnetic Resonance Imaging. Korean J. Radiol. 2020, 21, 1210–1219. [Google Scholar] [CrossRef]
- Lv, W.; Zheng, D.; Guan, W.; Wu, P. Contribution of Diffusion-Weighted Imaging and ADC Values to Papillary Breast Lesions. Front. Oncol. 2022, 12, 911790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, J.; Jin, L.; Jia, C.; Shi, Q.; Wu, R. Comparison of the diagnostic value of contrast-enhanced ultrasound combined with conventional ultrasound versus magnetic resonance imaging in malignant non-mass breast lesions. Br. J. Radiol. 2023, 96, 20220880. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Z.; Lv, W.; Qin, Y.; Tang, C.; Yan, X.; Yin, T.; Ai, T.; Xia, L. Role of combined clinical-radiomics model based on contrast-enhanced MRI in predicting the malignancy of breast non-mass enhancements without an additional diffusion-weighted imaging sequence. Quant. Imaging Med. Surg. 2023, 13, 5974–5985. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Xie, T.; Fu, C.; Chen, L.; Bai, Q.; Grimm, R.; Peng, W.; Wang, S. Differentiation between idiopathic granulomatous mastitis and invasive breast carcinoma, both presenting with non-mass enhancement without rim-enhanced masses: The value of whole-lesion histogram and texture analysis using apparent diffusion coefficient. Eur. J. Radiol. 2020, 123, 108782. [Google Scholar] [CrossRef]
- Yang, Q.X.; Ji, X.; Feng, L.L.; Zheng, L.; Zhou, X.Q.; Wu, Q.; Chen, X. Significant MRI indicators of malignancy for breast non-mass enhancement. J. X-Ray Sci. Technol. 2017, 25, 1033–1044. [Google Scholar] [CrossRef]
- Chen, S.T.; Covelli, J.; Okamoto, S.; Daniel, B.L.; DeMartini, W.B.; Ikeda, D.M. Clumped vs non-clumped internal enhancement patterns in linear non-mass enhancement on breast MRI. Br. J. Radiol. 2021, 94, 20201166. [Google Scholar] [CrossRef]
- Asada, T.; Yamada, T.; Kanemaki, Y.; Fujiwara, K.; Okamoto, S.; Nakajima, Y. Grading system to categorize breast MRI using BI-RADS 5th edition: A statistical study of non-mass enhancement descriptors in terms of probability of malignancy. Jpn. J. Radiol. 2018, 36, 200–208. [Google Scholar] [CrossRef]
- Spick, C.; Pinker-Domenig, K.; Rudas, M.; Helbich, T.H.; Baltzer, P.A. MRI-only lesions: Application of diffusion-weighted imaging obviates unnecessary MR-guided breast biopsies. Eur. Radiol. 2014, 24, 1204–1210. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Gao, J.; Jiang, L.; Chen, M. Quantitative apparent diffusion coefficient metrics for MRI-only suspicious breast lesions: Any added clinical value? Quant. Imaging Med. Surg. 2023, 13, 7092–7104. [Google Scholar] [CrossRef]
- Mohamed, S.; Elhamd, E.A.; Attia, N.M. Non-mass enhancement on breast MRI: Clues to a more confident diagnosis. Egypt. J. Radiol. Nucl. Med. 2024, 55, 87. [Google Scholar] [CrossRef]
- Niu, R.L.; Li, J.K.; Wang, B.; Jiang, Y.; Li, S.Y.; Fu, N.Q.; Liu, G.; Wang, Z.L. Combination of Breast Ultrasound With Magnetic Resonance Imaging in the Diagnosis of Non-mass-like Breast Lesions Detected on Ultrasound: A New Integrated Strategy to Improve Diagnostic Performance. Ultrasound Med. Biol. 2024, 50, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ahmadinejad, N.; Azizinik, F.; Khosravi, P.; Torabi, A.; Mohajeri, A.; Arian, A. Evaluation of Features in Probably Benign and Malignant Nonmass Enhancement in Breast MRI. Int. J. Breast Cancer 2024, 2024, 6661849. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zong, M.; Gong, H.Y.; Ling, L.J.; Ye, X.H.; Wang, S.; Li, C.Y. Comparison of Diagnostic Efficacy Between Contrast-Enhanced Ultrasound and DCE-MRI for Mass- and Non-Mass-Like Enhancement Types in Breast Lesions. Cancer Manag. Res. 2020, 12, 13567–13578. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.A.; Shimauchi, A.; Zak, L.; Fan, X.; Karczmar, G.S.; Newstead, G.M. The diverse pathology and kinetics of mass, nonmass, and focus enhancement on MR imaging of the breast. J. Magn. Reson. Imaging 2011, 33, 1382–1389. [Google Scholar] [CrossRef]
- Tang, W.; Chen, L.; Jin, Z.; Liang, Y.; Zuo, W.; Wei, X.; Guo, Y.; Kong, Q.; Jiang, X. The diagnostic dilemma with the plateau pattern of the time-intensity curve: Can the relative apparent diffusion coefficient (rADC) optimise the ADC parameter for differentiating breast lesions? Clin. Radiol. 2021, 76, 688–695. [Google Scholar] [CrossRef]
- Liberman, L.; Morris, E.A.; Lee, M.J.; Kaplan, J.B.; LaTrenta, L.R.; Menell, J.H.; Abramson, A.F.; Dashnaw, S.M.; Ballon, D.J.; Dershaw, D.D. Breast lesions detected on MR imaging: Features and positive predictive value. AJR Am. J. Roentgenol. 2002, 179, 171–178. [Google Scholar] [CrossRef]
- Lunkiewicz, M.; Forte, S.; Freiwald, B.; Singer, G.; Leo, C.; Kubik-Huch, R.A. Interobserver variability and likelihood of malignancy for fifth edition BI-RADS MRI descriptors in non-mass breast lesions. Eur. Radiol. 2020, 30, 77–86. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, H.K.; Park, A.Y.; Ko, K.H.; Jang, H. Diagnostic value of mammography for accompanying non-mass enhancement on preoperative breast MRI. Acta Radiol. 2022, 63, 1032–1042. [Google Scholar] [CrossRef]
- Clauser, P.; Krug, B.; Bickel, H.; Dietzel, M.; Pinker, K.; Neuhaus, V.F.; Marino, M.A.; Moschetta, M.; Troiano, N.; Helbich, T.H.; et al. Diffusion-weighted Imaging Allows for Downgrading MR BI-RADS 4 Lesions in Contrast-enhanced MRI of the Breast to Avoid Unnecessary Biopsy. Clin. Cancer Res. 2021, 27, 1941–1948. [Google Scholar] [CrossRef]
- Cho, Y.H.; Cho, K.R.; Park, E.K.; Seo, B.K.; Woo, O.H.; Cho, S.B.; Bae, J.W. Significance of Additional Non-Mass Enhancement in Patients with Breast Cancer on Preoperative 3T Dynamic Contrast Enhanced MRI of the Breast. Iran. J. Radiol. 2016, 13, e30909. [Google Scholar] [CrossRef]
- Uematsu, T.; Kasami, M. High-spatial-resolution 3-T breast MRI of nonmasslike enhancement lesions: An analysis of their features as significant predictors of malignancy. AJR Am. J. Roentgenol. 2012, 198, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Ballesio, L.; Di Pastena, F.; Gigli, S.; D’ambrosio, I.; Aceti, A.; Pontico, M.; Manganaro, L.; Porfiri, L.M.; Tardioli, S. Non mass-like enhancement categories detected by breast MRI and histological findings. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 910–917. [Google Scholar] [PubMed]
- Tozaki, M.; Fukuma, E. 1H MR spectroscopy and diffusion-weighted imaging of the breast: Are they useful tools for characterizing breast lesions before biopsy? AJR Am. J. Roentgenol. 2009, 193, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Tozaki, M.; Igarashi, T.; Fukuda, K. Breast MRI using the VIBE sequence: Clustered ring enhancement in the differential diagnosis of lesions showing non-masslike enhancement. AJR Am. J. Roentgenol. 2006, 187, 313–321. [Google Scholar] [CrossRef]
- Wilhelm, A.; McDonough, M.D.; DePeri, E.R. Malignancy rates of non-masslike enhancement on breast magnetic resonance imaging using American College of Radiology Breast Imaging Reporting and Data System descriptors. Breast J. 2012, 18, 523–526. [Google Scholar] [CrossRef]
- Gity, M.; Ghazi Moghadam, K.; Jalali, A.H.; Shakiba, M. Association of Different MRI BIRADS Descriptors With Malignancy in Non Mass-Like Breast Lesions. Iran. Red. Crescent. Med. J. 2014, 16, e26040. [Google Scholar] [CrossRef]
- Chikarmane, S.A.; Michaels, A.Y.; Giess, C.S. Revisiting Nonmass Enhancement in Breast MRI: Analysis of Outcomes and Follow-Up Using the Updated BI-RADS Atlas. AJR Am. J. Roentgenol. 2017, 209, 1178–1184. [Google Scholar] [CrossRef]
- Liberman, L.; Morris, E.A.; Dershaw, D.D.; Abramson, A.F.; Tan, L.K. Ductal enhancement on MR imaging of the breast. AJR Am. J. Roentgenol. 2003, 181, 519–525. [Google Scholar] [CrossRef]
- Yuen, S.; Uematsu, T.; Masako, K.; Uchida, Y.; Nishimura, T. Segmental enhancement on breast MR images: Differential diagnosis and diagnostic strategy. Eur. Radiol. 2008, 18, 2067–2075. [Google Scholar] [CrossRef]
- Tokuda, Y.; Kuriyama, K.; Nakamoto, A.; Choi, S.; Yutani, K.; Kunitomi, Y.; Haneda, T.; Kawai, M.; Masuda, N.; Takeda, M.; et al. Evaluation of suspicious nipple discharge by magnetic resonance mammography based on breast imaging reporting and data system magnetic resonance imaging descriptors. J. Comput. Assist. Tomogr. 2009, 33, 58–62. [Google Scholar] [CrossRef]
- Goto, M.; Ito, H.; Akazawa, K.; Kubota, T.; Kizu, O.; Yamada, K.; Nishimura, T. Diagnosis of breast tumors by contrast-enhanced MR imaging: Comparison between the diagnostic performance of dynamic enhancement patterns and morphologic features. J. Magn. Reson. Imaging 2007, 25, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Baltzer, P.A.; Dietzel, M.; Kaiser, W.A. Nonmass lesions in magnetic resonance imaging of the breast: Additional T2-weighted images improve diagnostic accuracy. J. Comput. Assist. Tomogr. 2011, 35, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Baltzer, P.A.; Benndorf, M.; Dietzel, M.; Gajda, M.; Runnebaum, I.B.; Kaiser, W.A. False-positive findings at contrast-enhanced breast MRI: A BI-RADS descriptor study. AJR Am. J. Roentgenol. 2010, 194, 1658–1663. [Google Scholar] [CrossRef]
- Moukhtar, F.Z.; Abu El Maati, A.A. Apparent diffusion coefficient values as an adjunct to dynamic contrast enhanced MRI for discriminating benign and malignant breast lesions presenting as mass and non-mass like enhancement. Egypt. J. Radiol. Nucl. Med. 2014, 45, 597–604. [Google Scholar] [CrossRef]
- Spak, D.A.; Plaxco, J.S.; Santiago, L.; Dryden, M.J.; Dogan, B.E. BI-RADS(®) fifth edition: A summary of changes. Diagn. Interv. Imaging 2017, 98, 179–190. [Google Scholar] [CrossRef]
- Zarcaro, C.; Santonocito, A.; Zeitouni, L.; Ferrara, F.; Kapetas, P.; Milos, R.I.; Helbich, T.H.; Baltzer, P.A.T.; Clauser, P. Inter-reader agreement of the BI-RADS CEM lexicon. Eur. Radiol. 2024, 35, 2378–2386. [Google Scholar] [CrossRef]
- Chadashvili, T.; Ghosh, E.; Fein-Zachary, V.; Mehta, T.S.; Venkataraman, S.; Dialani, V.; Slanetz, P.J. Nonmass enhancement on breast MRI: Review of patterns with radiologic-pathologic correlation and discussion of management. AJR Am. J. Roentgenol. 2015, 204, 219–227. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Liu, D.; Sheng, F.; Cai, J. Differential Diagnosis of Benign and Malignant Breast Papillary Neoplasms on MRI With Non-mass Enhancement. Acad. Radiol. 2023, 30 (Suppl. 2), S127–S132. [Google Scholar] [CrossRef]
- Cheng, L.; Li, X. Breast magnetic resonance imaging: Kinetic curve assessment. Gland. Surg. 2013, 2, 50–53. [Google Scholar] [CrossRef]
- Cheng, L.; Li, X. Breast magnetic resonance imaging: Non-mass-like enhancement. Gland Surg. 2012, 1, 176–188. [Google Scholar]
- Kessler, L.G.; Barnhart, H.X.; Buckler, A.J.; Choudhury, K.R.; Kondratovich, M.V.; Toledano, A.; Guimaraes, A.R.; Filice, R.; Zhang, Z.; Sullivan, D.C. The emerging science of quantitative imaging biomarkers terminology and definitions for scientific studies and regulatory submissions. Stat. Methods Med. Res. 2015, 24, 9–26. [Google Scholar] [CrossRef]
- Qu, R.F.; Guo, D.R.; Chang, Z.X.; Meng, J.; Sun, Y.; Hao, S.H.; Shi, G.; Sun, J. Differential Diagnosis of Benign and Malignant Breast Tumors Using Apparent Diffusion Coefficient Value Measured Through Diffusion-Weighted Magnetic Resonance Imaging. J. Comput. Assist. Tomogr. 2015, 39, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Dorrius, M.D.; Dijkstra, H.; Oudkerk, M.; Sijens, P.E. Effect of b value and pre-admission of contrast on diagnostic accuracy of 1.5-T breast DWI: A systematic review and meta-analysis. Eur. Radiol. 2014, 24, 2835–2847. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, M.; Sghedoni, R.; Iacconi, C.; Iori, M.; Traino, A.C.; Guerrisi, M.; Mascalchi, M.; Toschi, N.; Diciotti, S. MR Scanner Systems Should Be Adequately Characterized in Diffusion-MRI of the Breast. PLoS ONE 2014, 9, e86280. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-R.; Chen, K.-Y.; Li, P.; Xia, Z.-Y.; Wang, B. Accuracy of multiparametric MRI in distinguishing the breast malignant lesions from benign lesions: A meta-analysis. Acta Radiol. 2021, 62, 1290–1297. [Google Scholar] [CrossRef]
- Marino, M.A.; Helbich, T.; Baltzer, P.; Pinker-Domenig, K. Multiparametric MRI of the breast: A review. J. Magn. Reson. Imaging 2018, 47, 301–315. [Google Scholar] [CrossRef]
- Chopier, J.; Dratwa, C.; Antoine, M.; Gonin, J.; Thomassin Naggara, I. Radiopathological correlations: Masses, non-masslike enhancements and MRI-guided biopsy. Diagn. Interv. Imaging 2014, 95, 213–225. [Google Scholar] [CrossRef]
- Newburg, A.R.; Chhor, C.M.; Young Lin, L.L.; Heller, S.L.; Gillman, J.; Toth, H.K.; Moy, L. Magnetic Resonance Imaging-Directed Ultrasound Imaging of Non-Mass Enhancement in the Breast: Outcomes and Frequency of Malignancy. J. Ultrasound Med. 2017, 36, 493–504. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G. Chapter 10: Analysing data and undertaking meta-analysis. In Cochrane Handbook for Systematic Reviews for Intervention, 2nd ed.; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Singapore, 2019; pp. 241–284. [Google Scholar]
- Shah, J.S.; Sequeira, L.; Andrews, P.; Fultineer, K.; Martin, D.; Kwapisz, L. S1525 The File Drawer Effect in the Gastroenterology and Hepatology Literature. Off. J. Am. Coll. Gastroenterol. ACG 2022, 117, e1093. [Google Scholar] [CrossRef]
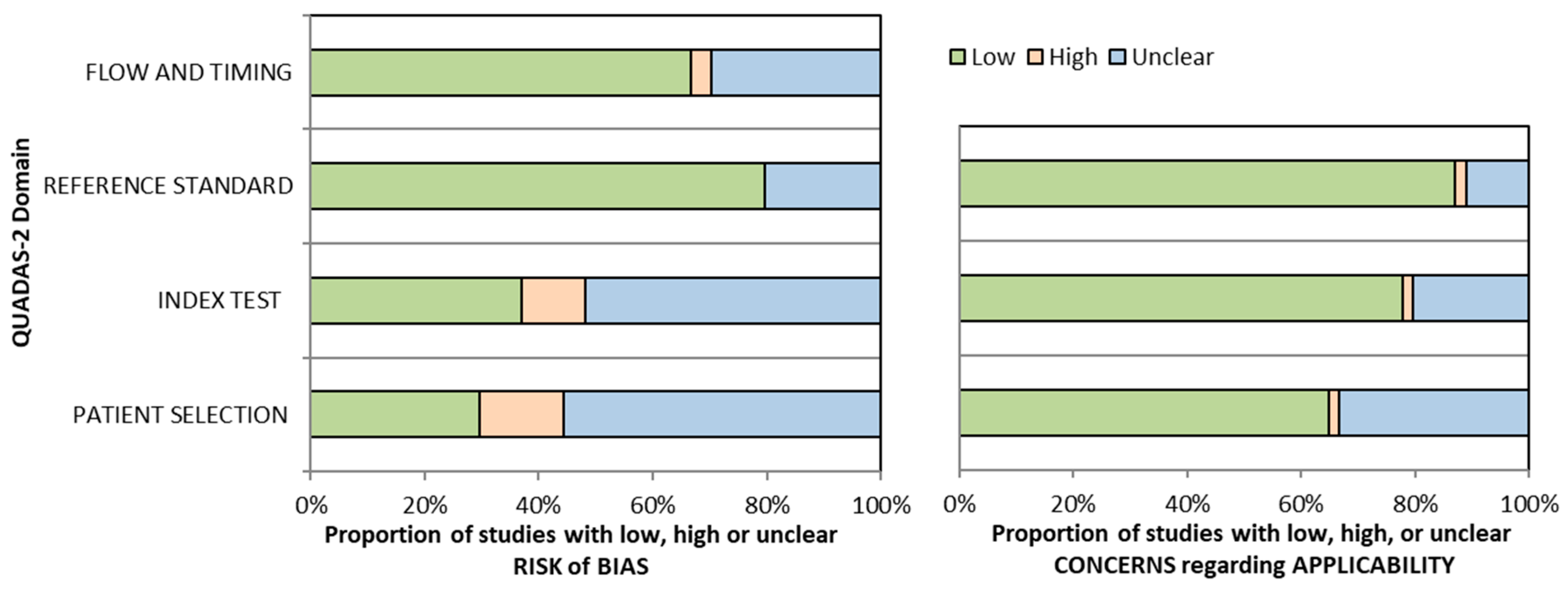
| Study | Risk of Bias | Applicability Concerns | Conclusions | |||||
|---|---|---|---|---|---|---|---|---|
| Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | ||
| Yabuuchi (2009) [27] |  |  |  |  |  |  |  | High risk of bias |
| Baltzer (2011) [75] |  |  |  |  |  |  |  | At risk of bias |
| Sakamoto (2008) [29] |  |  |  |  |  |  |  | At risk of bias |
| Imamura (2010) [28] |  |  |  |  |  |  |  | High risk of bias |
| Sotome (2007) [30] |  |  |  |  |  |  |  | High risk of bias |
| Baltzer (2010) [76] |  |  |  |  |  |  |  | At risk of bias |
| Goto (2007) [74] |  |  |  |  |  |  |  | At risk of bias |
| Thomassin-Naggara (2011) [31] |  |  |  |  |  |  |  | At risk of bias |
| Tozaki (2005) [67] |  |  |  |  |  |  |  | Low risk of bias |
| Tokuda (2009) [73] |  |  |  |  |  |  |  | High risk of bias |
| Yuen (2008) [72] |  |  |  |  |  |  |  | High risk of bias |
| Liberman (2002) [59] |  |  |  |  |  |  |  | At risk of bias |
| Liberman (2003) [71] |  |  |  |  |  |  |  | Low risk of bias |
| Di Ninno (2021) [32] |  |  |  |  |  |  |  | At risk of bias |
| Moukhtar (2014) [77] |  |  |  |  |  |  |  | High risk of bias |
| Liu (2022) [33] |  |  |  |  |  |  |  | At risk of bias |
| Aydin (2019) [34] |  |  |  |  |  |  |  | At risk of bias |
| Zhou (2021) [35] |  |  |  |  |  |  |  | At risk of bias |
| Lunkiewicz (2020) [60] |  |  |  |  |  |  |  | High risk of bias |
| Uematsu & Kasami (2012) [64] |  |  |  |  |  |  |  | High risk of bias |
| Chikarmane (2017) [70] |  |  |  |  |  |  |  | At risk of bias |
| Cheng (2013) [36] |  |  |  |  |  |  |  | High risk of bias |
| Marino (2022) [37] |  |  |  |  |  |  |  | At risk of bias |
| Kul (2013) [38] |  |  |  |  |  |  |  | At risk of bias |
| Yang (2020) [39] |  |  |  |  |  |  |  | High risk of bias |
| Liu (2023) [40] |  |  |  |  |  |  |  | High risk of bias |
| Zang (2022) [41] |  |  |  |  |  |  |  | At risk of bias |
| Bilge (2022) [42] |  |  |  |  |  |  |  | At risk of bias |
| Kwon (2020) [43] |  |  |  |  |  |  |  | High risk of bias |
| Liu (2020) [56] |  |  |  |  |  |  |  | At risk of bias |
| Lv (2022) [44] |  |  |  |  |  |  |  | High risk of bias |
| Zhao (2020) [47] |  |  |  |  |  |  |  | At risk of bias |
| Zhang (2022) [45] |  |  |  |  |  |  |  | At risk of bias |
| Li (2023) [46] |  |  |  |  |  |  |  | At risk of bias |
| Yang (2017) [48] |  |  |  |  |  |  |  | At risk of bias |
| Chen (2021) [49] |  |  |  |  |  |  |  | At risk of bias |
| Ballesio (2014) [65] |  |  |  |  |  |  |  | High risk of bias |
| Avendano (2019) [7] |  |  |  |  |  |  |  | At risk of bias |
| Asada (2017) [50] |  |  |  |  |  |  |  | At risk of bias |
| Bayoumi (2024) [5] |  |  |  |  |  |  |  | At risk of bias |
| Spick (2014) [51] |  |  |  |  |  |  |  | At risk of bias |
| Tang (2021) [58] |  |  |  |  |  |  |  | At risk of bias |
| Clauser (2021) [62] |  |  |  |  |  |  |  | At risk of bias |
| Jansen (2011) [57] |  |  |  |  |  |  |  | At risk of bias |
| Li (2023) [52] |  |  |  |  |  |  |  | At risk of bias |
| Cho (2016) [63] |  |  |  |  |  |  |  | At risk of bias |
| Partridge (2010) [10] |  |  |  |  |  |  |  | At risk of bias |
| Niu (2023) [54] |  |  |  |  |  |  |  | At risk of bias |
| Mohamed (2024) [53] |  |  |  |  |  |  |  | At risk of bias |
| Ahmadinejad (2024) [55] |  |  |  |  |  |  |  | At risk of bias |
| Kim (2021) [61] |  |  |  |  |  |  |  | At risk of bias |
| Tozaki & Fukuma (2009) [66] |  |  |  |  |  |  |  | At risk of bias |
| Gity (2014) [69] |  |  |  |  |  |  |  | At risk of bias |
| Wilhelm (2012) [68] |  |  |  |  |  |  |  | At risk of bias |
 Low Risk;
Low Risk;  High Risk;
High Risk;  Unclear Risk.
Unclear Risk.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarigan, V.N.; Kusumaningtyas, N.; Supit, N.I.S.H.; Sanjaya, E.; Chandra, M.; Sulay, C.B.H.; Octavius, G.S. An Updated Systematic Review and Meta-Analysis of Diagnostic Accuracy of Dynamic Contrast Enhancement and Diffusion-Weighted MRI in Differentiating Benign and Malignant Non-Mass Enhancement Lesions. J. Clin. Med. 2025, 14, 4628. https://doi.org/10.3390/jcm14134628
Tarigan VN, Kusumaningtyas N, Supit NISH, Sanjaya E, Chandra M, Sulay CBH, Octavius GS. An Updated Systematic Review and Meta-Analysis of Diagnostic Accuracy of Dynamic Contrast Enhancement and Diffusion-Weighted MRI in Differentiating Benign and Malignant Non-Mass Enhancement Lesions. Journal of Clinical Medicine. 2025; 14(13):4628. https://doi.org/10.3390/jcm14134628
Chicago/Turabian StyleTarigan, Vera Nevyta, Nungky Kusumaningtyas, Nina I. S. H. Supit, Edwin Sanjaya, Malvin Chandra, Callistus Bruce Henfry Sulay, and Gilbert Sterling Octavius. 2025. "An Updated Systematic Review and Meta-Analysis of Diagnostic Accuracy of Dynamic Contrast Enhancement and Diffusion-Weighted MRI in Differentiating Benign and Malignant Non-Mass Enhancement Lesions" Journal of Clinical Medicine 14, no. 13: 4628. https://doi.org/10.3390/jcm14134628
APA StyleTarigan, V. N., Kusumaningtyas, N., Supit, N. I. S. H., Sanjaya, E., Chandra, M., Sulay, C. B. H., & Octavius, G. S. (2025). An Updated Systematic Review and Meta-Analysis of Diagnostic Accuracy of Dynamic Contrast Enhancement and Diffusion-Weighted MRI in Differentiating Benign and Malignant Non-Mass Enhancement Lesions. Journal of Clinical Medicine, 14(13), 4628. https://doi.org/10.3390/jcm14134628






