1. Introduction
Atherosclerosis is a generalized chronic inflammatory disease that frequently affects different vascular territories. It is mainly characterized by thickening of the intimal and medial layers and loss of elasticity. Multisite artery disease (MSAD) is characterized by the simultaneous presence of clinically significant atherosclerotic lesions in at least two major vascular regions. MSAD ranges from 10 to 15% in patients with coronary artery disease (CAD) to 60 to 70% in patients with severe carotid stenosis or lower extremity arterial disease (LEAD) [
1].
The ankle-brachial index (ABI) is a quick and easy method to confirm the diagnosis and assess the severity of peripheral artery disease in the lower extremities. It is associated with the presence of cardiovascular risk factors and it is an indicator of generalized atherosclerosis. Ankle-brachial index represents the ratio between the systolic blood pressure measured at the ankle and the brachial arteries [
2]. Both the American College of Cardiology (ACC)/American Heart Association (AHA) and European Society of Cardiology (ESC) guidelines emphasize that a low ABI (≤0.90) serves as a marker of systemic atherosclerosis and is independently associated with heightened cardiovascular morbidity and mortality. An ankle-brachial index (ABI) of 0.90 or lower has been linked to over a twofold increase in the 10-year incidence of coronary events and both cardiovascular (CV) and all-cause mortality [
3,
4].
Another non-invasive index of arterial stiffness is the ambulatory arterial stiffness index (AASI), an index calculated from ambulatory 24-h blood pressure (BP) measurements. It is calculated as 1 minus the regression slope of diastolic BP (DBP) on systolic BP (SBP) during ambulatory measurements [
5,
6]. Evidence indicates that AASI is significantly related with markers of target organ damage. Among hypertensive patients, each standard deviation increase in AASI corresponds to twofold increase in the likelihood of microalbuminuria, carotid structural changes and hypertrophic remodeling of the left ventricle [
7]. AASI also independently predicts cardiovascular morbidity and mortality, especially stroke, beyond traditional risk factors [
8]. However, data on the prognostic role of ABI and AASI in patients hospitalized with acute myocardial infarction (AMI) remain limited. This study prospectively evaluated the prevalence and clinical significance of these indices in a large cohort of consecutive patients with AMI [
9].
2. Materials and Methods
2.1. Study Design and Population
This was a single-center, prospective, observational cohort study with a 3-year follow-up, enrolling hospitalized patients diagnosed with acute myocardial infarction (AMI), either with or without ST-segment elevation (STEMI: ST-elevation myocardial infarction; NSTEMI: non–ST-elevation myocardial infarction). All consecutive patients were fully informed about the objectives of the study, and written informed consent was obtained.
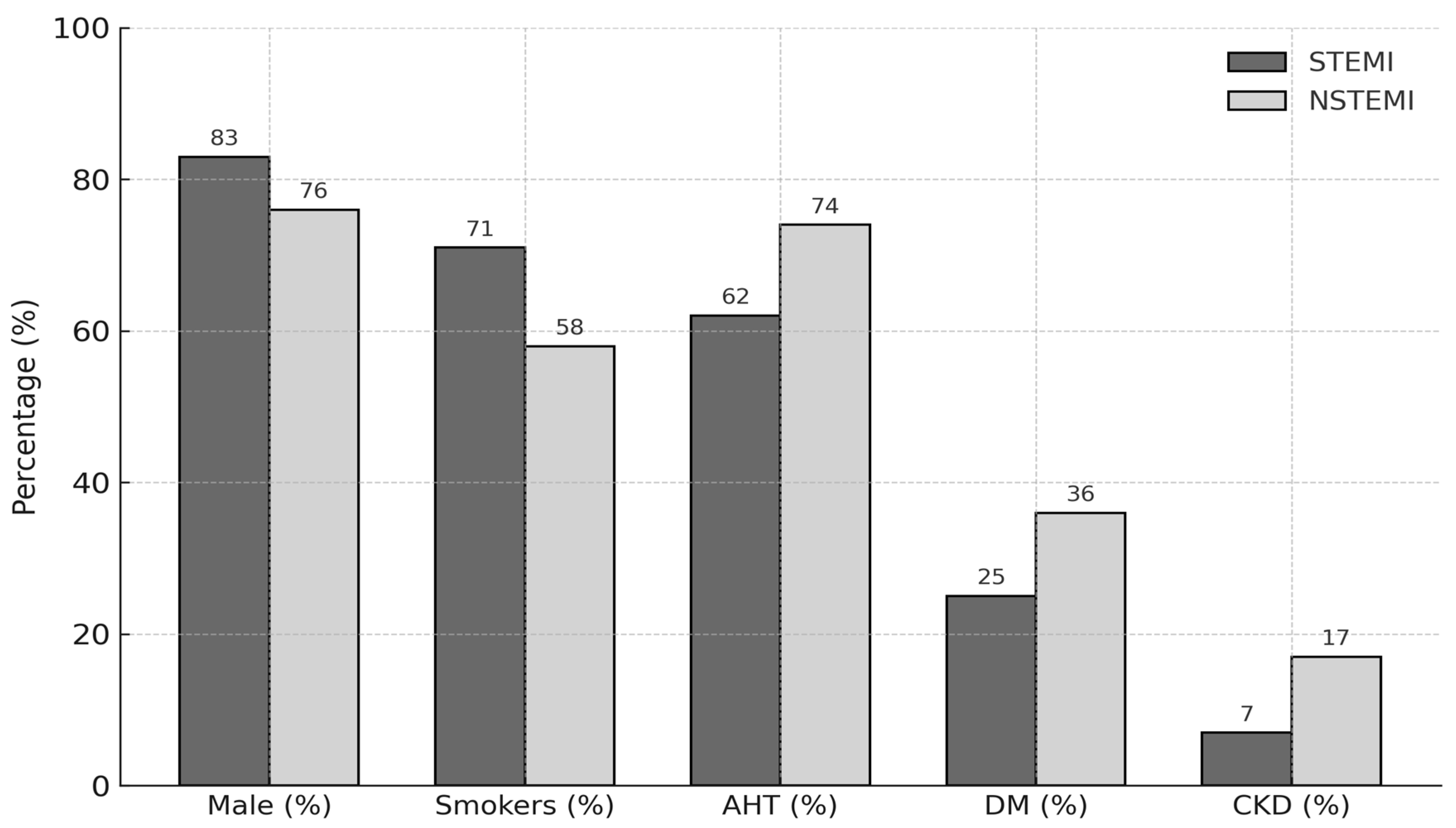
Eligible patients were adults aged between 18 and 85 years. Exclusion criteria included cardiogenic shock, cerebrovascular events, severe valvular heart disease, chronic heart failure, cardiomyopathy, chronic renal failure with an estimated glomerular filtration rate (GFR) < 30 mL/min/1.73 m
2, active malignancy, severe systemic disease, and psychiatric illness preventing sufficient cooperation (
Table 1).
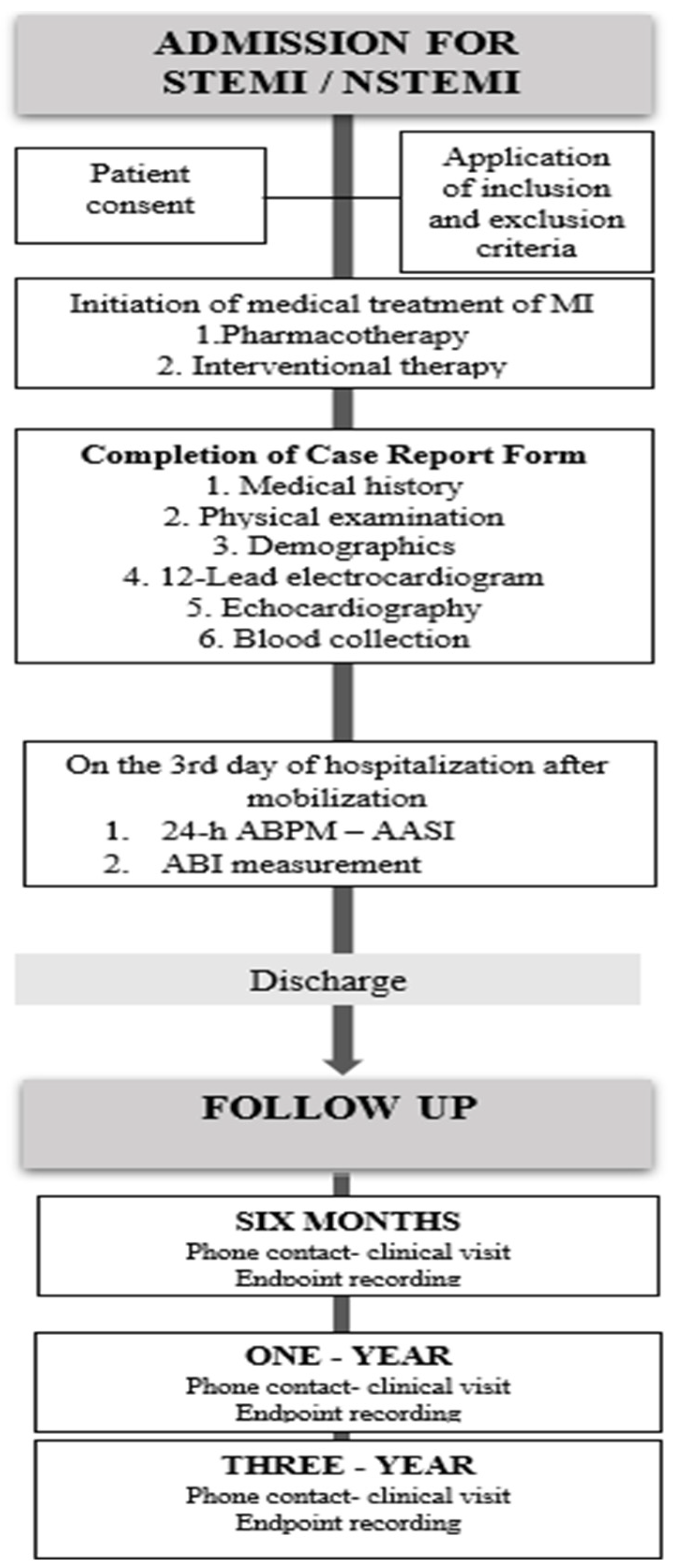
All participants received standard medical management for AMI and were followed during hospitalization, with subsequent scheduled follow-ups at 6, 12, and 36 months after the index event. (
Figure A1).
2.2. Study Plan and Measurements
Given the observational nature of this study, patient management for AMI remained consistent with prevailing guidelines and institutional standards for both pharmacological and interventional treatment. On the day of admission, demographic data, cardiovascular risk factors, complete medical history of patients and baseline medications were meticulously collected on prespecified forms. During hospitalization, results from laboratory tests, the coronary angiography and echocardiography were collected. ABI and AASI measurements were obtained after the third day of hospitalization and at least 24 h following reperfusion and mobilization, provided the patient was clinically stable and receiving standard medical therapy, during a 24-h ambulatory blood pressure monitoring period. The ABI measured according to established methodology using a certified automated device ABPI MD, MESI
® [
10,
11,
12]. An ABI value of 0.9 or less was considered abnormal. AASI was derived from the 24-h blood pressure recordings obtained with a Spacelabs Medical Model 90207 automatic non-invasive blood pressure monitor. Blood pressure data were analyzed using Spacelabs ABP Monitoring Software, version 2.00.03 (Spacelabs Medical).
2.3. Study Endpoints and Follow-Up
The prespecified follow-up period for each patient included monitoring during hospitalization (average hospital stay: 4 days) and subsequent follow-up for a total duration of 36 months. Throughout hospitalization and at 6, 12, and 36 months after the index event, patients and/or their relatives were contacted via telephone to complete a structured questionnaire documenting patient status and potential clinical endpoints. In cases of reported hospitalization, clinic visits were arranged to verify events through hospital records, while official death certificates were requested to confirm cause of death in deceased patients.
Over the 36-month follow-up, the primary endpoint consisted of a composite of fatal and non-fatal cardiovascular and renal events, including myocardial infarction (MI), unstable angina, coronary revascularization, admission due to decompensated heart failure, cerebrovascular events as stroke and transient ischemic attack (TIA), acute limb ischemia, peripheral vascular interventions, serum creatinine doubling and/or progression to end-stage renal disease. Secondary endpoints included individual evaluations of in-hospital clinical events, long-term cardiovascular and renal outcomes, cardiovascular death, and all-cause mortality (
Table 2).
In-hospital major adverse cardiovascular events (MACE) were separately defined as a composite of all-cause mortality, MI (including type 4, type 5, and reinfarction), cardiogenic shock, acute pulmonary edema (PE), cerebrovascular events, and life-threatening arrhythmias. Atrial fibrillation (AF) occurring during hospitalization was recorded as a separate predefined event but was not included in the MACE composite.
Long-term outcomes assessed included all-cause and cardiovascular mortality, acute coronary syndrome (ACS)—defined as recurrent myocardial infarction (MI) or urgent coronary revascularization—stroke (ischemic, hemorrhagic, or transient), life-threatening arrhythmias, and rehospitalization for heart failure decompensation.
Event definitions followed established European Society of Cardiology (ESC) guidelines. Myocardial reinfarction, recurrent MI, cardiogenic shock, and pulmonary edema were classified according to ESC criteria [
13]. Cerebrovascular events were defined as brain ischemia or hemorrhage confirmed by CT or MRI imaging. Life-threatening arrhythmias encompassed ventricular tachycardia, ventricular fibrillation, and complete heart block.
Of the 488 patients initially enrolled, 441 (90.4%) successfully completed the 36-month follow-up period, resulting in a dropout rate of 9.6%. Patients lost to follow-up were mainly due to withdrawal of consent, inability to establish contact despite repeated attempts, or relocation without updated information. These dropouts were evenly distributed across STEMI and NSTEMI groups, minimizing potential selection bias. All available patient data were included in the final analysis according to the intention-to-follow principle.
2.4. Statistical Analysis
Sample Size Calculation and Data Management
Continuous variables were described as mean ± standard deviation (SD) or median with interquartile range (IQR), depending on data distribution, while categorical variables were expressed as absolute numbers and percentages. Group comparisons were performed using parametric tests for normally distributed variables and non-parametric tests otherwise. Statistical significance was defined as a two-sided p-value ≤ 0.05, with adjustments for multiple comparisons made using the Bonferroni correction.
In-hospital events were assessed using multivariable binary logistic regression models to estimate odds ratios (ORs) with 95% confidence intervals (CIs). Longitudinal outcomes were analyzed using Cox proportional hazards models to derive hazard ratios (HRs) and 95% CIs.
In both cases, we originally built a full model, adjusted for the explanatory variables among age, sex, body mass index [BMI], SBP, DBP, smoking status, medication use, ACS type, previous MI, ejection fraction [EF], low-density lipoproteins [LDL], high-density lipoproteins [HDL], triglycerides [TG], troponin at presentation, peak troponin, and creatinine at presentation, that were statistically significant in univariable analysis. A stepwise backward elimination method was employed, sequentially removing the variable with the highest p-value until only significant predictors remained. We used ROC analysis and calculated Youden’s Index area under the curve (AUC) to determine optimal classification cutoffs for the in-hospital and longitudinal outcomes that were significantly associated with ABI.
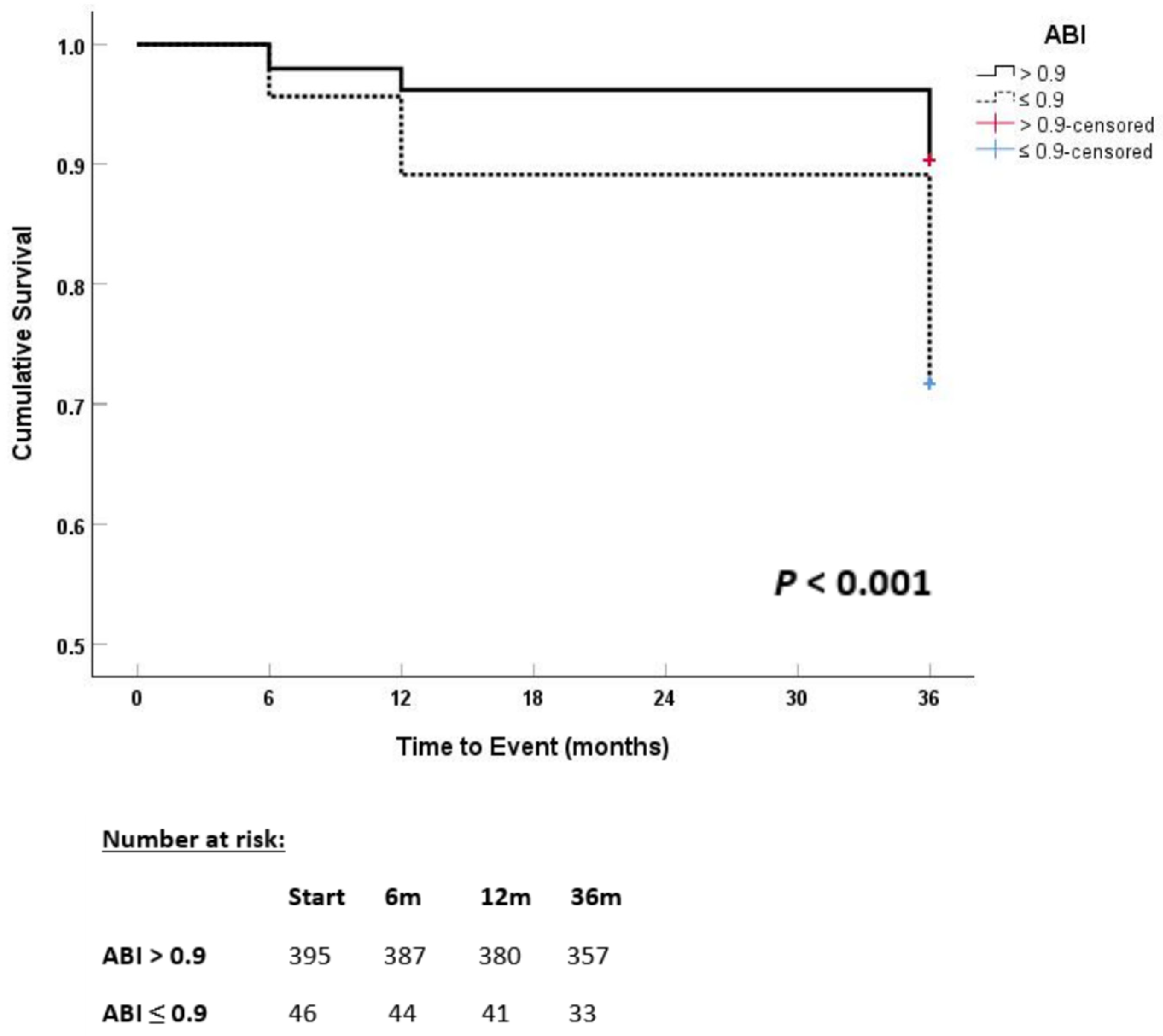
Kaplan–Meier survival analyses were performed to evaluate time-to-event outcomes, including all-cause mortality and major cardiovascular events, over the 36-month follow-up period. Survival curves were generated for the overall cohort and predefined subgroups based on baseline ankle-brachial index (ABI) categories. Differences between survival curves were assessed using the log-rank test.
Sample size determination was based on expected event rates from the Global Registry of Acute Coronary Events (GRACE), targeting a 45% composite event rate at 6 months. A minimum sample size of 367 patients was initially estimated. To account for an anticipated 20–25% attrition rate due to missing data, patient withdrawal, or loss to follow-up, the final enrollment target was set at approximately 450 patients. This sample size was calculated to provide 80% power to detect significant intergroup differences with a two-sided alpha of 0.05 over the 36-month follow-up period.
All clinical endpoints and adverse events were systematically recorded in standardized case report forms and entered into a secure digital database, with rigorous electronic and on-site quality control procedures in place to ensure data integrity. Statistical analyses were performed using SPSS software, version 29 (IBM Corp., Armonk, NY, USA).
Sensitivity analyses were conducted to assess the robustness of the main findings. These included repeating multivariable models after excluding patients lost to follow-up, performing analyses stratified by ACS type (STEMI vs. NSTEMI), and adjusting for additional covariates such as hospitalization duration and revascularization status. Results remained consistent across all sensitivity analyses, reinforcing the stability of the associations observed.
4. Discussion
This was the first study to investigate the relationship between two non-invasive tools, the ankle–brachial index and the ambulatory arterial stiffness index, with adverse cardiovascular and renal outcomes in patients hospitalized for acute myocardial infarction. Most previous studies examined ABI or AASI individually; in contrast, we evaluated both parameters simultaneously within the same population, providing a broader and more comprehensive assessment of vascular risk. This parallel evaluation, coupled with a prolonged follow-up, offers exploratory insights with potential clinical relevance.
Patients presenting with AMI often exhibit concurrent atherosclerotic conditions, such as peripheral artery disease. Growing evidence indicates that PAD is linked to worse long-term outcomes after AMI. Dinser et al. and Kirchberger et al. reported higher short- and long-term mortality rates in patients with AMI and PAD, while Attar et al. confirmed PAD as an independent predictor of adverse post-AMI outcomes [
14,
15,
16]. These findings highlight the relevance of PAD in this clinical context.
The ABI Collaboration, which included 16 international cohort studies, demonstrated that a low ABI is a strong predictor of future cardiovascular disease. Likewise, AASI has been established through prior meta-analyses as a biomarker predictive of future cardiovascular events, stroke, and all-cause mortality in hypertensive populations [
17,
18].
Our study supports the prognostic significance of abnormal ABI values in patients hospitalized with AMI. An ABI ≤ 0.9 was strongly associated with long-term adverse cardiovascular outcomes, independent of traditional risk factors. These findings align with previous reports, including Ban et al., who demonstrated a significant association between low ABI and major adverse cardiovascular events in patients with AMI without a prior history of peripheral artery disease [
19]. Similarly, Berkovitch et al. observed an incremental increase in MACE and one-year mortality in patients with low ABI and asymptomatic PAD [
20].
Further supporting these findings, large cohort studies have shown consistent results. Xu et al. reported increased all-cause mortality below an ABI of 1.11, even within the normal range, while a Thai nationwide study found that ABI ≤ 0.9 was linked to a twofold higher mortality risk and greater renal decline [
21,
22]. Likewise, Masini et al. demonstrated increased mortality in patients with AMI and low ABI, independent of multivessel or carotid disease, and the PATHOS study linked abnormal ABI to a twofold higher one-year mortality in ACS patients [
23,
24]. A recent study by Verdoia et al. found that over 20% of patients with AMI undergoing percutaneous coronary intervention had an ABI ≤ 0.9 despite no prior diagnosis of PAD. Interestingly, higher platelet counts were independently associated with impaired ABI, suggesting a possible link between thrombotic burden and subclinical peripheral vascular disease. This adds an emerging pathophysiological dimension that warrants further exploration [
25].
These data support the clinical utility of ABI not only in identifying PAD but as a broader prognostic tool in acute cardiovascular care.
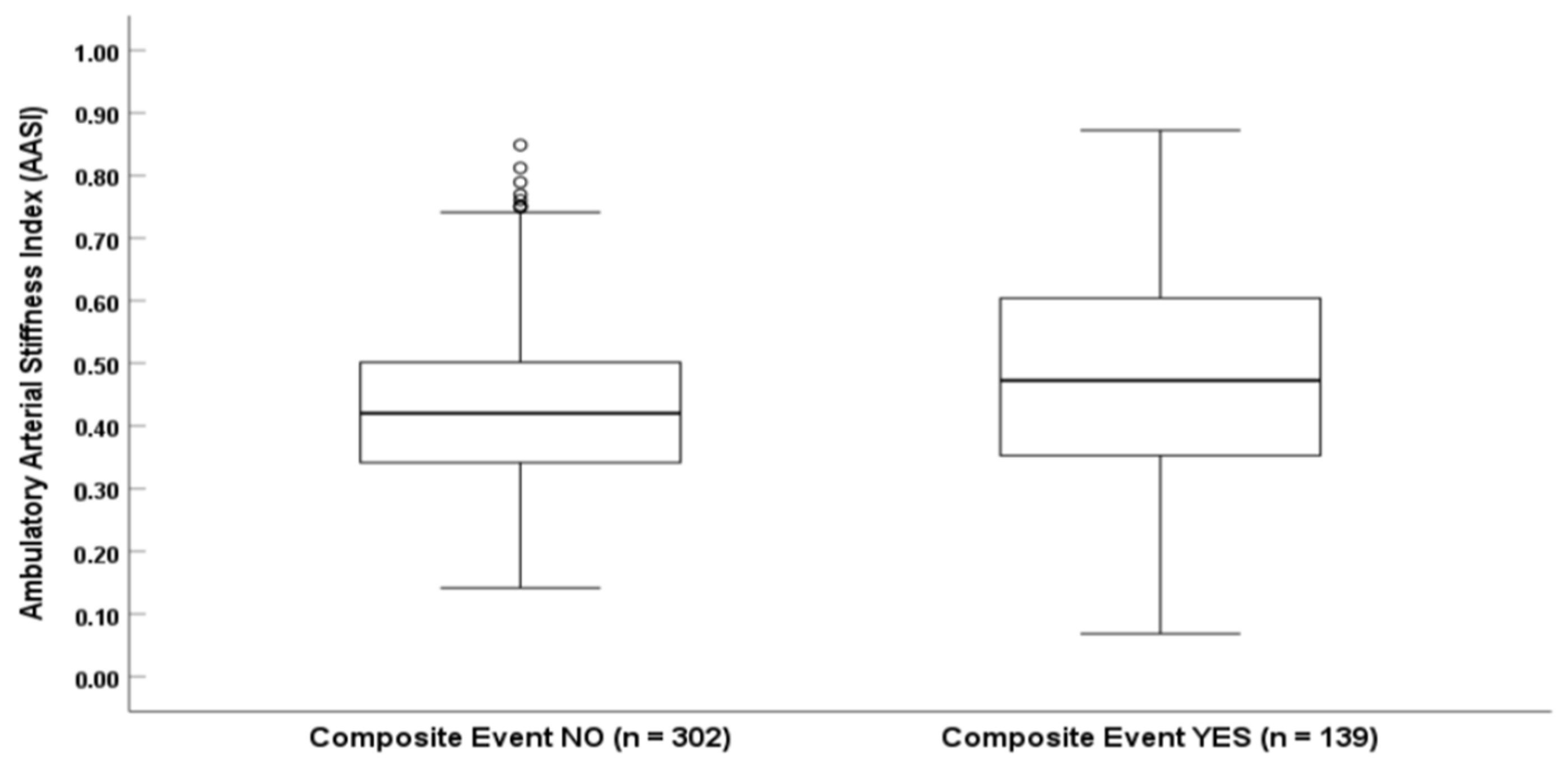
In addition, we introduced the evaluation of AASI, highlighting arterial stiffness as a complementary risk marker. To our knowledge, this is one of the first studies to demonstrate a statistically significant association between AASI and adverse cardiovascular outcomes in AMI. While AASI has been identified as a prognostic marker in broader coronary artery disease populations, its role in AMI remains underexplored. In 2019, Cieślik-Guerra et al. evaluated 90 post-MI patients and reported that an AASI > 0.4235 was an independent predictor of cardiovascular death, recurrent infarction, unstable angina, coronary artery bypass surgery, and implantable cardioverter-defibrillator placement for heart failure [
26]. Our findings are consistent with that study and extend its observations to a larger cohort.
We found no significant sex-based differences in long-term survival, consistent with previous epidemiologic data [
27].
In light of these findings, incorporating additional non-invasive tools—such as multimodality imaging—could further enhance prognostic accuracy in patients with AMI. Recent studies have underscored the value of advanced imaging techniques, including cardiac MRI, echocardiography, and nuclear imaging, in providing comprehensive assessments of myocardial viability, infarct size, and left ventricular function.
Canton et al. highlighted the pivotal role of multimodality imaging in ischemic heart disease, emphasizing its ability to guide revascularization decisions by assessing myocardial viability [
28]. Their findings suggest that integrating functional imaging into clinical pathways may improve patient outcomes through more individualized therapeutic strategies. Furthermore, they demonstrated that combining anatomical and functional imaging modalities can detect subtle myocardial dysfunction that may be overlooked with standard evaluations. This aligns with a growing body of literature advocating for multimodality imaging as a key component in advanced risk stratification.
Bergamaschi et al. further supported this approach by emphasizing the diagnostic and prognostic value of non-invasive anatomical and functional imaging in patients with chronic coronary syndromes [
29]. Complementing these findings, a meta-analysis by Lipinski et al. confirmed the prognostic utility of stress cardiac MRI in individuals with suspected or known coronary artery disease, reinforcing its role alongside vascular markers in comprehensive risk assessment [
30].
While these modalities are highly informative, they may not always be readily accessible. In contrast, ABI and AASI are cost-effective, non-invasive bedside tools that can be used routinely to support early post-AMI risk assessment.
Taken together, our findings provide early evidence that ABI and AASI serve as independent markers of long-term prognosis in AMI. Nonetheless, validation in larger, multicenter studies is essential before ABI and AASI can be routinely integrated into guideline-directed AMI risk stratification.