One-Stage Versus Two-Stage Gastrectomy for Perforated Gastric Cancer: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
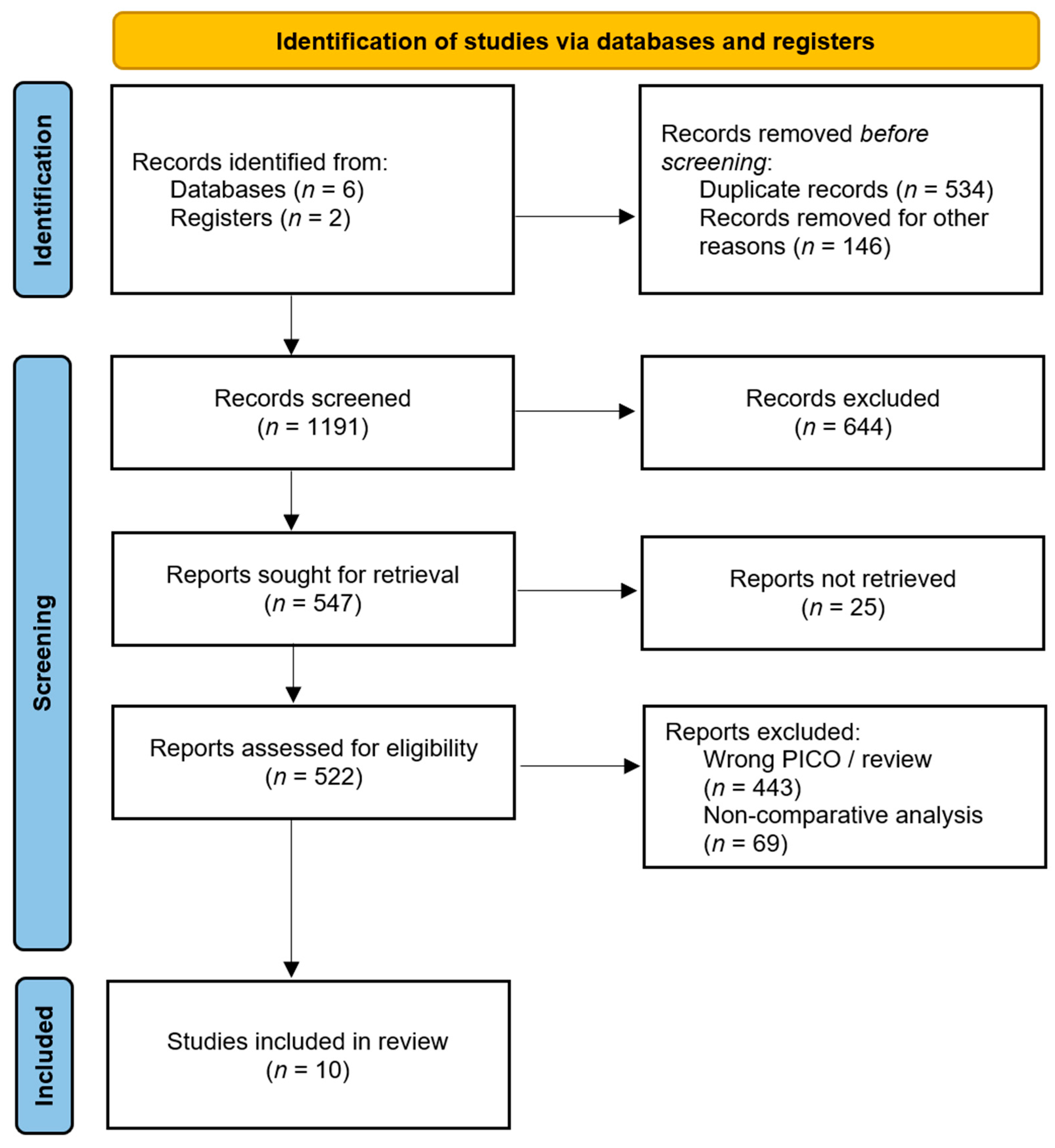
2.2. Selection Process
2.3. Data Collection Process
2.4. Outcome of Interest and Definition
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Systematic Review
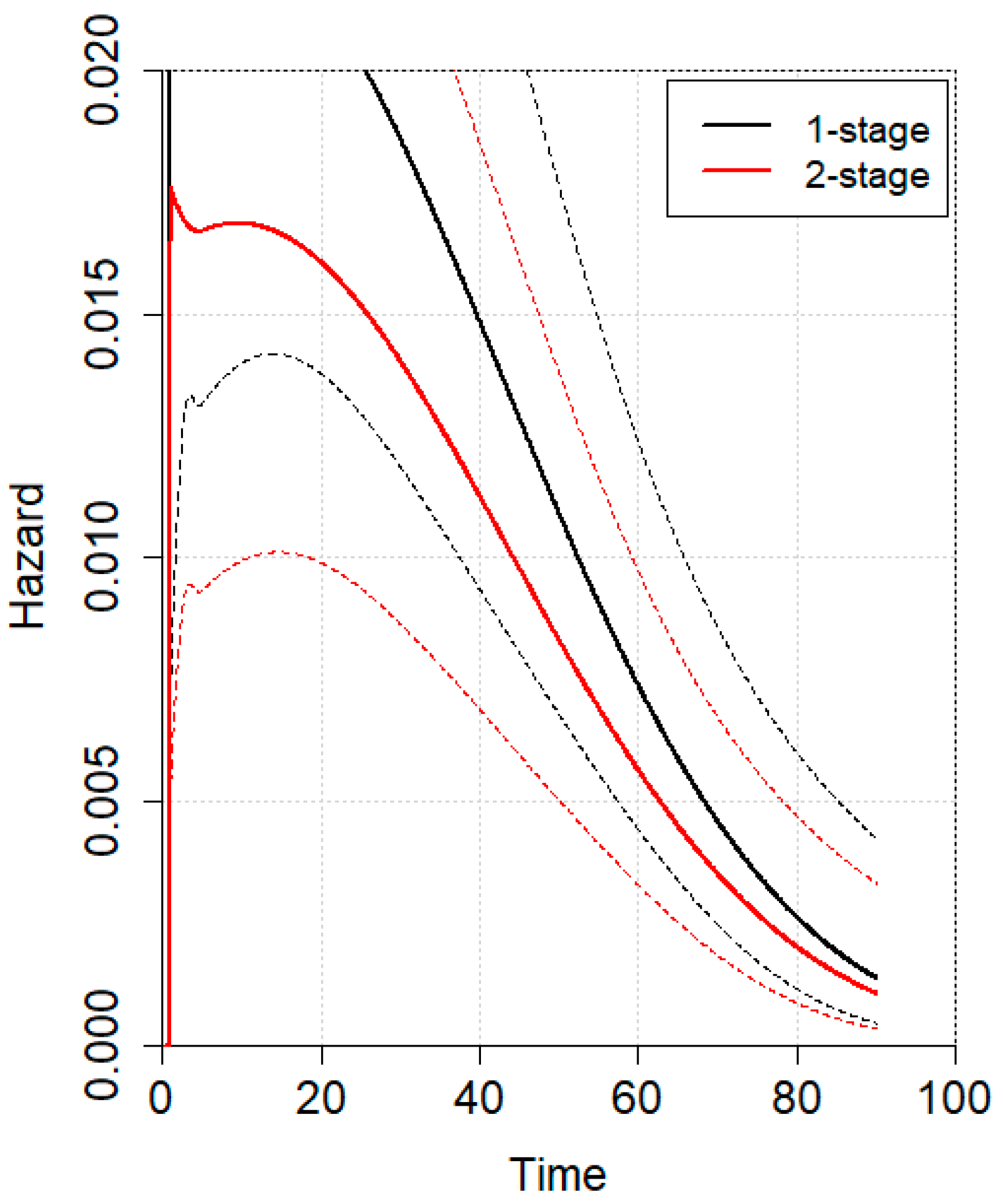
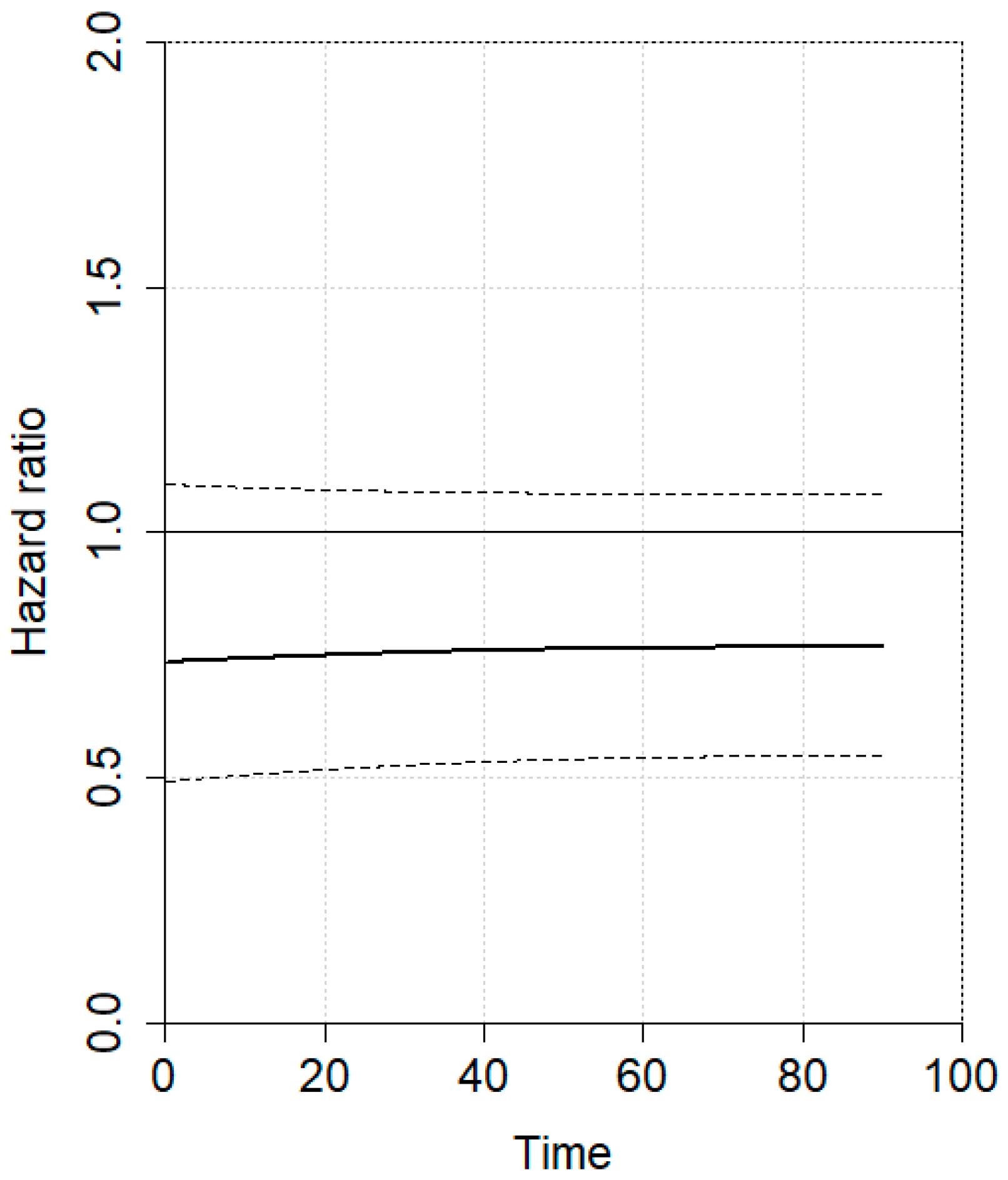
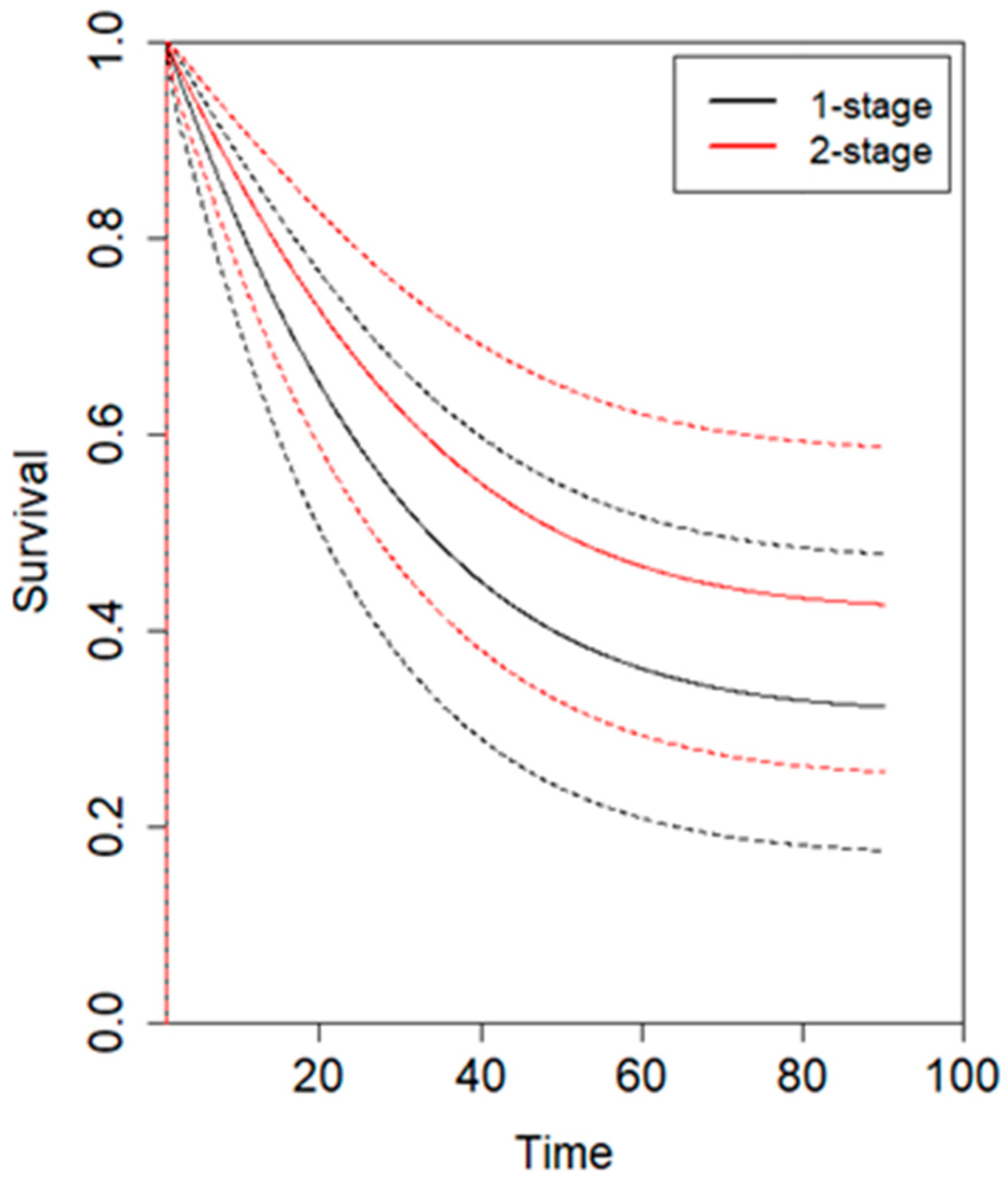
3.2. Meta-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PGC | Perforated gastric carcinoma |
| 1SG | One-stage gastrectomy |
| 2SG | Two-stage gastrectomy |
| OS | Overall survival |
| IPD | Individual patient time-to-event data |
| TG | Total gastrectomy |
| STG | Subtotal gastrectomy |
Appendix A
Appendix A.1
References
- Lau, J.Y.; Sung, J.; Hill, C.; Henderson, C.; Howden, C.W.; Metz, D.C. Systematic Review of the Epidemiology of Complicated Peptic Ulcer Disease: Incidence, Recurrence, Risk Factors and Mortality. Digestion 2011, 84, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Tarasconi, A.; Coccolini, F.; Biffl, W.L.; Tomasoni, M.; Ansaloni, L.; Picetti, E.; Molfino, S.; Shelat, V.; Cimbanassi, S.; Weber, D.G.; et al. Perforated and Bleeding Peptic Ulcer: WSES Guidelines. World J. Emerg. Surg. 2020, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.W.; Fluck, M.; Young, K.; Shabahang, M.; Blansfield, J.; Arora, T.K. Urgent Surgery for Gastric Adenocarcinoma: A Study of the National Cancer Database. J. Surg. Res. 2020, 245, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, J.H.; Kim, M.G. Outcomes of Laparoscopic Primary Gastrectomy with Curative Intent for Gastric Perforation: Experience from a Single Surgeon. Surg. Endosc. 2021, 35, 4206–4213. [Google Scholar] [CrossRef]
- Zhang, J.; Li, K.; Zhang, Z.; Zhang, G.; Zhang, S.; Zhao, Y.; Gao, Z.; Ma, H.; Xie, Y.; Han, J.; et al. Short-and Long-Term Outcomes of One-Stage versus Two-Stage Gastrectomy for Perforated Gastric Cancer: A Multicenter Retrospective Propensity Score-Matched Study. World J. Surg. Oncol. 2024, 22, 7. [Google Scholar] [CrossRef]
- Hata, T.; Sakata, N.; Kudoh, K.; Shibata, C.; Unno, M. The Best Surgical Approach for Perforated Gastric Cancer: One-Stage vs. Two-Stage Gastrectomy. Gastric Cancer 2014, 17, 578–587. [Google Scholar] [CrossRef]
- Melloni, M.; Bernardi, D.; Asti, E.; Bonavina, L. Perforated Gastric Cancer: A Systematic Review. J. Laparoendosc. Adv. Surg. Tech. A 2020, 30, 156–162. [Google Scholar] [CrossRef]
- Di Carlo, S.; Franceschilli, M.; Rossi, P.; Cavallaro, G.; Cardi, M.; Vinci, D.; Sibio, S. Perforated Gastric Cancer: A Critical Appraisal. Discov. Oncol. 2021, 12, 15. [Google Scholar] [CrossRef]
- Mahar, A.L.; Brar, S.S.; Coburn, N.G.; Law, C.; Helyer, L.K. Surgical Management of Gastric Perforation in the Setting of Gastric Cancer. Gastric Cancer 2012, 15 (Suppl. 1), 146–152. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Goossen, K.; Tenckhoff, S.; Probst, P.; Grummich, K.; Mihaljevic, A.L.; Büchler, M.W.; Diener, M.K. Optimal Literature Search for Systematic Reviews in Surgery. Langenbecks Arch. Surg. 2018, 403, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological Quality and Synthesis of Case Series and Case Reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef]
- Gertsch, P.; Yip, S.K.H.; Chow, L.W.C.; Lauder, I.J. Free Perforation of Gastric Carcinoma. Results of Surgical Treatment. Arch. Surg. 1995, 130, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, T.; Buhl, K.; Dueck, M.; Hinz, U.; Herfarth, C. Two-Stage Radical Gastrectomy for Perforated Gastric Cancer. Eur. J. Surg. Oncol. 2000, 26, 780–784. [Google Scholar] [CrossRef]
- Ozmen, M.M.; Zulfikaroglu, B.; Kece, C.; Aslar, A.K.; Ozalp, N.; Koc, M. Factors Influencing Mortality in Spontaneous Gastric Tumour Perforations. J. Int. Med. Res. 2002, 30, 180–184. [Google Scholar] [CrossRef]
- Kasakura, Y.; Ajani, J.A.; Fujii, M.; Mochizuki, F.; Takayama, T. Management of Perforated Gastric Carcinoma: A Report of 16 Cases and Review of World Literature. Am. Surg. 2002, 68, 434–440. [Google Scholar] [CrossRef]
- Pyrc, J.; Kersting, S.; Dobrowolski, F.; Denz, A.; Kuhlisch, E.; Saeger, H.D. Tumor−Related Bleeding, Perforation, and Stenosis as Prognostic Factors of Gastric Cancer. Adv. Clin. Exp. Med. 2006, 15, 1015–1022. [Google Scholar]
- Kandel, B.P.; Singh, Y.; Singh, K.P.; Khakurel, M. Gastric Cancer Perforation: Experience from a Tertiary Care Hospital. J. Nepal Med. Assoc. 2013, 52, 489–493. [Google Scholar] [CrossRef]
- Ignjatovic, N.; Stojanov, D.; Djordjevic, M.; Ignjatovic, J.; Stojanov, D.B.; Milojkovic, B. Perforation of Gastric Cancer—What Should the Surgeon Do? Bosn. J. Basic Med. Sci. 2016, 16, 222. [Google Scholar] [CrossRef]
- Kasakura, Y.; Ajani, J.A.; Mochizuki, F.; Morishita, Y.; Fujii, M.; Takayama, T. Outcomes after Emergency Surgery for Gastric Perforation or Severe Bleeding in Patients with Gastric Cancer. J. Surg. Oncol. 2002, 80, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kim, D.J.; Kim, W. The Role of Laparoscopic Management in Perforated Gastric Cancer. Ann. Surg. Treat. Res. 2021, 101, 151–159. [Google Scholar] [CrossRef]
- Ikehara, H.; Gotoda, T.; Ono, H.; Oda, I.; Saito, D. Gastric Perforation during Endoscopic Resection for Gastric Carcinoma and the Risk of Peritoneal Dissemination. Br. J. Surg. 2007, 94, 992–995. [Google Scholar] [CrossRef]
- Huh, C.W.; Kim, G.J.; Kim, B.W.; Seo, M.; Kim, J.S. Long-Term Clinical Outcomes and Risk of Peritoneal Seeding after Endoscopic Submucosal Dissection for Early Gastric Cancer: A Focus on Perforation during the Procedure. Gut Liver 2019, 13, 515–521. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Shitara, K.; Di Bartolomeo, M.; Lonardi, S.; Al-Batran, S.-E.; Van Cutsem, E.; Ilson, D.H.; Alsina, M.; Chau, I.; Lacy, J.; et al. Ramucirumab with Cisplatin and Fluoropyrimidine as First-Line Therapy in Patients with Metastatic Gastric or Junctional Adenocarcinoma (RAINFALL): A Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2019, 20, 420–435. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, Y.; Leung, L.-H.; Liu, L.; Yang, F.; Yao, X. Efficacy and Safety of Angiogenesis Inhibitors in Advanced Gastric Cancer: A Systematic Review and Meta-Analysis. J. Hematol. Oncol. 2016, 9, 111. [Google Scholar] [CrossRef]
- Ergul, E.; Gozetlik, E.O. Emergency Spontaneous Gastric Perforations: Ulcus versus Cancer. Langenbecks Arch. Surg. 2009, 394, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Steyn, P.F.; Karusseit, O. Gastric Perforation Biopsy: Is It Obsolete? Langenbecks Arch. Surg. 2024, 409, 139. [Google Scholar] [CrossRef]
- Weledji, E.P. An Overview of Gastroduodenal Perforation. Front. Surg. 2020, 7, 573901. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, C.; Liu, Z.; Ai, S.; Guan, W.; Liu, S. Controlling Nutritional Status (CONUT) Score as a Predictive Marker for Short-Term Complications Following Gastrectomy of Gastric Cancer: A Retrospective Study. BMC Gastroenterol. 2021, 21, 107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Wu, T.; Zhang, Y.; Yan, K.; Sun, X. Modified Glasgow Prognostic Score as a Prognostic Factor in Gastric Cancer Patients: A Systematic Review and Meta-Analysis. Int. J. Clin. Exp. Med. 2015, 8, 15222–15229. [Google Scholar] [PubMed]
- Luo, Z.; Zhou, L.; Balde, A.I.; Li, Z.; He, L.; ZhenWei, C.; Zou, Z.N.; Huang, S.Y.; Han, S.; Zhou, M.W.; et al. Prognostic Impact of Preoperative Prognostic Nutritional Index in Resected Advanced Gastric Cancer: A Multicenter Propensity Score Analysis. Eur. J. Surg. Oncol. 2019, 45, 425–431. [Google Scholar] [CrossRef]
- Kubota, T.; Shoda, K.; Konishi, H.; Okamoto, K.; Otsuji, E. Nutrition Update in Gastric Cancer Surgery. Ann. Gastroenterol. Surg. 2020, 4, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Manara, M.; Aiolfi, A.; Bonitta, G.; Schlanger, D.; Popa, C.; Lombardo, F.; Manfredini, L.; Biondi, A.; Bonavina, L.; Bona, D. Short-Term Outcomes Analysis Comparing Open, Lap-Assisted, Totally Laparoscopic, and Robotic Total Gastrectomy for Gastric Cancer: A Network Meta-Analysis. Cancers 2024, 16, 3404. [Google Scholar] [CrossRef]
- Fisher, B.W.; Jammula, S.; Wang, S.; Fluck, M.; Young, K.; Shabahang, M.; Blansfield, J. Short-Term Outcomes of Emergent Versus Elective Gastrectomy for Gastric Adenocarcinoma: A National Surgical Quality Improvement Program Study. Am. Surg. 2022, 89, 1758–1763. [Google Scholar] [CrossRef]
- Calì, M.; Bona, D.; Kim, Y.M.; Hyung, W.; Cammarata, F.; Bonitta, G.; Bonavina, L.; Aiolfi, A. Effect of Minimally Invasive versus Open Distal Gastrectomy on Long-Term Survival in Patients with Gastric Cancer: Individual Patient Data Meta-Analysis. Ann. Surg. Oncol. 2024, 32, 2161–2171. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, C.; Cai, Z.; Shen, C.; Yin, Y.; Yin, X.; Zhao, Z.; Mu, M.; Yin, Y.; Zhang, B. Impact of Surgical Margin Status on Survival in Gastric Cancer: A Systematic Review and Meta-Analysis. Cancer Control 2021, 28, 10732748211043665. [Google Scholar] [CrossRef]
- Aiolfi, A.; Bona, D.; Bonitta, G.; Lombardo, F.; Manara, M.; Sozzi, A.; Schlanger, D.; Popa, C.; Cavalli, M.; Campanelli, G.; et al. Long-Term Impact of D2 Lymphadenectomy during Gastrectomy for Cancer: Individual Patient Data Meta-Analysis and Restricted Mean Survival Time Estimation. Cancers 2024, 16, 424. [Google Scholar] [CrossRef]
- Mocellin, S.; Mcculloch, P.; Kazi, H.; Gama-Rodrigues, J.J.; Yuan, Y.; Nitti, D. Extent of Lymph Node Dissection for Adenocarcinoma of the Stomach. Cochrane Database Syst. Rev. 2015, 2015, CD001964. [Google Scholar] [CrossRef]
- Pelc, Z.; Sędłak, K.; Endo, Y.; Van Sandick, J.; Gisbertz, S.; Pera, M.; Baiocchi, G.L.; Morgagni, P.; Framarini, M.; Hoelscher, A.; et al. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Gastric Cancer with Peritoneal Metastasis—Joint Analysis of European GASTRODATA and American National Cancer Database. Am. J. Surg. 2025, 242, 116235. [Google Scholar] [CrossRef]
- Manara, M.; Aiolfi, A.; Sozzi, A.; Calì, M.; Grasso, F.; Rausa, E.; Bonitta, G.; Bonavina, L.; Bona, D. Short-Term Outcomes Analysis Comparing Open, Laparoscopic, Laparoscopic-Assisted, and Robotic Distal Gastrectomy for Locally Advanced Gastric Cancer: A Randomized Trials Network Analysis. Cancers 2024, 16, 1620. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, D.; Asti, E.; Punturieri, M.; Luporini, A.; Bonavina, L. Laparoscopic Gastrectomy and Adjuvant Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Using a Closed System with Turbulent-Flow Circuit: Technical Aspects and Preliminary Results of a Pilot Study. Eur. Surg. 2018, 50, 209–214. [Google Scholar] [CrossRef]
- Roviello, F.; Caruso, S.; Neri, A.; Marrelli, D. Treatment and Prevention of Peritoneal Carcinomatosis from Gastric Cancer by Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Overview and Rationale. Eur. J. Surg. Oncol. 2013, 39, 1309–1316. [Google Scholar] [CrossRef]
- Aiolfi, A.; Lombardo, F.; Matsushima, K.; Sozzi, A.; Cavalli, M.; Panizzo, V.; Bonitta, G.; Bona, D. Systematic Review and Updated Network Meta-Analysis of Randomized Controlled Trials Comparing Open, Laparoscopic-Assisted, and Robotic Distal Gastrectomy for Early and Locally Advanced Gastric Cancer. Surgery 2021, 170, 942–951. [Google Scholar] [CrossRef]
- Lordick, F.; Rha, S.Y.; Muro, K.; Yong, W.P.; Lordick Obermannová, R. Systemic Therapy of Gastric Cancer—State of the Art and Future Perspectives. Cancers 2024, 16, 3337. [Google Scholar] [CrossRef] [PubMed]
- Yasufuku, I.; Tsuchiya, H.; Fujibayashi, S.; Okumura, N.; Sengoku, Y.; Fukada, M.; Asai, R.; Sato, Y.; Tajima, J.Y.; Kiyama, S.; et al. Oligometastasis of Gastric Cancer: A Review. Cancers 2024, 16, 673. [Google Scholar] [CrossRef]
- Guan, W.L.; He, Y.; Xu, R.H. Gastric Cancer Treatment: Recent Progress and Future Perspectives. J. Hematol. Oncol. 2023, 16, 57. [Google Scholar] [CrossRef]
- Sun, F.; Feng, M.; Guan, W. Mechanisms of Peritoneal Dissemination in Gastric Cancer. Oncol. Lett. 2017, 14, 6991–6998. [Google Scholar] [CrossRef]
- Ning, F.L.; Gu, W.J.; Zhao, Z.M.; Du, W.Y.; Sun, M.; Cao, S.Y.; Zeng, Y.J.; Abe, M.; Zhang, C.D. Association between Hospital Surgical Case Volume and Postoperative Mortality in Patients Undergoing Gastrectomy for Gastric Cancer: A Systematic Review and Meta-Analysis. Int. J. Surg. 2023, 109, 936. [Google Scholar] [CrossRef]
- Yang, W.J.; Zhao, H.P.; Yu, Y.; Wang, J.H.; Guo, L.; Liu, J.Y.; Pu, J.; Lv, J. Updates on Global Epidemiology, Risk and Prognostic Factors of Gastric Cancer. World J. Gastroenterol. 2023, 29, 2452. [Google Scholar] [CrossRef] [PubMed]
| Author, Contry, Year | Surgical Procedure | No. pts | Age (yrs) | M–F Ratio | Tumor Site (up–Mid/Low) | pStage 0–I | pStage II–III | pStage IV | Adjuvant Therapy | Resection (TG–STG) | QoE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gertsch, China 1995 [15] | 1SG | 30 | nr | nr | 7–23 | nr | nr | nr | 0 | nr | M |
| 2SG | 2 | nr | nr | 0–2 | nr | nr | nr | 0 | nr | ||
| Lehnert, Germany 2000 [16] | 1SG | 2 | nr | nr | nr | nr | nr | nr | nr | 2–0 | M |
| 2SG | 6 | nr | nr | nr | nr | nr | nr | nr | 2–4 | ||
| Ozmen, Turkey 2002 [17] | 1SG | 9 | nr | 9–0 | nr | nr | nr | nr | nr | 4–5 | S |
| 2SG | 3 | nr | 3–0 | nr | nr | nr | nr | nr | 3–0 | ||
| Kasakura, Japan 2002 [18,22] | 1SG | 12 | 60.2 ± 14.9 | 10–2 | 8–4 | 2 | 3 | 7 | 7 | 3–9 | M |
| 2SG | 2 | 43.5 ± 5.5 | 2–0 | 0–2 | 1 | 1 | 0 | 2 | 0–2 | ||
| Pyrc, Germany 2006 [19] | 1SG | 1 | 70 | 0–1 | 0–1 | 0 | 1 | 0 | nr | 0–1 | M |
| 2SG | 4 | 52 ± 12.3 | 3–1 | 1–3 | 0 | 2 | 2 | nr | 1–3 | ||
| Kandel, Nepal 2013 [20] | 1SG | 5 | nr | nr | nr | nr | nr | nr | 5 | nr | S |
| 2SG | 1 | nr | nr | nr | nr | nr | nr | 1 | nr | ||
| Hata, Japan 2014 [6] | 1SG | 376 | 64 ± 11.3 | nr | nr | nr | nr | nr | nr | nr | L |
| 2SG | 54 | 56 ± 10.8 | 44–10 | nr | nr | nr | nr | nr | nr | ||
| Ignjatovic, Serbia 2016 [21] | 1SG | 3 | nr | nr | nr | 0 | 3 | 0 | nr | nr | S |
| 2SG | 5 | nr | nr | nr | 0 | 5 | 0 | nr | nr | ||
| Kim, Korea 2021 [23] | 1SG | 15 | nr | 14–1 | 1–14 | 3 | 12 | 0 | 10 | 1–14 | M |
| 2SG | 5 | Nr | 3–2 | 1–4 | 0 | 5 | 0 | 4 | 0–5 | ||
| Zhang, China 2024 [5] | 1SG | 29 | 63.7 ± 12 | 22–7 | 6–23 | 0 | 29 | 0 | nr | nr | L |
| 2SG | 15 | 62.7 ± 12.2 | 12–3 | 2–13 | 0 | 15 | 0 | nr | nr |
| Author, Year | Surgical Procedure | No. pts | Curative Resection | Lymphadenectomy Extension (D0/1–D2/3) | No. lymph Nodes Retrieved | Overall Complications | Anastomotic Leak | In-Hospital Mortality |
|---|---|---|---|---|---|---|---|---|
| Gertsch, 1995 [15] | 1SG | 30 | 23 | 30–0 | nr | 1 (3) | nr | 6 (20) |
| 2SG | 2 | 2 | 0–2 | nr | 0 (0) | nr | 1 (50) | |
| Lehnert, 2000 [16] | 1SG | 2 | 2 | nr | nr | nr | nr | 1 (50) |
| 2SG | 6 | 6 | nr | nr | nr | nr | 0 (0) | |
| Ozmen, 2002 [17] | 1SG | 9 | 0 | 9–0 | nr | 8 (89) | 2 (22) | 5 (55) |
| 2SG | 3 | 0 | 0–3 | nr | 0 (0) | 0 (0) | 0 (0) | |
| Kasakura, 2002 [18,22] | 1SG | 12 | 2 | 12–0 | nr | 4 (33) | 2 (17) | 0 (0) |
| 2SG | 2 | 2 | 0–2 | nr | 1 (50) | 0 (0) | 0 (0) | |
| Pyrc, 2006 [19] | 1SG | 1 | nr | nr | nr | nr | nr | 0 (0) |
| 2SG | 4 | nr | nr | nr | nr | nr | 0 (0) | |
| Kandel, 2013 [20] | 1SG | 5 | nr | nr | nr | nr | nr | 0 (0) |
| 2SG | 1 | nr | nr | nr | nr | nr | 0 (0) | |
| Hata, 2014 [6] | 1SG | 376 | nr | nr | nr | nr | nr | 42 (11) |
| 2SG | 54 | nr | nr | nr | nr | 2 (4) | 1 (2) | |
| Ignjatovic, 2016 [21] | 1SG | 3 | nr | 3–0 | nr | nr | nr | nr |
| 2SG | 5 | nr | 2–3 | nr | nr | nr | nr | |
| Kim, 2021 [23] | 1SG | 15 | 15 | 6–9 | 17 ± 3.8 | nr | nr | nr |
| 2SG | 5 | 5 | 0–5 | 33 ± 4.3 | 0 (0) | nr | nr | |
| Zhang, 2024 [5] | 1SG | 29 | 29 | 11–18 | 17 ± 3 | nr | nr | nr |
| 2SG | 15 | 15 | 0–15 | 31 ± 2.8 | nr | nr | nr |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manara, M.; Aiolfi, A.; Wang, Q.; Bonitta, G.; Shabat, G.; Biondi, A.; Calì, M.; Bona, D.; Bonavina, L. One-Stage Versus Two-Stage Gastrectomy for Perforated Gastric Cancer: Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 4603. https://doi.org/10.3390/jcm14134603
Manara M, Aiolfi A, Wang Q, Bonitta G, Shabat G, Biondi A, Calì M, Bona D, Bonavina L. One-Stage Versus Two-Stage Gastrectomy for Perforated Gastric Cancer: Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(13):4603. https://doi.org/10.3390/jcm14134603
Chicago/Turabian StyleManara, Michele, Alberto Aiolfi, Quan Wang, Gianluca Bonitta, Galyna Shabat, Antonio Biondi, Matteo Calì, Davide Bona, and Luigi Bonavina. 2025. "One-Stage Versus Two-Stage Gastrectomy for Perforated Gastric Cancer: Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 13: 4603. https://doi.org/10.3390/jcm14134603
APA StyleManara, M., Aiolfi, A., Wang, Q., Bonitta, G., Shabat, G., Biondi, A., Calì, M., Bona, D., & Bonavina, L. (2025). One-Stage Versus Two-Stage Gastrectomy for Perforated Gastric Cancer: Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(13), 4603. https://doi.org/10.3390/jcm14134603








