Evolution and Predictors of Right Ventricular Failure in Fontan Patients: A Case-Control Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Study Population
2.3. Echocardiographic Examination
2.4. Assessment of RV Function
2.5. Assessment of RV-Vascular Coupling
2.6. Index of RV Remodeling
2.7. Statistical Analyses
3. Results
3.1. Patients and Clinical Characteristics
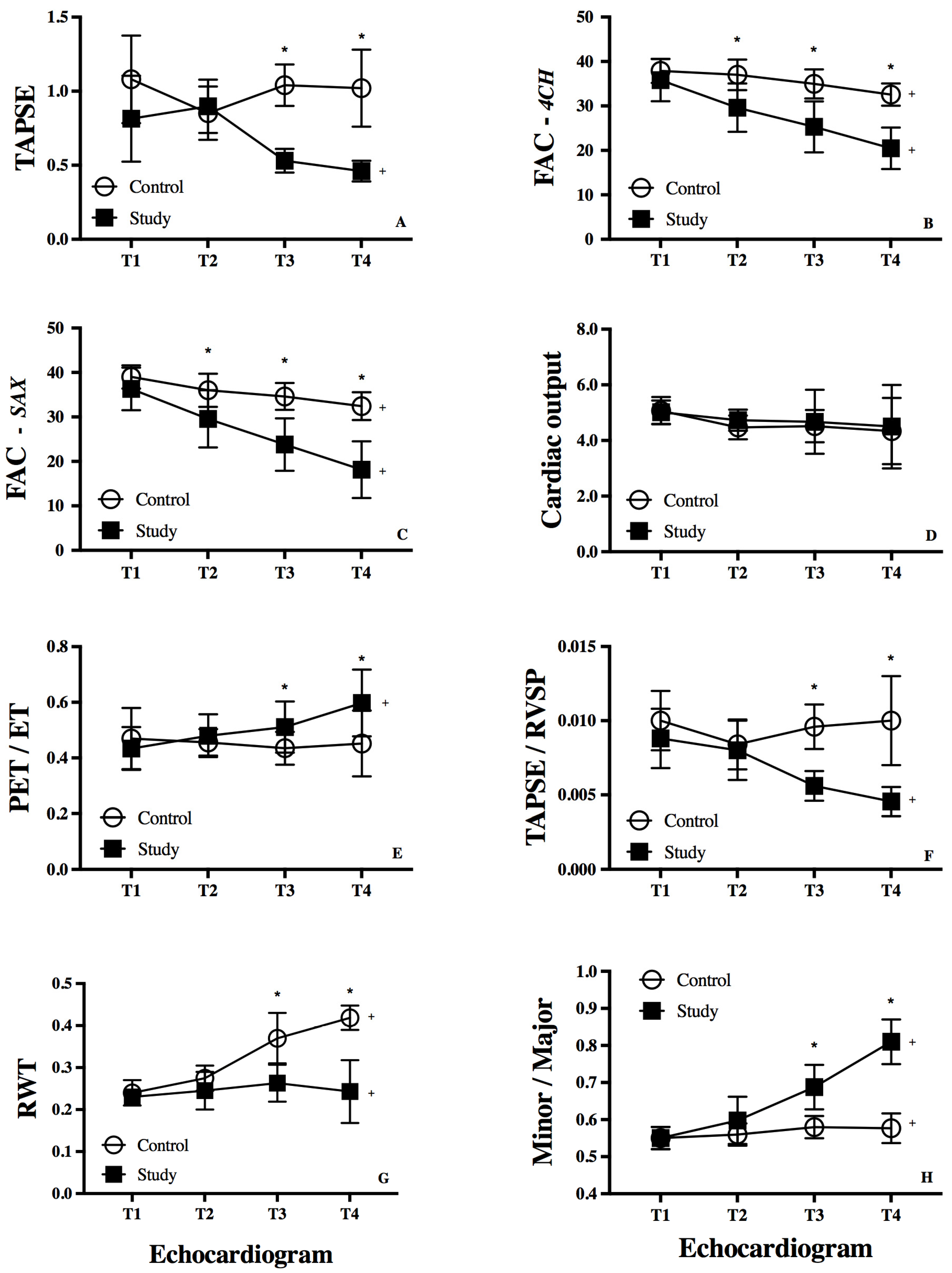
3.2. RV Function
3.3. RV-Systemic Vascular Coupling
3.4. RV Remodeling
3.5. Receiver Operating Characteristic (ROC) Curve Analysis
3.6. Reproducibility
4. Discussion
4.1. RV Remodeling and RV-Systemic Vascular Coupling
4.2. Systemic RV Systolic Function in Fontan Patients
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RV | Right ventricular |
| LV | Left ventricle |
| SRV | Single right ventricular morphology |
| TAPSE | Tricuspid annular plane systolic excursion |
| OHT | Orthotopic heart transplant |
| DORV | Double outlet right ventricle |
| UAVC | Unbalanced atrioventricular canal |
| HLHS | Hypoplastic left heart syndrome |
| FAC | Fractional area of change |
| RVESA | RV end-systolic area |
| RVEDS | RV end-diastolic area |
| AV | Atrioventricular valve |
| RVET | RV ejection time |
| RV-PET | RV pre-ejection time |
| RVSP | RV systolic pressure |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| RWT | Relative wall thickness |
| RVID | RV internal diameter |
| ILWT | Inferolateral wall thickness |
| ACE | Angiotensin-converting enzyme inhibitor |
| ROC | Receiver operating characteristic |
| RVEDS | RV end-diastolic area |
References
- Jayakumar, K.A.; Addonizio, L.J.; Kichuk-Chrisant, M.R.; Galantowicz, M.E.; Lamour, J.M.; Quaegebeur, J.M.; Hsu, D.T. Cardiac transplantation after the Fontan or Glenn procedure. J. Am. Coll. Cardiol. 2004, 44, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Khoo, N.S.; Smallhorn, J.F.; Tham, E.B. Single right ventricles have impaired systolic and diastolic function compared to those of left ventricular morphology. J. Am. Soc. Echocardiogr. 2012, 25, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Piran, S.; Veldtman, G.; Siu, S.; Webb, G.D.; Liu, P.P. Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation 2002, 105, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Senzaki, H.; Masutani, S.; Kobayashi, J.; Kobayashi, T.; Sasaki, N.; Asano, H.; Kyo, S.; Yokote, Y.; Ishizawa, A. Ventricular afterload and ventricular work in fontan circulation: Comparison with normal two-ventricle circulation and single-ventricle circulation with blalock-taussig shunts. Circulation 2002, 105, 2885–2892. [Google Scholar] [CrossRef]
- Vonk Noordegraaf, A.; Westerhof, B.E.; Westerhof, N. The Relationship Between the Right Ventricle and its Load in Pulmonary Hypertension. J. Am. Coll. Cardiol. 2017, 69, 236–243. [Google Scholar] [CrossRef]
- Breatnach, C.R.; Bussmann, N.; Smith, A.; Levy, P.; McCallion, N.; Franklin, O.; El-Khuffash, A. Cardiac mechanics in infants with Down syndrome in the early neonatal period. J. Perinatol. 2019, 39, 626–633. [Google Scholar] [CrossRef]
- Forfia, P.R.; Fisher, M.R.; Mathai, S.C.; Housten-Harris, T.; Hemnes, A.R.; Borlaug, B.A.; Chamera, E.; Corretti, M.C.; Champion, H.C.; Abraham, T.P.; et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2006, 174, 1034–1041. [Google Scholar] [CrossRef]
- Colan, S.D.; Borow, K.M.; Neumann, A. Left ventricular end-systolic wall stress-velocity of fiber shortening relation: A load-independent index of myocardial contractility. J. Am. Coll. Cardiol. 1984, 4, 715–724. [Google Scholar] [CrossRef]
- Simsek, E.; Nalbantgil, S.; Ceylan, N.; Zoghi, M.; Kemal, H.S.; Engin, C.; Yagdi, T.; Ozbaran, M. Assessment of right ventricular systolic function in heart transplant patients: Correlation between echocardiography and cardiac magnetic resonance imaging. Investigation of the accuracy and reliability of echocardiography. Echocardiography 2017, 34, 1432–1438. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Burwash, I.G.; Otto, C.M.; Pearlman, A.S. Use of Doppler-derived left ventricular time intervals for noninvasive assessment of systolic function. Am. J. Cardiol. 1993, 72, 1331–1333. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Bandera, F.; Pelissero, G.; Castelvecchio, S.; Menicanti, L.; Ghio, S.; Temporelli, P.L.; Arena, R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: An index of right ventricular contractile function and prognosis. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1373–H1381. [Google Scholar] [CrossRef] [PubMed]
- Groh, G.K.; Levy, P.T.; Holland, M.R.; Murphy, J.J.; Sekarski, T.J.; Myers, C.L.; Hartman, D.P.; Roiger, R.D.; Singh, G.K. Doppler echocardiography inaccurately estimates right ventricular pressure in children with elevated right heart pressure. J. Am. Soc. Echocardiogr. 2014, 27, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Reesink, H.J.; Marcus, J.T.; Tulevski, II; Jamieson, S.; Kloek, J.J.; Vonk Noordegraaf, A.; Bresser, P. Reverse right ventricular remodeling after pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: Utility of magnetic resonance imaging to demonstrate restoration of the right ventricle. J. Thorac. Cardiovasc. Surg. 2007, 133, 58–64. [Google Scholar] [CrossRef]
- Lewis, J.F.; Webber, J.D.; Sutton, L.L.; Chesoni, S.; Curry, C.L. Discordance in degree of right and left ventricular dilation in patients with dilated cardiomyopathy: Recognition and clinical implications. J. Am. Coll. Cardiol. 1993, 21, 649–654. [Google Scholar] [CrossRef]
- Hilde, J.M.; Skjorten, I.; Grotta, O.J.; Hansteen, V.; Melsom, M.N.; Hisdal, J.; Humerfelt, S.; Steine, K. Right ventricular dysfunction and remodeling in chronic obstructive pulmonary disease without pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, 1103–1111. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713; quiz 786–788. [Google Scholar] [CrossRef]
- Hunt, S.A.; Abraham, W.T.; Chin, M.H.; Feldman, A.M.; Francis, G.S.; Ganiats, T.G.; Jessup, M.; Konstam, M.A.; Mancini, D.M.; Michl, K.; et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J. Am. Coll. Cardiol. 2009, 53, e1–e90. [Google Scholar] [CrossRef]
- Seliem, M.A.; Baffa, J.M.; Vetter, J.M.; Chen, S.L.; Chin, A.J.; Norwood, W.I., Jr. Changes in right ventricular geometry and heart rate early after hemi-Fontan procedure. Ann. Thorac. Surg. 1993, 55, 1508–1512. [Google Scholar] [CrossRef]
- Voelkel, N.F.; Quaife, R.A.; Leinwand, L.A.; Barst, R.J.; McGoon, M.D.; Meldrum, D.R.; Dupuis, J.; Long, C.S.; Rubin, L.J.; Smart, F.W.; et al. Right ventricular function and failure: Report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 2006, 114, 1883–1891. [Google Scholar] [CrossRef]
- Aggarwal, M.; Grady, R.M.; Choudhry, S.; Anwar, S.; Eghtesady, P.; Singh, G.K. Potts Shunt Improves Right Ventricular Function and Coupling With Pulmonary Circulation in Children With Suprasystemic Pulmonary Arterial Hypertension. Circ. Cardiovasc. Imaging 2018, 11, e007964. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.T.; El Khuffash, A.; Woo, K.V.; Hauck, A.; Hamvas, A.; Singh, G.K. A Novel Noninvasive Index to Characterize Right Ventricle Pulmonary Arterial Vascular Coupling in Children. JACC Cardiovasc. Imaging 2019, 12, 761–763. [Google Scholar] [CrossRef] [PubMed]
- de Leval, M.R. The Fontan circulation: What have we learned? What to expect? Pediatr. Cardiol. 1998, 19, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Buhmann, V.; Graf, A.; Melnitschuk, S.; Bahrle, S.; Vahl, C.F.; Hagl, S. Ventricular energetics after the Fontan operation: Contractility-afterload mismatch. J. Thorac. Cardiovasc. Surg. 2003, 125, 1061–1069. [Google Scholar] [CrossRef]
- Senzaki, H.; Masutani, S.; Ishido, H.; Taketazu, M.; Kobayashi, T.; Sasaki, N.; Asano, H.; Katogi, T.; Kyo, S.; Yokote, Y. Cardiac rest and reserve function in patients with Fontan circulation. J. Am. Coll. Cardiol. 2006, 47, 2528–2535. [Google Scholar] [CrossRef]
- Kouzu, H.; Yuda, S.; Muranaka, A.; Doi, T.; Yamamoto, H.; Shimoshige, S.; Hase, M.; Hashimoto, A.; Saitoh, S.; Tsuchihashi, K.; et al. Left ventricular hypertrophy causes different changes in longitudinal, radial, and circumferential mechanics in patients with hypertension: A two-dimensional speckle tracking study. J. Am. Soc. Echocardiogr. 2011, 24, 192–199. [Google Scholar] [CrossRef]
- Buckberg, G.D.; Coghlan, H.C.; Hoffman, J.I.; Torrent-Guasp, F. The structure and function of the helical heart and its buttress wrapping. VII. Critical importance of septum for right ventricular function. Semin. Thorac. Cardiovasc. Surg. 2001, 13, 402–416. [Google Scholar] [CrossRef]
- Sallin, E.A. Fiber orientation and ejection fraction in the human left ventricle. Biophys. J. 1969, 9, 954–964. [Google Scholar] [CrossRef]
- Khoo, N.S.; Smallhorn, J.F.; Kaneko, S.; Myers, K.; Kutty, S.; Tham, E.B. Novel insights into RV adaptation and function in hypoplastic left heart syndrome between the first 2 stages of surgical palliation. JACC Cardiovasc. Imaging 2011, 4, 128–137. [Google Scholar] [CrossRef]
- Lin, L.Q.; Conway, J.; Alvarez, S.; Goot, B.; Serrano-Lomelin, J.; Colen, T.; Tham, E.B.; Kutty, S.; Li, L.; Khoo, N.S. Reduced Right Ventricular Fractional Area Change, Strain, and Strain Rate before Bidirectional Cavopulmonary Anastomosis is Associated with Medium-Term Mortality for Children with Hypoplastic Left Heart Syndrome. J. Am. Soc. Echocardiogr. 2018, 31, 831–842. [Google Scholar] [CrossRef]
- Ugaki, S.; Khoo, N.S.; Ross, D.B.; Rebeyka, I.M.; Adatia, I. Tricuspid valve repair improves early right ventricular and tricuspid valve remodeling in patients with hypoplastic left heart syndrome. J. Thorac. Cardiovasc. Surg. 2013, 145, 446–450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bharucha, T.; Khan, R.; Mertens, L.; Friedberg, M.K. Right ventricular mechanical dyssynchrony and asymmetric contraction in hypoplastic heart syndrome are associated with tricuspid regurgitation. J. Am. Soc. Echocardiogr. 2013, 26, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Inage, A.; Rebeyka, I.M.; Ross, D.B.; Thompson, R.B.; Mackie, A.S.; Smallhorn, J.F. Real-time 3-dimensional echocardiography provides new insight into mechanisms of tricuspid valve regurgitation in patients with hypoplastic left heart syndrome. Circulation 2009, 120, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, K.R.; Goldberg, D.J. Biomarkers and the Fontan Circulation. J. Am. Heart Assoc. 2016, 5, e002926. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Braga, M.; Villa, M.C.; Ferrulli, A.; Nicolosi, G.L.; Lombardo, M.; Migliori, C.; Luzi, L. Comprehensive assessment of biventricular myocardial function by two-dimensional speckle tracking echocardiography in infants of gestational diabetic mothers. Acta Diabetol. 2022, 59, 1145–1156. [Google Scholar] [CrossRef]
- Aly, S.; Mertens, L.; Friedberg, M.K.; Dragulescu, A. Longitudinal Changes in Ventricular Mechanics in Adolescents After the Fontan Operation. J. Am. Soc. Echocardiogr. 2023, 36, 998–1007. [Google Scholar] [CrossRef]
- Pozza, A.; Avesani, M.; Cattapan, I.; Reffo, E.; Cavaliere, A.; Sabatino, J.; Piana, S.; Molinaroli, A.; Sirico, D.; Castaldi, B.; et al. Multimodality imaging and functional assessment in patients with systemic right ventricle and biventricular physiology: A retrospective single-center study. Monaldi Arch. Chest Dis. 2024, 94, 3085. [Google Scholar] [CrossRef]
- Pozza, A.; Zanella, L.; Castaldi, B.; Di Salvo, G. How Will Artificial Intelligence Shape the Future of Decision-Making in Congenital Heart Disease? J. Clin. Med. 2024, 13, 2996. [Google Scholar] [CrossRef]


| Timing for Echocardiogram | Time 1 | Time 2 | Time 3 | Time 4 |
|---|---|---|---|---|
| Control Patients (n = 26) | ||||
| Age at Echo (Years) | 3.7 (3.1–7.5) | 3.8 (3.2–6.7) | 6.2 (5.2–7.9) | 7.8 (4.6–14.8) |
| Weight (kilograms) | 15.8 (14.8–37.7) * | 16.2 (15.3–20.1) | 18.1 (16.0–27.4) | 26.5 (17.8–48.6) |
| Body Surface Area (m2) | 0.82 (0.64–1.0) * | 0.68 (0.63–0.83) | 0.9 (0.7–1.0) | 1.0 (0.7–1.5) |
| Heart Rate (bpm) | 93 (74–109) | 95 (77–103) | 77 (66–98) | 76 (71–94) * |
| SBP (mmHg) | 96 (86–104) | 92 (91–98) | 104 (100–110) | 99 (90–114) |
| DBP (mmHg) | 54 (54–65) | 52 (50–57) | 52 (51–63) | 60 (54–69) |
| Study Patients (n = 26) | ||||
| Age at Echo (Years) | 3.2 (3.0–4.2) | 4.0 (3.7–7.4) | 7.8 (6.5–12.5) | 9.0 (5.6–16.4) |
| Weight (grams) | 18.1 (15.6–20.5) | 19 (18–26) | 22.3 (19.0–34.0) | 28.4 (18.0–46.8) |
| Body Surface Area (m2) | 1.4 (1.1–1.4) | 0.9 (0.7–1.0) | 0.9 (0.7–1.1) | 1.0 (0.7–1.4) |
| Heart Rate (bpm) | 79 (78–85) | 90 (85–116) | 105 (87–118) | 98 (85–118) |
| SBP (mmHg) | 87 (87–165) | 100 (93–115) | 95 (91–105) | 99 (86–108) |
| DBP (mmHg) | 53 (53–67) | 56 (53–58) | 56 (54–59) | 59 (51–67) |
| Timing for Echocardiogram | Time 1 | Time 2 | Time 3 | Time 4 | p-Value |
|---|---|---|---|---|---|
| Control Patients (n = 26) | |||||
| RV performance | |||||
| FAC 4CH (%) | 38 (36–39) | 36 (35–39) * | 34 (32–38) * | 31 (30–33) * | 0.01 |
| FAC SAX (%) | 39 (38–40) | 35 (34–38) * | 35 (33–36) * | 30 (30–34) * | <0.01 |
| TAPSE (cm) | 1.1 (0.8–1.2) | 0.8 (0.7–1.1) | 1.0 (0.9–1.1) * | 0.9 (0.8–1.0) * | 0.09 |
| TAPSE/RVSP | 0.1 (0.08–0.12) | 0.08 (0.07–0.09) | 0.08 (0.08–0.10) * | 0.08 (0.08–0.1) * | 0.17 |
| RV-PET/ET | 0.43 (0.40–0.49) | 0.43 (0.42–0.49) | 0.45 (0.41–0.48) * | 0.46 (0.40–0.50) * | 0.10 |
| Hemodynamics | |||||
| CO (L/min) | 4.9 (4.8–5.5) | 4.5 (4.2–4.5) | 4.3 (4.1–4.8) | 4.2 (3.3–5.1) | 0.22 |
| SV (ml/m2) | 69 (65–81) | 55 (51–67) * | 54.4 (48.0–65.8) | 53.0 (39.7–68.4) | 0.04 |
| RV geometry | |||||
| RWT (cm) | 0.25 (0.23–026) | 0.27 (0.25–0.27) | 0.31 (0.29–0.34) * | 0.42 (0.41–0.43) * | <0.01 |
| Minor/Major Axis | 0.57 (0.51–0.58) | 0.57 (0.55–0.59) | 0.57 (0.56–0.59) * | 0.57 (0.55–0.62) * | 0.09 |
| Study Patients (n = 26) | |||||
| RV performance | |||||
| FAC 4CH (%) | 34 (32–40) | 28 (26–32) | 28 (21–30) | 21 (18–24) | <0.01 |
| FAC SAX (%) | 38 (35–38) | 30 (24–33) | 21 (19–30) | 19 (12–22) | <0.01 |
| TAPSE (cm) | 0.9 (0.8–1) | 0.7 (0.6–1.1) | 0.6 (0.5–0.6) | 0.45 (0.4–0.5) | <0.01 |
| TAPSE/RVSP | 0.08(0.07–0.08) | 0.08 (0.05–0.10) | 0.05 (0.05–0.0 | 0.04 (0.04–0.05) | <0.01 |
| RV-PET/ET | 0.44 (0.38–0.49) | 0.47 (0.43–0.51) | 0.51 (0.45–0.57) | 0. 60 (0.53–0.67) | <0.01 |
| Hemodynamics | |||||
| CO (L/min) | 4.8 (4.6–5.5) | 4.4 (4.4–5.1) | 4.5 (4.1–5.3) | 4.4 (3.5–5.4) | 0.66 |
| SV (ml/m2) | 72 (69–78) | 45 (42–58) | 48.2 (42.6–59.5) | 42.3 (34.8–53.7) | <0.01 |
| RV geometry | |||||
| RWT (cm) | 0.24 (0.22–0.24) | 0.24 (0.21–0.27) | 0.26 (0.22–0.30) | 0.24 (0.20–0.28) | 0.62 |
| Minor/Major Axis | 0.56 (0.53–0.58) | 0.59 (0.58–0.63) | 0.68 (0.64–0.71) | 0.80 (0.77–0.85) | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.S.; Abarbanell, G.; Simpson, K.; Abarbanell, A.M.; Eghtesady, P.; Levy, P.T.; Singh, G.K. Evolution and Predictors of Right Ventricular Failure in Fontan Patients: A Case-Control Study. J. Clin. Med. 2025, 14, 4602. https://doi.org/10.3390/jcm14134602
Kim HS, Abarbanell G, Simpson K, Abarbanell AM, Eghtesady P, Levy PT, Singh GK. Evolution and Predictors of Right Ventricular Failure in Fontan Patients: A Case-Control Study. Journal of Clinical Medicine. 2025; 14(13):4602. https://doi.org/10.3390/jcm14134602
Chicago/Turabian StyleKim, Hannah S., Ginnie Abarbanell, Kathleen Simpson, Aaron M. Abarbanell, Pirooz Eghtesady, Philip T. Levy, and Gautam K. Singh. 2025. "Evolution and Predictors of Right Ventricular Failure in Fontan Patients: A Case-Control Study" Journal of Clinical Medicine 14, no. 13: 4602. https://doi.org/10.3390/jcm14134602
APA StyleKim, H. S., Abarbanell, G., Simpson, K., Abarbanell, A. M., Eghtesady, P., Levy, P. T., & Singh, G. K. (2025). Evolution and Predictors of Right Ventricular Failure in Fontan Patients: A Case-Control Study. Journal of Clinical Medicine, 14(13), 4602. https://doi.org/10.3390/jcm14134602






