Abstract
Background/Objectives: Nonvalvular atrial fibrillation (NVAF) is a prevalent arrhythmia associated with elevated risks of stroke, systemic embolism, and mortality. Emerging evidence underscores the pivotal role of inflammation in NVAF pathogenesis. The CHA2DS2-VA score is currently the most powerful tool used in the management of patients with atrial fibrillation, and integrating novel inflammatory biomarkers—neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammation index (SII), and systemic inflammation response index (SIRI)—into this score may enhance prognostic accuracy and guide personalized therapy. Methods: In this observational case–control study, a cohort of 330 NVAF patients and 201 controls, inflammatory and biochemical parameters were measured and compared, we employed multivariate logistic regression and ROC analyses to validate the discriminative power of novel inflammatory indexes and novel CHA2DS2-VA score, setting a new benchmark for biomarker integration in NVAF management. Results: Inflammatory indexes (NLR, PLR, SII, SIRI) were significantly higher in NVAF patients compared to controls (p < 0.001). Multivariate analysis identified NLR (OR = 4.02), PLR (OR = 1.04), SII (OR = 1.01), and SIRI (OR = 1.87) as independent NVAF risk markers. The CHA2DS2-VA score showed the strongest association with NVAF (OR = 5.55), and an optimal cutoff of ≥2 yielded 88.18% sensitivity and 74.63% specificity. Conclusions: Inflammatory markers NLR, PLR, SII, and SIRI, when assessed alongside the CHA2DS2-VA score, offer significant and complementary prognostic insight for patients with NVAF. These findings support the integration of inflammatory indexes into routine clinical risk assessment models to enhance early identification of high-risk individuals and inform personalized therapeutic strategies. Moreover, our findings provide a rationale for developing composite risk scores in future studies that integrate inflammatory biomarkers with the CHA2DS2-VA score (e.g., a CHA2DS2-VA-Inflammation Score). Further large-scale, longitudinal studies are warranted to validate these results and explore the benefits of inflammation-targeted interventions.
1. Introduction
Nonvalvular atrial fibrillation (NVAF) is a common arrhythmia involving disorganized atrial activity, resulting in heightened stroke risk and mortality [1]. Its rising prevalence parallels aging populations and comorbidities like hypertension and diabetes [2,3]. Inflammatory processes, including oxidative stress, cytokine release, and immune cell infiltration, appear integral to both NVAF onset and persistence [4,5].
Inflammatory markers, such as C-reactive protein (CRP), have been linked to more severe NVAF outcomes [6,7]. Recently, emerging composite indexes including the systemic inflammation response index (SIRI), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and the systemic immune-inflammation index (SII) have attracted increasing attention for their potential to capture broader, dynamic inflammatory pathways [8,9,10]. These indexes may offer enhanced predictive insight by combining multiple hematologic parameters, reflecting not only short-lived immune responses but also the chronic inflammatory milieu that can fuel NVAF-related complications [6]. Moreover, preliminary evidence suggests these biomarkers might help identify patients at heightened risk of bleeding events and overall mortality, further underscoring their potential clinical significance [10,11].
Whether these markers refine or augment standard clinical tools, such as CHA2DS2-VASc and HAS-BLED, remains uncertain. Established scores predominantly focus on clinical risk factors like heart failure, age, and prior stroke, but may not fully account for evolving inflammatory processes that contribute to NVAF burden [12]. Recent modifications to conventional risk stratification models for NVAF have led to the introduction of the CHA2DS2-VA score, an updated parameter that excludes the female sex category from its calculation, in contrast to the traditional CHA2DS2-VASc score [13,14]. Recent studies suggest that, in the absence of other concomitant risk factors, female sex does not independently increase the risk of thromboembolic events, thereby challenging its inclusion as a weighted component in risk prediction models [15,16]. By omitting the sex variable, the CHA2DS2-VA score aims to refine risk stratification by more accurately identifying patients at elevated risk for stroke, potentially reducing the risk of overtreatment in women and enabling more individualized anticoagulation management strategies [13,17]. Integrating composite indexes like NLR, PLR, and SII into CHA2DS2-VA score could potentially optimize anticoagulation strategies by highlighting individuals more susceptible to adverse outcomes. Early intervention or more aggressive treatment regimens might then be selectively applied to these higher-risk patients, improving their long-term prognosis and reducing hospitalizations. Therefore, the aim of this observational case–control study was to evaluate the ability of NLR, PLR, and SII, as well as the CHA2DS2-VA score, to detect the occurrence of NVAF in patients.
2. Patients, Methods, and Ethics Committee Approval
2.1. Patient Selection and Methods
This observational case–control study was conducted on 330 patients with NVAF, and 201 individuals without NVAF as a control (NSR) group at Amasya University, Faculty of Medicine, Department of Cardiology outpatient clinic.
Patients who were under 18 years of age; being pregnant or diagnosed with atrial fibrillation due to heart valve disease such as moderate to severe mitral stenosis, mechanical mitral valves, bioprosthetic valves, or mitral valve repair; patients with acute or chronic infectious disease; patients being treated with immunosuppressive drugs or patients with known or suspected cancer; patients with liver failure, hepatitis, kidney failure, arthritis, systemic diseases; and patients with a history of injury or surgery within 2 months were excluded from study.
After obtaining consent from the patient diagnosed with NVAF, the patient’s laboratory tests at the time of diagnosis were noted; the patient was taken for additional evaluation at the cardiology clinic; the patient’s physical examination was performed; electrocardiography and transthoracic echocardiography were applied to each patient.
During the same period, participants also began recruiting for the control group for the study. Exclusion criteria for the control group were patients younger than 18 years of age; diagnosed with AF at any time point; patients with acute decompensated heart failure (HF); acute coronary syndromes; pulmonary embolism; acute renal failure; coagulation disorders; severe valvular hearth disease (moderate mitral stenosis and all other serious valve diseases and prosthetic valve disease); severe anemia; active thyroid disorders; pregnancy; malignancy; epilepsy; major surgery within the preceding two months; and active infection and/or sepsis. After obtaining consent from the participant, relevant laboratory tests were performed, and each participant’s physical examination was performed similarly to the NVAF patient group; electrocardiography and transthoracic echocardiography were applied to each participant.
A 12-lead ECG was taken with all participants in the supine position at rest.
Echocardiographic evaluation was performed using a PHILIPS EPIQ 7G (is manufactured by Philips Healthcare in Andover, Massachusetts, USA) ultrasound system color Doppler echocardiography device in the left decubitus position for all participants. Ejection fractions (EFs) of all participants were measured using the modified Simpson’s method as per the American Society of Echocardiography and the European Society of Cardiovascular Imaging criteria [18].
2.2. Definitions
AF is reflected on the surface electrocardiogram (ECG) by the absence of discernible and regular P waves, and irregular activation of the ventricles. This results in no specific pattern to RR intervals, in the absence of an atrioventricular block [19]. We included patients diagnosed with permanent AF in the study of patients aged over 18 years.
Diagnosis of non-valvular atrial fibrillation in the absence of moderate to severe mitral stenosis or mechanical heart valves, bioprosthetic valves, or mitral valve repair. The definition of AF by temporal pattern is permanent AF which no further attempts at restoration of sinus rhythm are planned, after a shared decision between the patient and physician [19].
We used the 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes criteria, including fasting glucose, 2 h glucose (during the glucose tolerance test), random glucose, and glycated hemoglobin (HbA1c) to diagnose diabetes mellitus (DM) [20]. Hypertension is defined according to the 2024 ESC Guidelines for the management of elevated blood pressure and hypertension as a confirmed office systolic BP of ≥140 mmHg or diastolic BP of ≥90 mmHg. For this diagnosis to be made, confirmation is recommended with out-of-office measurements (HBPM or ABPM) or at least one repeat office measurement at a subsequent visit, or those who were previously diagnosed with hypertension and started treatment were considered hypertensive [21].
The presence of stroke/TIA in patients was determined based on the criteria outlined in “A consensus report from the European Society of Cardiology Cardiovascular Round Table” [22].
Vascular disease was defined as coronary artery disease, including prior myocardial infarction, angina, history of coronary revascularization (surgical or percutaneous), and evident CAD on angiography or cardiac imaging or peripheral vascular disease (PVD), including the following: intermittent claudication, prior revascularization for PVD, percutaneous or surgical intervention on the abdominal aorta, and complex aortic plaque (defined as mobility, ulceration, pedunculation, or thickness ≥ 4 mm) on imaging [23,24,25].
Demographic characteristics (age and gender), blood and serum parameters (e.g., total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglyceride (TG), C reactive protein (CRP), serum albumin, creatinine (Cr), alanine aminotransferase (ALT), aspartate aminotransferase (AST), hemoglobin (HG), uric acid, Na+, K+, white blood cell counts); LVEF values and presence of diabetes mellitus (DM), hypertension (HT), cerebrovascular accident (SVA), Vascular Disease (VD), were analyzed. CHA2DS2-VA score [C: congestive HF or left ventricular systolic dysfunction, H: hypertension, A: ≥75 years, D: diabetes mellitus, S: previous stroke, V: vascular disease, A: 65–74 years] scores of both groups were calculated.
Inflammatory indexes defined and calculated as SII, the systemic immune-inflammation index (platelet count x neutrophil count/lymphocytes count); SIRI, the systemic inflammation response index (neutrophil count × monocyte count/lymphocyte count; BAR, Blood Urea Nitrogen to Serum Albumin (g/L) Ratio; NLR, neutrophil count/lymphocytes count ratio; CAR, C-reactive protein to albumin (g/L) ratio; UAR, Uric acid to albumin (g/L) ratio; PNI, prognostic nutritional index (albumin level (g/L) + 0.005 × lymphocyte count); MHR, the monocyte count/high-density lipoprotein cholesterol (mg/dL) ratio; NHR, neutrophil count-to-high-density lipoprotein cholesterol (mg/dL) ratio; PLR, the platelet count/lymphocyte count ratio; MLR, monocyte count/lymphocyte count ratio; LCR, lymphocyte count to C-reactive protein ratio; LMR, lymphocyte count/monocyte count ratio; TyG, The triglyceride–glucose index (Ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2]); TG/HDL, triglycerides (mg/dL) to high-density lipoprotein cholesterol (mg/dL) ratio.
2.3. Ethics Committee Approval
The study was approved by the ethics committee of the Amasya University Rectorate Non-Interventional Clinical Research Ethics Committee; Board Decision Number: E-30640013-050.04-246857.
2.4. Statistical Analysis
The statistical analysis of the study data was performed using the SPSS software (Version 22.0, SPSS, Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation (SD), while categorical variables were presented as frequencies and percentages. The Kolmogorov–Smirnov test was used to assess the distribution pattern of the variables. For group comparisons, both the t-test and chi-square test were employed. p < 0.05 was considered statistically significant.
For the intergroup statistical analysis, categorical variables were compared using the χ2 test or Fisher’s exact test, while continuous variables were analyzed using either the t-test or Mann–Whitney U test, based on the normality of distribution. To explore the independent association between inflammatory markers and NVAF, multivariate logistic regression analysis was employed. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each associated variable. Furthermore, a receiver operating characteristic (ROC) curve analysis was conducted to assess the sensitivity and specificity of the CHA2DS2-VA score for detecting NVAF. A p-value of 0.05 was considered statistically significant across all analyses. Optimal cut-off value for the CHA2DS2-VA score score was determined using ROC curves, with the area under the curve (AUC) and 95% CI calculated for the marker. All statistical analyses were performed using R software version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria), MedCalc Programme (trial version), and IBM SPSS Statistics version 26.0. A two-tailed p-value of 0.05 was deemed significant in all analyses.
3. Results
The mean age of the study group was 77.70 ± 5.94 years. The mean age in the NVAF group (79.29 ± 6.64 years) was significantly higher compared to the NSR group (75.09 ± 3.14 years) (p < 0.001). Gender distribution among the groups was similar (p = 0.465; Table 1). There was no significant difference in gender distribution between the NVAF group (188 females, 142 males) and the NSR group (121 females, 80 males) (p = 0.465).

Table 1.
Participants’ demographic and disease data.
The prevalence of hypertension (HT) in the NVAF group (95.2%) was significantly higher than in the NSR group (81.1%) (p < 0.001). Similarly, the prevalence of cerebrovascular disease (CVD) in the NVAF group (17.9%) was significantly higher than in the NSR group (5.5%) (p < 0.001). However, no significant difference was observed between the groups regarding the prevalence of diabetes mellitus (DM) (p = 0.80).
The mean LVEF of the patient group was 48.81 ± 10.34, and the mean LVEF of the control group was 58,59 ± 5.31 (p < 0.0001; Table 1).
The incidence of vascular disease in the patient group was (%) 9.1 (30/330), and the incidence of vascular disease in the control group was (%) 3.0 (6/195) (p-value: 0.007; Table 1).
The mean CHA2DS2-VA score of the patient group was 3.66 ± 1.14, and the mean CHA2DS2-VA score of the control group was 2.10 ± 0.98 (p < 0.0001; Table 1).
The CHA2DS2-VA score was significantly higher in the NVAF group (3.66 ± 1.14) compared to the NSR group (2.10 ± 0.98) (p < 0.001). In the NVAF group, the proportions of patients with CHA2DS2-VA scores of 1 and 2 were 0.9% and 10.9%, respectively, whereas in the NSR group, these rates were 22.9% and 51.7%, respectively. The proportions of patients with CHA2DS2-VA scores of 3 and 4 in the NVAF group were 37.6% and 31.8%, respectively, while in the NSR group, they were 19.4% and 4.0%, respectively. In the NVAF group, 12.1% and 4.8% of patients had CHA2DS2-VA scores of 5 and 6, respectively, compared to 2.0% and 0.0% in the NSR group. Additionally, 1.2% and 0.6% of patients in the NVAF group had CHA2DS2-VA scores of 7 and 8, respectively, whereas no patients in the NSR group had scores of 7 or 8. These findings indicate that the stroke risk detected by the CHA2DS2-VA score was higher in the NVAF group.
Glucose, Cr, ALT, Na+, K+, TG, Hgb, WBC, PLT, and MPV levels were similar in the control and patient groups (Table 2). BUN, AST, CRP, uric acid, neutrophil, and monocyte levels were significantly higher in the patient group compared to the control group (p < 0.001; Table 2). While albumin, total cholesterol, HDL, LDL (p-value: 0.001), and lymphocyte levels were significantly higher in the control (NSR) group compared to the NVAF group (p < 0.001; Table 2). On the other hand, while TyG levels were similar in both groups, TG/HDL, SII, SIRI, BAR, UAR, MHR, NLR, PLR, and CAR levels were significantly higher in the patient group compared to the control group (p < 0.001; Table 2). Additionally, PNI, LCR, and LMR levels were significantly higher in the control (NSR) group compared to the NVAF group (p < 0.001; Table 2).

Table 2.
Participants’ laboratory and inflammatory index data.
In the NVAF group, most inflammatory markers were found to be significantly higher compared to the NSR group. CRP levels were significantly elevated in the NVAF group compared to the NSR group (p < 0.001). Similarly, the NLR was significantly higher in the NVAF group than in the NSR group (p < 0.001). The PLR was also significantly elevated in the NVAF group (263.84 ± 130.7) compared to the NSR group (p < 0.001).
The SII was significantly higher in the NVAF group than in the NSR group (p < 0.001). Furthermore, LCR was significantly lower in the NVAF group (0.14 ± 0.06) compared to the NSR group (p < 0.001). Similarly, the prognostic PNI was significantly lower in the NVAF group than in the NSR group (p < 0.001), mirroring the trend observed with the LCR. These findings indicate that systemic inflammation is more pronounced in patients with NVAF.
3.1. Multivariate Analysis of CHA2DS2-VA Score and Hematological Indexes in NVAF Group
Table 3 shows multivariate analyses of risk markers for NVAF. A multivariate logistic regression analysis was conducted to evaluate the independent detective value of inflammatory markers and clinical parameters in the association between the NVAF and NSR groups. According to the results of the analysis, age (OR: 1.15, 95% CI: 1.14–1.20, p < 0.001), the presence of cerebrovascular disease (OR: 3.76, 95% CI: 1.92–7.34, p < 0.001), and vascular disease (OR: 3.25, 95% CI: 1.32–7.95, p = 0.010) were identified as significant risk factors for the development of NVAF. The CHA2DS2-VA score demonstrated the strongest association with NVAF (OR: 5.55, 95% CI: 4.11–7.50, p < 0.001), further emphasizing the importance of this score in risk stratification.

Table 3.
Multivariate logistic regression model for the association between NVAF group and NSR group.
Among the inflammatory markers, the SII (OR: 1.01, 95% CI: 1.01–1.02, p < 0.001), SIRI (OR: 1.87, 95% CI: 1.65–2.12, p < 0.001), PLR (OR: 1.04, 95% CI: 1.03–1.04, p < 0.001), and NLR (OR: 4.02, 95% CI: 2.05–8.03, p < 0.001) were all found to be significantly associated with the presence of NVAF. These findings underscore the important role of both clinical risk factors and systemic inflammation in the pathogenesis of NVAF.
Table 3 demonstrates that, in multivariate logistic regression analysis, inflammatory markers possess independent risk markers for the development of NVAF and suggest that integrating these markers into existing risk scoring systems may enhance clinical decision-making processes. In particular, composite indexes such as SII, SIRI, PLR, and NLR may serve as potential biomarkers for the early diagnosis and risk stratification of NVAF. Finally, these results indicate that the CHA2DS2-VA score can be used with high accuracy to identify patients with NVAF (Table 3).
3.2. ROC Curve Analyses of CHA2DS2-VA Score in NVAF
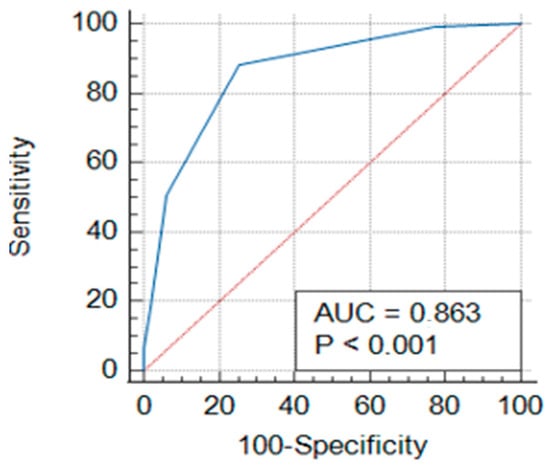
Figure 1.
Receiver operating characteristic (ROC) curve of CHA2DS2-VA score.

Table 4.
ROC curve and prognostic accuracy of the CHA2DS2-VA score.
The performance of the CHA2DS2-VA score in detecting NVAF diagnosis was evaluated using receiver operating characteristic (ROC) curve analysis. Figure 1 shows that the area under the curve (AUC) value for the CHA2DS2-VA score was found to be 0.863 (p < 0.001). The cut-off value for the CHA2DS2-VA score was determined as 2. At this threshold, the sensitivity of the score was calculated to be 88.18%, and the specificity was 74.63% (Table 4).
4. Discussion
The pathophysiology underlying NVAF is multifaceted, encompassing structural, electrical, and inflammatory mechanisms. Among these, systemic inflammation has been increasingly recognized as a critical contributor to both the initiation and perpetuation of atrial fibrillation (AF) [26]. Elevated levels of inflammatory biomarkers, such as CRP, interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), have been consistently associated with the occurrence and prognosis of AF, underscoring the role of inflammatory processes in atrial remodeling, fibrosis, and thrombogenesis [27,28].
Recent research has shifted toward exploring readily available and cost-effective hematological parameters as potential indicators of systemic inflammation and predictors of NVAF risk [10,11]. Specifically, NLR, PLR, SII, and SIRI have emerged as promising biomarkers due to their ease of measurement and integration into routine clinical practice. These indexes not only reflect inflammatory status but also provide insights into immune dysregulation and prothrombotic states, which are integral to NVAF pathogenesis [10].
Our results demonstrated that NLR, SII, SIRI, PLR, and CHA2DS2-VA scores were significantly higher in the NVAF group than in the NSR group. In our multivariate analysis, risk factors were identified in the NVAF group. In particular, the CHA2DS2-VA score showed the strongest association with NVAF (OR: 5.55, 95% CI: 4.11–7.50), with each incremental increase in the score markedly elevating the risk. Additionally, well-known risk factors, including age, hypertension, and previous cerebrovascular events, were significantly associated with NVAF. Furthermore, an elevated NLR increased the odds of NVAF almost fourfold (OR: 4.02, 95% CI: 2.05–8.03). Similarly, SIRI was also identified as a risk factor for NVAF (OR: 1.87, 95% CI: 1.65–2.12), while PLR (OR: 1.04, 95% CI: 1.03–1.04) and SII (OR: 1.01, 95% CI: 1.01–1.02) were statistically significantly detected with more modest risk increases. Considering the broad range of these parameters, markedly elevated PLR or SII may indicate a substantially increased NVAF risk. Collectively, these findings suggest that both traditional clinical risk factors and systemic inflammation contribute complementarily to the pathogenesis of NVAF.
The association between AF and systemic inflammation has long been reported in the literature [29]. Previous studies have shown that inflammatory markers such as CRP and interleukin-6 are closely related to both the presence and duration of AF, with inflammatory cell infiltrates being detected in atrial tissue specimens [29]. Inflammation is considered to trigger atrial remodeling through pathways involving oxidative stress, apoptosis, and fibrosis, thereby predisposing to the development of AF [30,31]. Moreover, inflammation may also contribute to the thrombotic complications associated with AF by promoting endothelial dysfunction, platelet activation, and coagulation [32].
Regarding the neutrophil-to-lymphocyte ratio, a recent meta-analysis reported that elevated NLR is associated with increased AF recurrence and stroke risk in patients with AF [33]. Additionally, higher NLR values have been linked to left atrial thrombus formation, emphasizing its value as a prognostic biomarker [33]. Our finding of significantly elevated NLR in the NVAF group further supports the clinical importance of NLR in risk stratification. Similarly, the platelet-to-lymphocyte ratio (PLR) is recognized as an indicator of both inflammatory and thrombotic states, which are mechanistically involved in AF pathogenesis. The high PLR values in the NVAF group of our study align with reports in the literature indicating PLR as an independent predictor of AF recurrence post-ablation and incorporating PLR into conventional risk scores enhances predictive accuracy for AF recurrence, as reflected by improved model discrimination [34].
The systemic immune-inflammation index (SII) emerged as another significant parameter in our work. SII, which integrates neutrophil, platelet, and lymphocyte counts, has been validated as a prognostic marker for cardiovascular events. Recent studies have highlighted that elevated SII levels in NVAF patients are linked to a higher risk of left atrial thrombus formation [19]. For instance, one study found that an SII value above 693 predicted left atrial thrombus with 71.6% sensitivity and 71.7% specificity, suggesting that SII may be as effective as—or even superior to—NLR and PLR in certain clinical settings [20]. Accordingly, the high SII levels observed in our NVAF cohort further reinforce its role as a comprehensive marker that reflects both inflammatory and prothrombotic states [21].
Regarding SIRI, although available data are more limited, our findings are in line with emerging evidence. SIRI reflects the balance between pro-inflammatory (neutrophils and monocytes) and regulatory (lymphocytes) immune components, and its significant elevation in NVAF patients in our study is consistent with observations in ischemic stroke populations where patients with concomitant AF demonstrated much higher SIRI levels (with an OR for log-SIRI approximating 6.2) [22]. Such findings underscore the potential of SIRI to serve as an indicator of the intense inflammatory processes that may underlie AF pathogenesis [23].
Traditionally, the CHA2DS2-VASc has been used to predict stroke, major bleeding, and mortality in NVAF patients; however, it relies solely on clinical demographic and comorbidity data without reflecting the inflammatory processes [35,36]. Our study found that while a high CHA2DS2-VA is a strong independent risk marker of NVAF (with each additional point conferring significant risk), the inflammatory indexes, particularly NLR and SII, add prognostic value beyond the clinical score. This observation is consistent with the recent literature that underlines the limitations of traditional risk scores and emphasizes the need to incorporate inflammatory biomarkers into risk stratification models [37,38,39]. Several studies suggested that including biomarkers in conventional risk scores can improve the model’s discriminative power [40]. Therefore, in predicting key clinical outcomes such as stroke, major bleeding, and mortality, integrating inflammatory indexes like NLR, PLR, and SII alongside the CHA2DS2-VA may offer a more precise risk stratification [41]. In addition, our results showed that factors such as age, history of cerebrovascular events, vascular disease, and CHA2DS2-VA, in addition to NLR, PLR, SII, and SIRI, were significantly associated with increased NVAF risk. These results underscore that the inflammatory response plays a critical role in the pathogenesis of NVAF beyond the traditional clinical risk factors.
Although our study has several strengths, including its cross sectional design, adequate sample size, and comprehensive laboratory analyses, it also has several limitations. First, despite the prospective approach, our analysis represents a cross sectional comparison between NVAF patients and a control group; therefore, it is not fully clear whether the elevated inflammatory indexes are a cause or a consequence of AF. Since AF itself or its associated comorbidities may affect inflammation levels, caution is warranted in inferring a causal relationship [42]. Long-term prospective cohort studies that measure inflammatory indexes prior to the onset of AF are needed to clarify this issue. Second, as the study was conducted at a single center, the generalizability of the results to different geographic regions or ethnic groups may be limited. Third, the inflammatory markers can be influenced by various factors such as chronic inflammatory conditions, acute infections, or medications, such as corticosteroids, which may affect the specificity of the measurements. Fourth, the intercorrelation among indexes like NLR, PLR, SII, and SIRI could introduce challenges in determining the most prognostically valuable marker when used together; thus, future studies may consider developing a composite score that integrates these measures. Additionally, left atrial strain parameters, particularly left atrial strain rate (LASr)—which have demonstrated incremental prognostic value in NVAF patients—were not evaluated in this study [43,44]. Recent data indicate that LASr is strongly associated with both myocardial fibrosis and inflammation and may further improve risk stratification in NVAF populations [45]. Nonetheless, the detailed evaluation of inflammatory indexes and their independent detection values for NVAF provided by our study represents a significant contribution to the literature.
5. Conclusions and Recommendations
This study is one of the first observational case–control study investigations to systematically evaluate the role of inflammatory processes in the pathogenesis of NVAF and to demonstrate the prognostic value of next-generation inflammatory indexes (NLR, PLR, SII, SIRI) when integrated with the CHA2DS2-VA score. Our findings fill a gap in the existing literature by providing strong evidence that these indexes can significantly enhance risk stratification in NVAF patients compared to traditional scoring systems. In particular, composite indexes such as SIRI and SII highlight the potential for an inflammation-focused paradigm in personalized treatment strategies.
The study offers a comprehensive cross sectional analysis of the diagnostic and prognostic utility of inflammatory indexes (NLR, PLR, SII, SIRI) alongside the CHA2DS2-VA score in NVAF patients. We demonstrated that these indexes are significantly associated with both the presence and severity of NVAF. Notably, NLR (OR = 4.02) and the CHA2DS2-VA score (OR = 5.55) exhibited the strongest associations, underscoring the critical role of inflammation in NVAF pathogenesis.
We recommend incorporating inflammatory indexes such as NLR, PLR, and SII together with the CHA2DS2-VA score into the routine evaluation of NVAF patients. This combined approach may be particularly valuable for the early identification of high-risk individuals and for optimizing anticoagulation strategies. Future studies should focus on developing composite risk scores that integrate inflammatory biomarkers (for example, a CHA2DS2-VA-Inflammation Score) and should validate these models in independent cohorts to assess their potential for improving clinical outcomes. Additionally, longitudinal studies and randomized controlled trials targeting inflammation (e.g., with IL-6 inhibitors) are needed to elucidate the causal role of inflammation in NVAF. Also, replication of these findings in multicenter studies will be essential to increase the generalizability of the results.
Author Contributions
Conceptualization, A.C.; methodology, S.C.; software, A.C. and S.C.; validation, A.C. and S.C.; formal analysis, A.C. and S.C.; investigation, A.C., M.C. and E.K.; resources, A.C.; data curation, A.C.; writing—original draft preparation, A.C. and S.C.; writing—review and editing, A.C., S.C., M.C. and E.K.; visualization, A.C.; supervision, M.C. and E.K.; project administration, A.C.; funding acquisition, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committee of the Amasya University Rectorate Non-Interventional Clinical Research Ethics Committee; protocol code: E-30640013-050.04-246857 and date of approval-24 February 2025.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are provided within the manuscript. If needed, the datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank all the authors who contributed to the research and our families who never stopped supporting us during this period.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Min, J.; Farooq, M.U. Detecting nonvalvular atrial fibrillation and anticoagulant therapy in cardioembolic ischemic stroke. Postgrad. Med. 2016, 128, 620–628. [Google Scholar] [CrossRef]
- Singh, B.; Pai, P.; Kumar, H.; George, S.; Mahapatra, S.; Garg, V.; Gupta, G.N.; Makineni, K.; Ganeshwala, G.; Narkhede, P.; et al. Expert Recommendations on the Usage of Non-vitamin K Antagonist Oral Anticoagulants (NOACs) from India: Current Perspective and Future Direction. Cardiol. Ther. 2022, 11, 49–79. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Murillo, M.F.; Brenner-Muslera, E.; Rodríguez-Carrillo, D.L.; Chua-López, C.A.; Torres-Tamayo, M. Type 2 Diabetes Mellitus and Nonvalvular Atrial Fibrillation in Mexico: National Registries Raise a Red Flag. Rev. Investig. Clin. 2023, 75, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef] [PubMed]
- Jannati, S.; Patnaik, R.; Banerjee, Y. Beyond Anticoagulation: A Comprehensive Review of Non-Vitamin K Oral Anticoagulants (NOACs) in Inflammation and Protease-Activated Receptor Signaling. Int. J. Mol. Sci. 2024, 25, 8727. [Google Scholar] [CrossRef]
- Martins, G.L.; Duarte, R.C.F.; Vieira É, L.M.; Rocha, N.P.; Figueiredo, E.L.; Silveira, F.R.; Caiaffa, J.R.S.; Lanna, R.P.; Carvalho, M.D.G.; Palotás, A.; et al. Evaluation of New Potential Inflammatory Markers in Patients with Nonvalvular Atrial Fibrillation. Int. J. Mol. Sci. 2023, 24, 3326. [Google Scholar] [CrossRef]
- Sharma, G.; Ghati, N.; Sharique, M.; Sharma, S.; Shetkar, S.; Karmakar, S.; Naik, N.; Lakshmy, R.; Thakur, B.; Agarwal, A.; et al. Role of inflammation in initiation and maintenance of atrial fibrillation in rheumatic mitral stenosis—An analytical cross-sectional study. J. Arrhythm. 2020, 36, 1007–1015. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Zhang, S.; Liu, Y.; Ma, L.; Zhu, J.; Xin, Y.; Wang, Y.; Yang, C.; Cheng, Y. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J. Clin. Lab. Anal. 2019, 33, e22964. [Google Scholar] [CrossRef]
- Yao, W.; Wang, W.; Tang, W.; Lv, Q.; Ding, W. Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune inflammation index (SII) to predict postoperative pneumonia in elderly hip fracture patients. J. Orthop. Surg. Res. 2023, 18, 673. [Google Scholar] [CrossRef]
- Islam, M.M.; Satici, M.O.; Eroglu, S.E. Unraveling the clinical significance and prognostic value of the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, systemic immune-inflammation index, systemic inflammation response index, and delta neutrophil index: An extensive literature review. Turk. J. Emerg. Med. 2024, 24, 8–19. [Google Scholar]
- Thottuvelil, S.R.; Chacko, M.; Warrier, A.R.; Nair, M.P.; Rajappan, A.K. Comparison of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII) as marker of adverse prognosis in patients with infective endocarditis. Indian. Heart J. 2023, 75, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Zhang, L.; Guo, Y.; Sun, H.; Zhang, X.; Bo, Y.; Zhou, X.; Tang, B. A Review of Biomarkers for Ischemic Stroke Evaluation in Patients With Non-valvular Atrial Fibrillation. Front. Cardiovasc. Med. 2021, 8, 682538. [Google Scholar] [CrossRef]
- Boriani, G.; Vitolo, M.; Mei, D.A. CHA2DS2-VA instead of CHA2DS2-VASc for stroke risk stratification in patients with atrial fibrillation: Not just a matter of sex. Europace 2024, 26, euae281. [Google Scholar] [CrossRef] [PubMed]
- Teppo, K.; Lip, G.Y.H.; Airaksinen, K.E.J.; Halminen, O.; Haukka, J.; Putaala, J.; Mustonen, P.; Linna, M.; Hartikainen, J.; Lehto, M. Comparing CHA(2)DS(2)-VA and CHA(2)DS(2)-VASc scores for stroke risk stratification in patients with atrial fibrillation: A temporal trends analysis from the retrospective Finnish AntiCoagulation in Atrial Fibrillation (FinACAF) cohort. Lancet Reg. Health Eur. 2024, 43, 100967. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, A.P.; Lindhardsen, J.; Lip, G.Y.; Gislason, G.H.; Torp-Pedersen, C.; Olesen, J.B. Female sex as a risk factor for stroke in atrial fibrillation: A nationwide cohort study. J. Thromb. Haemost. 2012, 10, 1745–1751. [Google Scholar] [CrossRef]
- Nielsen, P.B.; Overvad, T.F. Female Sex as a Risk Modifier for Stroke Risk in Atrial Fibrillation: Using CHA2DS2-VASc versus CHA2DS2-VA for Stroke Risk Stratification in Atrial Fibrillation: A Note of Caution. Thromb. Haemost. 2020, 120, 894–898. [Google Scholar] [CrossRef]
- Chao, T.F.; Lip, G.Y.; Liu, C.J.; Tuan, T.C.; Chen, S.J.; Wang, K.L.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; et al. Validation of a Modified CHA2DS2-VASc Score for Stroke Risk Stratification in Asian Patients With Atrial Fibrillation: A Nationwide Cohort Study. Stroke 2016, 47, 2462–2469. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. J. Echocardiogr. 2016, 17, 1321–1360. [Google Scholar]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes: Developed by the task force on the management of cardiovascular disease in patients with diabetes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension: Developed by the task force on the management of elevated blood pressure and hypertension of the European Society of Cardiology (ESC) and endorsed by the European Society of Endocrinology (ESE) and the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [PubMed]
- Doehner, W.; Mazighi, M.; Hofmann, B.M.; Lautsch, D.; Hindricks, G.; Bohula, E.A.; Byrne, R.A.; Camm, A.J.; Casadei, B.; Caso, V. Cardiovascular care of patients with stroke and high risk of stroke: The need for interdisciplinary action: A consensus report from the European Society of Cardiology Cardiovascular Round Table. Eur. J. Prev. Cardiol. 2020, 27, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A. 2024 ESC guidelines for the management of chronic coronary syndromes: Developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [PubMed]
- Halliday, A.; Bax, J.J. The 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 301–302. [Google Scholar] [CrossRef]
- Steensig, K.; Olesen, K.K.; Thim, T.; Nielsen, J.C.; Jensen, S.E.; Jensen, L.O.; Kristensen, S.D.; Bøtker, H.E.; Lip, G.Y.; Maeng, M. Should the presence or extent of coronary artery disease be quantified in the CHA2DS2-VASc score in atrial fibrillation? A report from the Western Denmark Heart Registry. Thromb. Haemost. 2018, 118, 2162–2170. [Google Scholar] [CrossRef]
- Tirandi, A.; Carbone, F.; Liberale, L.; Montecucco, F. Evaluating inflammatory status to predict atrial fibrillation recurrence following ablation: The role of systemic immune-inflammation index. World J. Cardiol. 2025, 17, 103074. [Google Scholar] [CrossRef]
- Giovannini, S.; Onder, G.; Liperoti, R.; Russo, A.; Carter, C.; Capoluongo, E.; Pahor, M.; Bernabei, R.; Landi, F. Interleukin-6, C-reactive protein, and tumor necrosis factor-alpha as predictors of mortality in frail, community-living elderly individuals. J. Am. Geriatr. Soc. 2011, 59, 1679–1685. [Google Scholar] [CrossRef]
- Marcus, G.M.; Whooley, M.A.; Glidden, D.V.; Pawlikowska, L.; Zaroff, J.G.; Olgin, J.E. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: Data from the Heart and Soul Study. Am. Heart J. 2008, 155, 303–309. [Google Scholar] [CrossRef]
- Harada, M.; Van Wagoner, D.R.; Nattel, S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ. J. 2015, 79, 495–502. [Google Scholar] [CrossRef]
- Nso, N.; Bookani, K.R.; Metzl, M.; Radparvar, F. Role of inflammation in atrial fibrillation: A comprehensive review of current knowledge. J. Arrhythm. 2021, 37, 1–10. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Vlachakis, P.K.; Korantzopoulos, P.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Atrial Fibrosis in Atrial Fibrillation: Mechanistic Insights, Diagnostic Challenges, and Emerging Therapeutic Targets. Int. J. Mol. Sci. 2024, 26, 209. [Google Scholar] [CrossRef]
- Guo, Y.; Lip, G.Y.; Apostolakis, S. Inflammatory Biomarkers and Atrial Fibrillation: Potential Role of Inflammatory Pathways in the Pathogenesis of Atrial Fibrillation-induced Thromboembolism. Curr. Vasc. Pharmacol. 2015, 13, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Liu, L.; Chai, M.; Cai, Z.; Wang, D. Predictive value of neutrophil to lymphocyte ratio for clinical outcome in patients with atrial fibrillation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2024, 11, 1461923. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Sun, H.; Tang, Y.; Luo, Y.; Liu, H. Platelet-to-Lymphocyte Ratio Improves the Predictive Ability of the Risk Score for Atrial Fibrillation Recurrence After Radiofrequency Ablation. J. Inflamm. Res. 2023, 16, 6023–6038. [Google Scholar] [CrossRef]
- Serna, M.J.; Rivera-Caravaca, J.M.; López-Gálvez, R.; Soler-Espejo, E.; Lip, G.Y.H.; Marín, F.; Roldán, V. Dynamic assessment of CHA(2)DS(2)-VASc and HAS-BLED scores for predicting ischemic stroke and major bleeding in atrial fibrillation patients. Rev. Esp. Cardiol. (Engl. Ed.) 2024, 77, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Morrone, D.; Kroep, S.; Ricci, F.; Renda, G.; Patti, G.; Kirchhof, P.; Chuang, L.H.; van Hout, B.; De Caterina, R. Mortality Prediction of the CHA(2)DS(2)-VASc Score, the HAS-BLED Score, and Their Combination in Anticoagulated Patients with Atrial Fibrillation. J. Clin. Med. 2020, 9, 3987. [Google Scholar] [CrossRef]
- Parsons, C.; Patel, S.I.; Cha, S.; Shen, W.K.; Desai, S.; Chamberlain, A.M.; Luis, S.A.; Aguilar, M.I.; Demaerschalk, B.M.; Mookadam, F.; et al. CHA(2)DS(2)-VASc Score: A Predictor of Thromboembolic Events and Mortality in Patients With an Implantable Monitoring Device Without Atrial Fibrillation. Mayo Clin. Proc. 2017, 92, 360–369. [Google Scholar] [CrossRef]
- Chao, T.F.; Liu, C.J.; Tuan, T.C.; Chen, S.J.; Wang, K.L.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Chen, T.J.; et al. Comparisons of CHADS2 and CHA2DS2-VASc scores for stroke risk stratification in atrial fibrillation: Which scoring system should be used for Asians? Heart Rhythm. 2016, 13, 46–53. [Google Scholar] [CrossRef]
- Laish-Farkash, A.; Sevilya, Z.; Perelshtein Brezinov, O.; Fortis, L.; Lev, E. Inflammatory cytokines differ between patients with high versus low CHA2DS2-VASc scores in sinus rhythm-a possible mechanism for adverse cardiovascular events. Int. J. Cardiol. Cardiovasc. Risk Prev. 2022, 15, 200155. [Google Scholar] [CrossRef]
- Kim, H.C. Clinical utility of novel biomarkers in the prediction of coronary heart disease. Korean Circ. J. 2012, 42, 223–228. [Google Scholar] [CrossRef]
- Mănescu, I.B.; Pál, K.; Lupu, S.; Dobreanu, M. Conventional Biomarkers for Predicting Clinical Outcomes in Patients with Heart Disease. Life 2022, 12, 2112. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Chen, Q.; Liu, Z.; Li, X.; Zhang, H.; Feng, X. Genetically predicted inflammatory proteins and the risk of atrial fibrillation: A bidirectional Mendelian randomization study. Front. Cardiovasc. Med. 2024, 11, 1375750. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, A.; Genovesi, S.; Sonaglioni, A.; Binda, G.; Rigamonti, E.; Lombardo, M.; Anza, C. Mechanical atrial recovery after cardioversion in persistent atrial fibrillation evaluated by bidimensional speckle tracking echocardiography. J. Cardiovasc. Med. 2019, 20, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.R.; Yakupoglu, H.Y.; Kralj-Hans, I.; Haldar, S.; Bahrami, T.; Clague, J.; De Souza, A.; Hussain, W.; Jarman, J.; Jones, D.G. Left atrial function predicts atrial arrhythmia recurrence following ablation of long-standing persistent atrial fibrillation. Circ. Cardiovasc. Imaging 2023, 16, e015352. [Google Scholar] [CrossRef]
- Yin, G.; Xie, R.; You, L.; Yin, H.; Sun, Y.; Wu, J.; Zhao, Y.; Geng, X.; Zhang, Y. Left atrial function, inflammation, and prothrombotic response after radiofrequency ablation for atrial fibrillation. J. Chin. Med. Assoc. 2018, 81, 409–415. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).