Systemic Inflammation and Metabolic Changes After Cardiac Surgery and Postoperative Delirium Risk
Abstract
1. Introduction
2. Methods
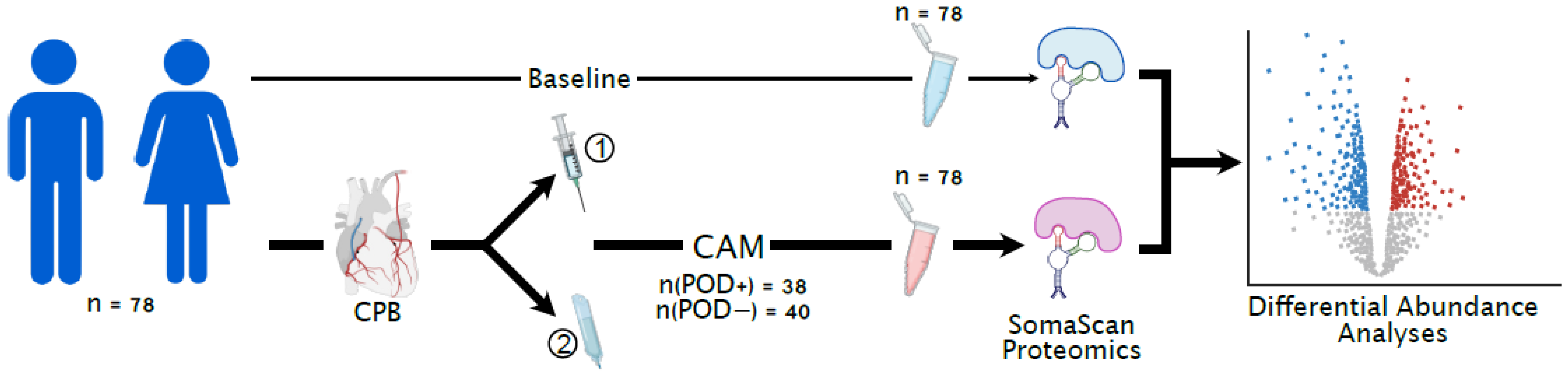
2.1. Study Design and Patient Selection
2.2. Sample Collection
2.3. Proteomic Profiling Using SOMAScan
2.4. Bioinformatics and Statistical Analyses
3. Results
3.1. Baseline Subject Characteristics
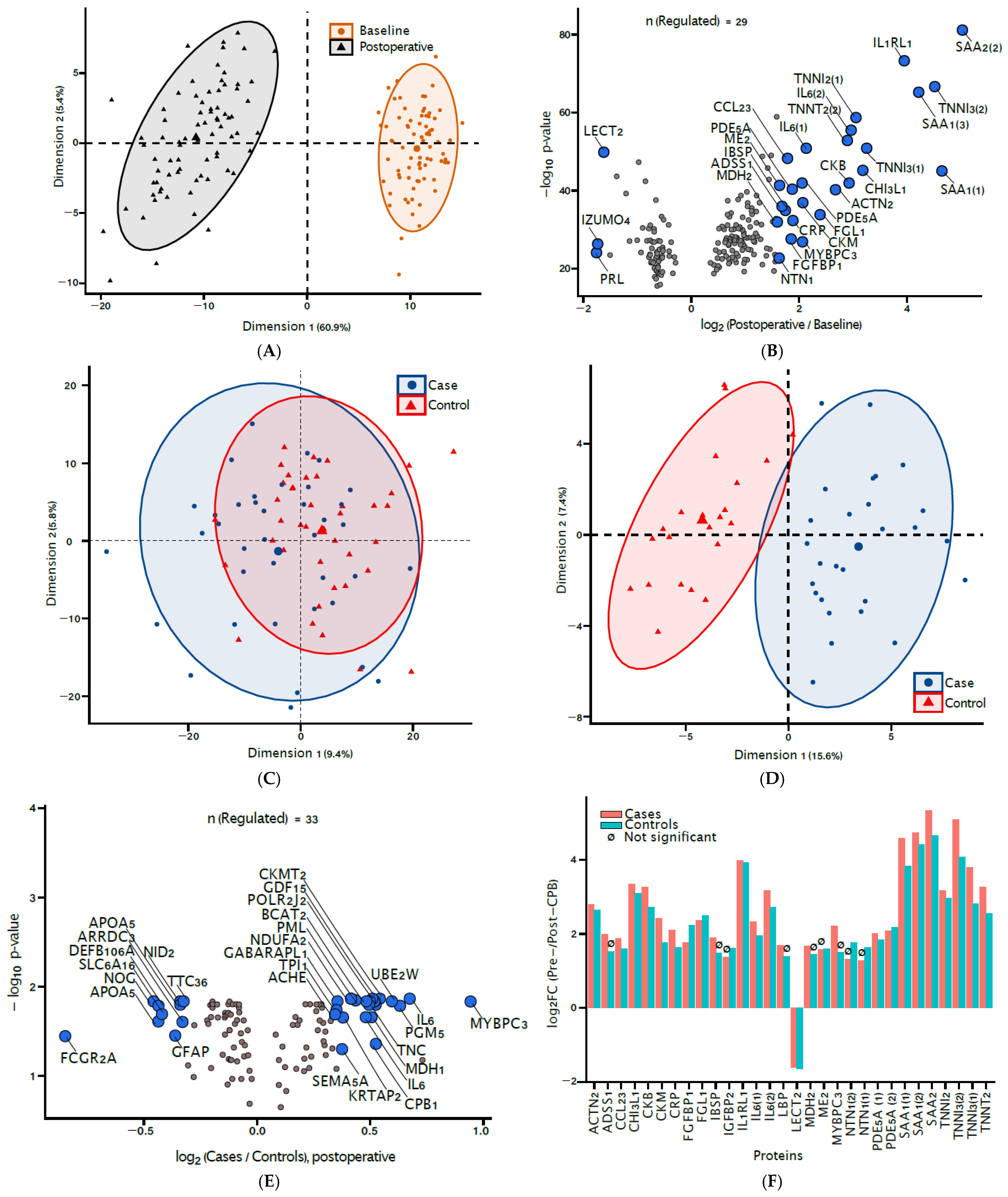
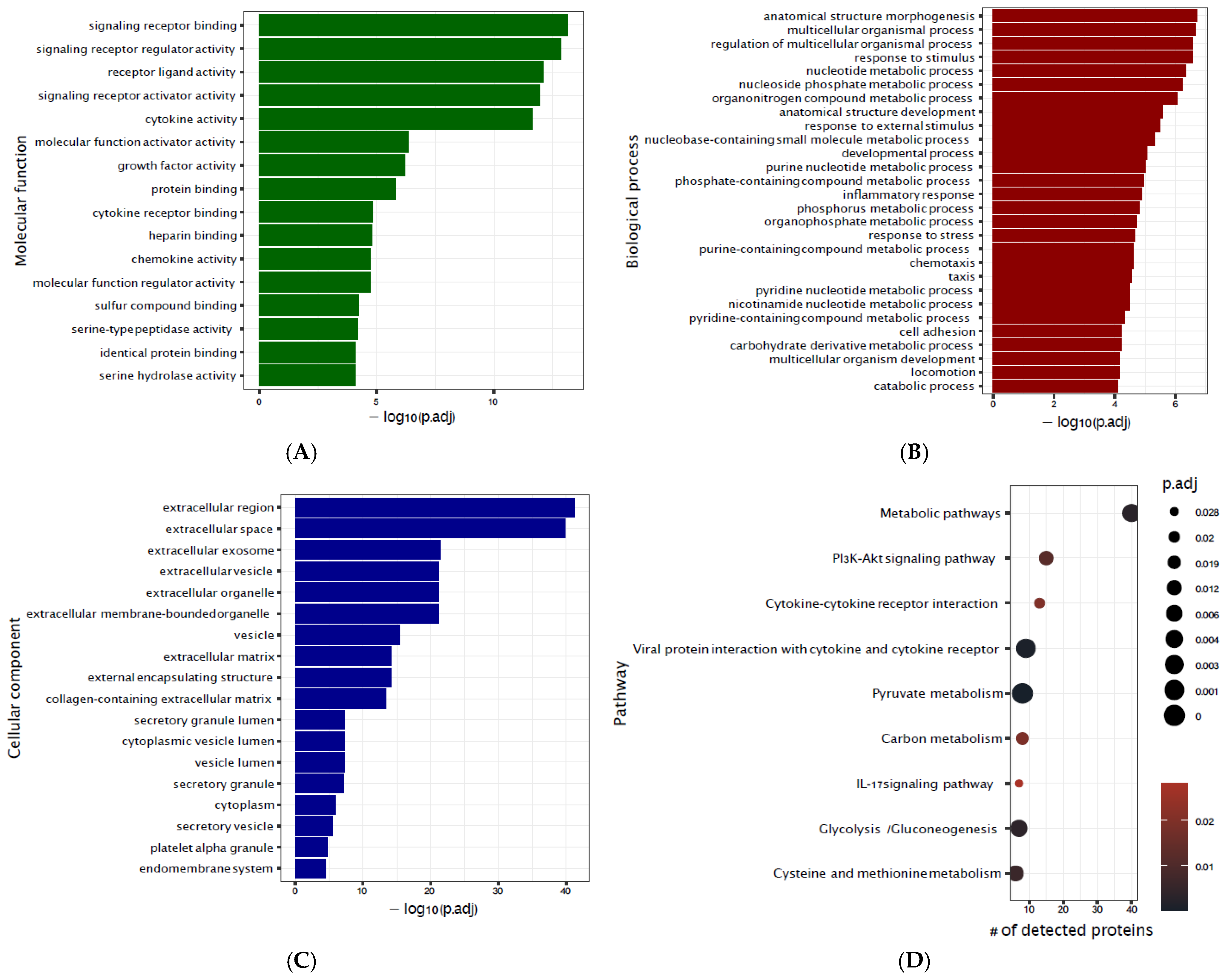
3.2. Signatures of CPB Exposure
3.3. Biomarkers of Delirium
3.4. Metabo-Inflammatory Model of Delirium
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, C.H., IV; Laflam, A.; Max, L.; Lymar, D.; Neufeld, K.J.; Tian, J.; Shah, A.S.; Whitman, G.J.; Hogue, C.W. The impact of delirium after cardiac surgical procedures on postoperative resource use. Ann. Thorac. Surg. 2016, 101, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Schürch, R.; Boettger, S.; Garcia Nuñez, D.; Schwarz, U.; Bettex, D.; Jenewein, J.; Bogdanovic, J.; Staehli, M.L.; Spirig, R. A hospital-wide evaluation of delirium prevalence and outcomes in acute care patients-a cohort study. BMC Health Serv. Res. 2018, 18, 550. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-C.; Yeh, T.Y.-C.; Wei, Y.-C.; Ku, S.-C.; Xu, Y.-J.; Chen, C.C.-H.; Inouye, S.; Boehm, L.M. Association of Incident Delirium With Short-term Mortality in Adults With Critical Illness Receiving Mechanical Ventilation. JAMA Netw. Open 2022, 5, e2235339. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.H., IV; Probert, J.; Healy, R.; Parish, M.; Nomura, Y.; Yamaguchi, A.; Tian, J.; Zehr, K.; Mandal, K.; Kamath, V. Cognitive decline after delirium in patients undergoing cardiac surgery. Anesthesiology 2018, 129, 406–416. [Google Scholar] [CrossRef]
- Wildes, T.S.; Mickle, A.M.; Abdallah, A.B.; Maybrier, H.R.; Oberhaus, J.; Budelier, T.P.; Kronzer, A.; McKinnon, S.L.; Park, D.; Torres, B.A. Effect of electroencephalography-guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: The ENGAGES randomized clinical trial. JAMA 2019, 321, 473–483. [Google Scholar] [CrossRef]
- Qu, J.Z.; Mueller, A.; McKay, T.B.; Westover, M.B.; Shelton, K.T.; Shaefi, S.; D’Alessandro, D.A.; Berra, L.; Brown, E.N.; Houle, T.T. Nighttime dexmedetomidine for delirium prevention in non-mechanically ventilated patients after cardiac surgery (MINDDS): A single-centre, parallel-arm, randomised, placebo-controlled superiority trial. eClinicalMedicine 2023, 56, 101796. [Google Scholar] [CrossRef]
- Deschamps, A.; Abdallah, A.B.; Jacobsohn, E.; Saha, T.; Djaiani, G.; El-Gabalawy, R.; Overbeek, C.; Palermo, J.; Courbe, A.; Cloutier, I. Electroencephalography-Guided Anesthesia and Delirium in Older Adults After Cardiac Surgery: The ENGAGES-Canada Randomized Clinical Trial. JAMA 2024, 332, 112–123. [Google Scholar] [CrossRef]
- Petersson, N.B.; Hansen, M.H.; Hjelmborg, J.V.; Instenes, I.; Christoffersen, A.S.; Larsen, K.L.; Schmidt, H.; Riber, L.P.S.; Norekvål, T.M.; Borregaard, B. Incidence and assessment of delirium following open cardiac surgery: A systematic review and meta-analysis. Eur. J. Cardiovasc. Nurs. 2024, 23, 825–832. [Google Scholar] [CrossRef]
- Rudolph, J.L.; Ramlawi, B.; Kuchel, G.A.; McElhaney, J.E.; Xie, D.; Sellke, F.W.; Khabbaz, K.; Levkoff, S.E.; Marcantonio, E.R. Chemokines are associated with delirium after cardiac surgery. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 184–189. [Google Scholar] [CrossRef]
- Day, J.; Taylor, K. The systemic inflammatory response syndrome and cardiopulmonary bypass. Int. J. Surg. 2005, 3, 129–140. [Google Scholar] [CrossRef]
- Hall, R.I.; Smith, M.S.; Rocker, G. The systemic inflammatory response to cardiopulmonary bypass: Pathophysiological, therapeutic, and pharmacological considerations. Anesth. Analg. 1997, 85, 766–782. [Google Scholar] [CrossRef] [PubMed]
- Aquino, A.; Abutalimova, N.; Ma, Y.; Ismail-Zade, I.; Grebennik, V.; Rubinstein, A.; Kudryavtsev, I.; Zaikova, E.; Sambur, D.; Marichev, A. Differences in Plasma Extracellular Vesicles of Different Origin in On-Pump Versus Off-Pump Cardiac Surgery. Curr. Issues Mol. Biol. 2024, 46, 13058–13077. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.B.; Khawaja, Z.Q.; Freedman, I.G.; Turco, I.; Wiredu, K.; Colecchi, T.; Akeju, O. Exploring the pathophysiology of delirium: An overview of biomarker studies, animal models, and tissue-engineered models. Anesth. Analg. 2022, 10, 1213. [Google Scholar] [CrossRef] [PubMed]
- Wiredu, K.; O’Connor, S.; Naseem, H.; Brauer, B.L.; Kettenbach, A.N.; Frost, H.R.; Shaefi, S.; Gerber, S.A. Intraoperative plasma proteomic changes in cardiac surgery: In search of biomarkers of post-operative delirium. Proteom.–Clin. Appl. 2023, 17, 2200066. [Google Scholar] [CrossRef]
- Wiredu, K.; Aduse-Poku, E.; Shaefi, S.; Gerber, S.A. Proteomics for the discovery of clinical delirium biomarkers: A systematic review of major studies. Anesth. Analg. 2023, 136, 422–432. [Google Scholar] [CrossRef]
- Devlin, J.W.; Sieber, F.; Akeju, O.; Khan, B.A.; MacLullich, A.M.; Marcantonio, E.R.; Oh, E.S.; Agar, M.R.; Avelino-Silva, T.J.; Berger, M. Advancing Delirium Treatment Trials in Older Adults: Recommendations for Future Trials From the Network for Investigation of Delirium: Unifying Scientists (NIDUS). Crit. Care Med. 2025, 53, e15–e28. [Google Scholar] [CrossRef]
- Vasunilashorn, S.M.; Lunardi, N.; Newman, J.C.; Crosby, G.; Acker, L.; Abel, T.; Bhatnagar, S.; Cunningham, C.; de Cabo, R.; Dugan, L. Preclinical and translational models for delirium: Recommendations for future research from the NIDUS delirium network. Alzheimer’s Dement. 2023, 19, 2150–2174. [Google Scholar] [CrossRef]
- Wiredu, K.; McKay, T.B.; Qu, J.; Akeju, O. Evaluating Neurological Biomarkers in Serum After Major Cardiac Surgery: A Study of Tau, Neurofilament Light Chain, Glial Fibrillary Acidic Protein, and Ubiquitin C-terminal Hydrolase L1. Anesth. Analg. 2024, 139, 1122–1124. [Google Scholar] [CrossRef]
- Simon, C.; Graves, O.K.; Akeju, O.; McKay, T.B. Elevated TDP-43 serum levels associated with postoperative delirium following major cardiac surgery. Brain Behav. Immun.-Health 2025, 45, 100974. [Google Scholar] [CrossRef]
- Lopez, M.G.; Hughes, C.G.; DeMatteo, A.; O’Neal, J.B.; McNeil, J.B.; Shotwell, M.S.; Morse, J.; Petracek, M.R.; Shah, A.S.; Brown, N.J. Intraoperative oxidative damage and delirium following cardiac surgery. Anesthesiology 2020, 132, 551. [Google Scholar] [CrossRef]
- Rhee, J.; Kuznetsov, A.; McKay, T.; Lyons, M.; Houstis, N.; Mekkonen, J.; Ethridge, B.; Ibala, R.; Hahm, E.; Gitlin, J.; et al. Serum Proteomics of Older Patients Undergoing Major Cardiac Surgery: Identification of Biomarkers Associated With Postoperative Delirium. Front. Aging Neurosci. 2021, 13, 699763. [Google Scholar] [CrossRef] [PubMed]
- Titlestad, I.; Watne, L.O.; Caplan, G.A.; McCann, A.; Ueland, P.M.; Neerland, B.E.; Myrstad, M.; Halaas, N.B.; Pollmann, C.T.; Henjum, K. Impaired glucose utilization in the brain of patients with delirium following hip fracture. Brain 2024, 147, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Kotfis, K.; Szylińska, A.; Listewnik, M.; Brykczyński, M.; Ely, E.W.; Rotter, I. Diabetes and elevated preoperative HbA1c level as risk factors for postoperative delirium after cardiac surgery: An observational cohort study. Neuropsychiatr. Dis. Treat. 2019, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Shelton, K.T.; Qu, J.; Bilotta, F.; Brown, E.N.; Cudemus, G.; D’Alessandro, D.A.; Deng, H.; DiBiasio, A.; Gitlin, J.A.; Hahm, E.Y.; et al. Minimizing ICU Neurological Dysfunction with Dexmedetomidine-induced Sleep (MINDDS): Protocol for a randomised, double-blind, parallel-arm, placebo-controlled trial. BMJ Open 2018, 8, e020316. [Google Scholar] [CrossRef]
- Hamadnalla, H.; Sessler, D.I.; Troianos, C.A.; Fang, J.; Rivas, E.; Ma, C.; Mascha, E.J.; Turan, A. Optimal interval and duration of CAM-ICU assessments for delirium detection after cardiac surgery. J. Clin. Anesth. 2021, 71, 110233. [Google Scholar] [CrossRef]
- Inouye, S.K.; van Dyck, C.H.; Alessi, C.A.; Balkin, S.; Siegal, A.P.; Horwitz, R.I. Clarifying confusion: The confusion assessment method: A new method for detection of delirium. Ann. Intern. Med. 1990, 113, 941–948. [Google Scholar] [CrossRef]
- Katz, M.J.; Wang, C.; Nester, C.O.; Derby, C.A.; Zimmerman, M.E.; Lipton, R.B.; Sliwinski, M.J.; Rabin, L.A. T-MoCA: A valid phone screen for cognitive impairment in diverse community samples. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2021, 13, e12144. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Gold, L.; Ayers, D.; Bertino, J.; Bock, C.; Bock, A.; Brody, E.; Carter, J.; Cunningham, V.; Dalby, A.; Eaton, B. Aptamer-based multiplexed proteomic technology for biomarker discovery. Nat. Preced. 2010, 5, e15004. [Google Scholar]
- Candia, J.; Daya, G.N.; Tanaka, T.; Ferrucci, L.; Walker, K.A. Assessment of Variability in the Plasma 7k SomaScan Proteomics Assay. Sci. Rep. 2022, 12, 17147. [Google Scholar] [CrossRef]
- Schneider, D.J.; Lynch, S.A.; Gelinas, A.D.; Ostroff, R.M.; Rohloff, J.C.; Williams, P.; Janjic, N.; Drolet, D.W. SOMAmer reagents and the SomaScan platform: Chemically modified aptamers and their applications in therapeutics, diagnostics, and proteomics. In RNA Therapeutics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 171–260. [Google Scholar]
- Rohloff, J.C.; Gelinas, A.D.; Jarvis, T.C.; Ochsner, U.A.; Schneider, D.J.; Gold, L.; Janjic, N. Nucleic acid ligands with protein-like side chains: Modified aptamers and their use as diagnostic and therapeutic agents. Mol. Ther.-Nucleic Acids 2014, 3, e201. [Google Scholar] [CrossRef] [PubMed]
- Billing, A.M.; Hamidane, H.B.; Bhagwat, A.M.; Cotton, R.J.; Dib, S.S.; Kumar, P.; Hayat, S.; Goswami, N.; Suhre, K.; Rafii, A. Complementarity of SOMAscan to LC-MS/MS and RNA-seq for quantitative profiling of human embryonic and mesenchymal stem cells. J. Proteom. 2017, 150, 86–97. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Rothrock, N.E.; Hays, R.D.; Spritzer, K.; Yount, S.E.; Riley, W.; Cella, D. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS). J. Clin. Epidemiol. 2010, 63, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Gershon, R.C.; Rothrock, N.; Hanrahan, R.; Bass, M.; Cella, D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J. Appl. Meas. 2010, 11, 304. [Google Scholar]
- Amaratunga, D.; Cabrera, J. Analysis of data from viral DNA microchips. J. Am. Stat. Assoc. 2001, 96, 1161–1170. [Google Scholar] [CrossRef]
- Bolstad, B.M.; Irizarry, R.A.; Åstrand, M.; Speed, T.P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003, 19, 185–193. [Google Scholar] [CrossRef]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef]
- Müller, C.; Schillert, A.; Röthemeier, C.; Trégouët, D.-A.; Proust, C.; Binder, H.; Pfeiffer, N.; Beutel, M.; Lackner, K.J.; Schnabel, R.B. Removing batch effects from longitudinal gene expression-quantile normalization plus ComBat as best approach for microarray transcriptome data. PLoS ONE 2016, 11, e0156594. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Algamal, Z.Y.; Lee, M.H. Regularized logistic regression with adjusted adaptive elastic net for gene selection in high dimensional cancer classification. Comput. Biol. Med. 2015, 67, 136–145. [Google Scholar] [CrossRef]
- Katrutsa, A.; Strijov, V. Comprehensive study of feature selection methods to solve multicollinearity problem according to evaluation criteria. Expert Syst. Appl. 2017, 76, 1–11. [Google Scholar] [CrossRef]
- Altelbany, S. Evaluation of Ridge, Elastic Net and Lasso Regression Methods in Precedence of Multicollinearity Problem: A Simulation Study. J. Appl. Econ. Bus. Stud. 2021, 5, 131–142. [Google Scholar] [CrossRef]
- Maldonado, J.R. Acute brain failure: Pathophysiology, diagnosis, management, and sequelae of delirium. Crit. Care Clin. 2017, 33, 461–519. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Wu, J.G.; Kunkel, D.; Parker, M.; Rivera, C.; Casey, C.; Naismith, S.; Teixeira-Pinto, A.; Maze, M.; Pearce, R.A. Resolution of elevated interleukin-6 after surgery is associated with return of normal cognitive function. Br. J. Anaesth. 2023, 131, 694–704. [Google Scholar] [CrossRef]
- Vasunilashorn, S.M.; Ngo, L.; Inouye, S.K.; Libermann, T.A.; Jones, R.N.; Alsop, D.C.; Guess, J.; Jastrzebski, S.; McElhaney, J.E.; Kuchel, G.A. Cytokines and postoperative delirium in older patients undergoing major elective surgery. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2015, 70, 1289–1295. [Google Scholar] [CrossRef]
- Capri, M.; Yani, S.L.; Chattat, R.; Fortuna, D.; Bucci, L.; Lanzarini, C.; Morsiani, C.; Catena, F.; Ansaloni, L.; Adversi, M. Pre-operative, high-IL-6 blood level is a risk factor of post-operative delirium onset in old patients. Front. Endocrinol. 2014, 5, 173. [Google Scholar] [CrossRef]
- Davis, D.H.; Skelly, D.T.; Murray, C.; Hennessy, E.; Bowen, J.; Norton, S.; Brayne, C.; Rahkonen, T.; Sulkava, R.; Sanderson, D.J. Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium. Am. J. Geriatr. Psychiatry 2015, 23, 403–415. [Google Scholar] [CrossRef]
- Tsui, A.; Searle, S.D.; Bowden, H.; Hoffmann, K.; Hornby, J.; Goslett, A.; Weston-Clarke, M.; Howes, L.H.; Street, R.; Perera, R. The effect of baseline cognition and delirium on long-term cognitive impairment and mortality: A prospective population-based study. Lancet Healthy Longev. 2022, 3, e232–e241. [Google Scholar] [CrossRef]
| Case (N = 38) | Non-Case (N = 40) | p-Value | |
|---|---|---|---|
| Age (years) | 72 (±6.2) | 70 (±6.1) | 0.174 |
| Biological sex | |||
| Female | 18 (47%) | 19 (48%) | 1.000 |
| Male | 20 (53%) | 21 (52%) | |
| BMI (Kg/m2) | 28 (±4.8) | 30 (±5.4) | 0.186 |
| Baseline neurocognition (MoCA scores) | 17 (±3.3) | 19 (±2.0) | 0.002 |
| Treatment | |||
| Dexmedetomidine | 9 (24%) | 17 (42%) | 0.128 |
| Placebo | 29 (76%) | 23 (58%) | |
| PROMIS Physical health | |||
| Poor | 8 (21%) | 2 (5%) | 0.196 |
| Fair | 4 (11%) | 9 (22%) | |
| Good | 11 (29%) | 10 (25%) | |
| Very good | 10 (26%) | 12 (30%) | |
| Excellent | 5 (13%) | 7 (18%) | |
| PROMIS Mental health | |||
| Fair | 4 (11%) | 2 (5%) | 0.236 |
| Good | 8 (21%) | 3 (8%) | |
| Very good | 15 (39%) | 21 (52%) | |
| Excellent | 11 (29%) | 14 (35%) | |
| PROMIS Pain interference | |||
| Moderate | 5 (13%) | 4 (10%) | 0.809 |
| Mild | 8 (21%) | 7 (18%) | |
| Normal | 25 (66%) | 29 (72%) | |
| PROMIS Applied cognition | |||
| Severe | 1 (3%) | 1 (2%) | 0.978 |
| Moderate | 6 (16%) | 5 (12%) | |
| Mild | 4 (11%) | 4 (10%) | |
| Normal | 27 (71%) | 30 (75%) | |
| Duration of CPB (mins) | 140 (±53) | 130 (±47) | 0.131 |
| Cross-clamp time (mins) | 100 (±42) | 93 (±35) | 0.228 |
| Duration of surgery (hours) | 6.3 (±1.5) | 6.0 (±1.2) | 0.390 |
| Hospital length of stay (days) | 8.2 (±5.5) | 6.5 (±1.9) | 0.073 |
| Duration of Ventilation (hours) | 10 (±19) | 6.5 (±6.7) | 0.282 |
| ICU Length of Stay (hours) | 55 (±56) | 34 (±22) | 0.040 |
| Discharge location * | |||
| Extended care †,* | 15 (39%) | 8 (20%) | 0.086 |
| Home | 22 (58%) | 32 (80%) | |
| Hospital readmission * | |||
| No | 33 (87%) | 37 (92%) | 0.914 |
| Yes | 4 (11%) | 3 (8%) | |
| ICU readmission | |||
| No | 35 (92%) | 39 (98%) | 0.571 |
| Yes | 3 (8%) | 1 (2%) |
| Variable | Beta | Std Error | Odds Ratio | p Value |
|---|---|---|---|---|
| Age (in years) | −0.025 | 0.04 | 0.97 | 0.600 |
| Sex (male) | 0.126 | 0.54 | 1.13 | 0.820 |
| Baseline neurocognition (tMoCA) | −0.303 | 0.13 | 0.74 | 0.019 |
| Composite biomarker profile | 0.012 | 0.01 | 1.03 | 0.013 |
| Treatment (Dexmedetomidine) | −1.358 | 0.62 | 0.26 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiredu, K.; Qu, J.; Turco, I.; McKay, T.B.; Akeju, O. Systemic Inflammation and Metabolic Changes After Cardiac Surgery and Postoperative Delirium Risk. J. Clin. Med. 2025, 14, 4600. https://doi.org/10.3390/jcm14134600
Wiredu K, Qu J, Turco I, McKay TB, Akeju O. Systemic Inflammation and Metabolic Changes After Cardiac Surgery and Postoperative Delirium Risk. Journal of Clinical Medicine. 2025; 14(13):4600. https://doi.org/10.3390/jcm14134600
Chicago/Turabian StyleWiredu, Kwame, Jason Qu, Isabella Turco, Tina B. McKay, and Oluwaseun Akeju. 2025. "Systemic Inflammation and Metabolic Changes After Cardiac Surgery and Postoperative Delirium Risk" Journal of Clinical Medicine 14, no. 13: 4600. https://doi.org/10.3390/jcm14134600
APA StyleWiredu, K., Qu, J., Turco, I., McKay, T. B., & Akeju, O. (2025). Systemic Inflammation and Metabolic Changes After Cardiac Surgery and Postoperative Delirium Risk. Journal of Clinical Medicine, 14(13), 4600. https://doi.org/10.3390/jcm14134600






