1. Introduction
Gingivitis or gingival inflammation is a mild infectious form of periodontal disease, leading to irritation, redness, oedema, high temperature, bleeding on probing, and increased gingival bleeding, often caused by the accumulation of bacterial plaque or tartar [
1]. Treating gingivitis in a timely manner is important, as it is reversible with professional treatment and improved outpatient hygiene measures (brushing teeth three times per day and using dental floss/interdental toothbrushes and antiseptic mouthwash). In the early stages, gingivitis is usually asymptomatic, which delays visits to the dentist. Untreated persistent gingivitis can progress to periodontitis, which is associated with the irreversible loss of attached periodontal tissues, including the teeth [
1,
2,
3,
4]. The most frequent cause of gingivitis is poor oral hygiene. There are two signs that are very often used to diagnose gingivitis, which are detected by visual examination and do not require invasive methods: intensification of gingival colour and increase in volume, beginning at the free gingival margin [
5]. The severity of gingivitis depends on the host inflammatory response as well as the pathogenic potential and quantity of anaerobic Gram-negative bacteria present in the gingival biofilm [
5]. Two of the main causes of gingival colour changes are vascular changes (the quantity, dilation, and permeability of vessels increase) and decreased keratinisation of the epithelium [
5,
6]. These underlying alterations are the primary drivers of the clinical colour changes described above. The gums usually become darker, redder/bluer, and bleed easily or spontaneously [
5]. In contrast, healthy gums are firm, pale pink in colour, and well attached around the teeth [
7]. Gingival colour changes can contribute to a diagnostic suspicion of clinical pathology, but their negative aesthetic impact should also be considered, particularly for patients with high smile lines [
8,
9,
10,
11,
12]. Gingival diseases are among the most prevalent intraoral pathologies [
12]. Gingivitis/periodontitis affects approximately 70% of the adult population at some point in their lives. Severe periodontal diseases are estimated to affect around 19% of adults, which translates to over a billion cases worldwide. [
13]. The primary risk factors are poor oral hygiene and smoking. Plaque control by patients is therefore essential, meaning that motivating and instructing them on improved oral hygiene is a particularly important part of treatment, both in the early stages and the long term [
14]. Smoking can cause smoker’s melanosis [
15], an increased risk of gingival fibrosis [
16], and reduced vascular density [
17].
There is no clear consensus about the full range of variables that define gingival colour or the relative impact of each upon the colour of natural healthy gingiva [
18]. Attempts have been made to quantify the influence made by variables such as gender, age, smoking habits, and frequency of toothbrushing, but such research has yet to produce firm or conclusive results [
19]. Further, gingival colour depends on certain anatomical components whose influence has yet to be scientifically studied, the most important of which include vascular number and size, epithelial thickness, degree of keratinisation, and pigments in the gingival epithelium [
20]. The main pigments that give the oral mucosa its colour are melanin, melanoid, carotene, reduced haemoglobin, and oxyhaemoglobin [
20,
21]. In almost all periodontal indices, colour is one of the main factors used to diagnose gingival conditions and determine their severity (with more marked colour changes indicating more severe periodontal pathology) [
22,
23,
24,
25,
26]. Two such examples are the Sulcus Bleeding Index [
27] and the Modified Gingival Index [
28].
While the use of digital electronic devices to objectively measure colour has been established in dentistry for many years, gingival classifications and indices are still based on a limited number of categories using narrative description of colour. For example, the Gingival Index [
22] classifies gums as having a normal colour, slight change in colour, redness, or marked redness. Such visual colour assessments are subjective, and publications using objective colour coordinates for this purpose are still lacking [
12]. Gingival colour is evidently an important factor for determining the presence of gingival inflammation as well as the severity of symptoms. Despite this, a protocol has yet to be established for relating gingival colour coordinates to gingival biotype or inflammation severity, although several options for digital analysis of gingival colour have been explored [
12,
29,
30]. Only a few studies have attempted to quantify colour changes in inflamed gingiva, and none of these have used a spectrophotometer, which is the preferred electronic device due to its superior reliability for gingival colour measurement [
31]. Using spectrophotometry for colour analysis has the advantage of increased precision and sensitivity to colour changes, improving the likelihood of detecting small alterations in colour. Unlike in periodontology, this technology is already well established in the dental field for measuring changes in colour (recording efficacy) after dental whitening treatments [
32].
Periodontal treatment with debridement provides many benefits to oral health: improving the aesthetics and functionality of teeth and gums [
33], removing tartar and bacterial plaque [
34], reducing the risk of mobility and loss of teeth [
35], preventing halitosis [
36,
37], reducing the risk of cardiovascular disease [
38] and bleeding gums [
39,
40], decreasing periodontal pocket depth, increasing clinical attachment levels, and reducing signs of inflammation such as oedema, texture, bleeding, and colour compared to pre-treatment levels [
12]. Clinical examination through periodontal probing is used to ascertain the probing pocket depth and bleeding on probing, providing cross-sectional and longitudinal data on periodontal health, which supports diagnosis and follow-up of the disease. While periodontal indices are a valuable tool for diagnosing periodontal disease, the intra- and inter-observer reproducibility and consistency of the periodontal assessments made have been called into question [
41,
42,
43,
44]. Consequently, these indices may underestimate or overestimate the frequency and severity of periodontal conditions. These potential errors [
42] could be eliminated through the use of objective methods such as gingival colour measurement with spectrophotometry, which was one of the motivations for conducting the present study. As described in narrative form by gingival indices, colour changes upon resolution of inflammation (when healthy gingival colour returns) are visually perceptible. Gingival colour usually becomes paler (redness is reduced) as inflammation goes down due to a decrease in vascularisation and increase in epithelial keratinisation, among other factors. In daily clinical practice, the methods used to quantify gingival colour are not appropriate, as they are often subjective, inaccurate, and difficult to communicate in a standardised way between professionals. This limitation represents a major challenge, especially considering that gingival colour is a basic and essential variable for the diagnosis of gingivitis. The absence of objective and reproducible tools hinders the reliable assessment of tissue changes, thus compromising the early detection and adequate follow-up of this periodontal disease.
The CIELAB colour system was developed by the CIE (Commission Internationale de l’Eclairage) in 1976 [
45] with the aim of standardising colour measurement, and it has become internationally recognised as the standard colorimetric system [
46]. The colour model contains three types of coordinates: an axis of L* coordinates ranges from black (0) to white (100), a* coordinates range from green (a* < 0) to red (a* > 0), and b* coordinates range from blue (b* < 0) to yellow (b* > 0). When it comes to quantifying colour differences, the following two formulae are most the commonly used in dentistry research [
46]. The Euclidean formula was the standard means of calculating colour difference:
The CIEDE2000 formula was developed by the CIE to better represent the visual perception of human observers [
47,
48,
49,
50]:
Based on the research context described above, the authors developed the following objectives concerning the use of spectrophotometry in periodontology to quantify colour changes related to gingival inflammation processes.
The objectives are (1) to assess the effect of biotype on the colour of inflamed gingiva, (2) to identify the relationship between the plaque index and gingival colour and compare the colour of moderately or severely inflamed gums to the colour of gums with mild inflammation, and (3) to assess the effect of periodontal treatment on gingival colour. The null hypotheses include the following: (1) The colour of inflamed gingiva in patients with a thin gingival biotype is no different from the colour of inflamed gingiva in patients with a thick gingival biotype. (2) There is not chromatic relationship between plaque index and gingival colour. The colour of moderately or severely inflamed gingiva is the same as that of mildly inflamed gingiva. (3) Periodontal treatment does not change the colour of the gingiva.
2. Materials and Methods
2.1. Patient Selection
All subjects above 18 years of age who attended the university’s dental clinic and met the following criteria were included: (1) visual signs of gingival inflammation: colour change and increase in volume (participants whose gingival colour change was perceptible to the operator—mainly increased redness), (2) no cognitive impairments, (3) without mucogingival surgery, (4) healthy natural tooth and gingival tissue in the aesthetic maxillary region, and (5) voluntarily acceptance of participation in the study. Participants were excluded who had systemic diseases and/or were taking long-term medication, were pregnant, had reduced upper-limb mobility, or had received orthodontic treatment. Also, participants with desquamative gingivitis or mucocutaneous conditions affecting the gingiva (such as lichen planus, pemphigoid, or pemphigus vulgaris) or recent use of antibiotics or anti-inflammatory drugs or smoking were excluded because these factors can independently influence gingival inflammation. The research protocol and study objectives were explained to each candidate. Participants who accepted inclusion in the study were required to sign the informed consent form. The research was approved by the university’s bioethics committee (BEC-594/21). The present study is non-randomised and longitudinal.
2.2. Collection of Data on Periodontal, Chromatic, and Hygiene-Related Variables
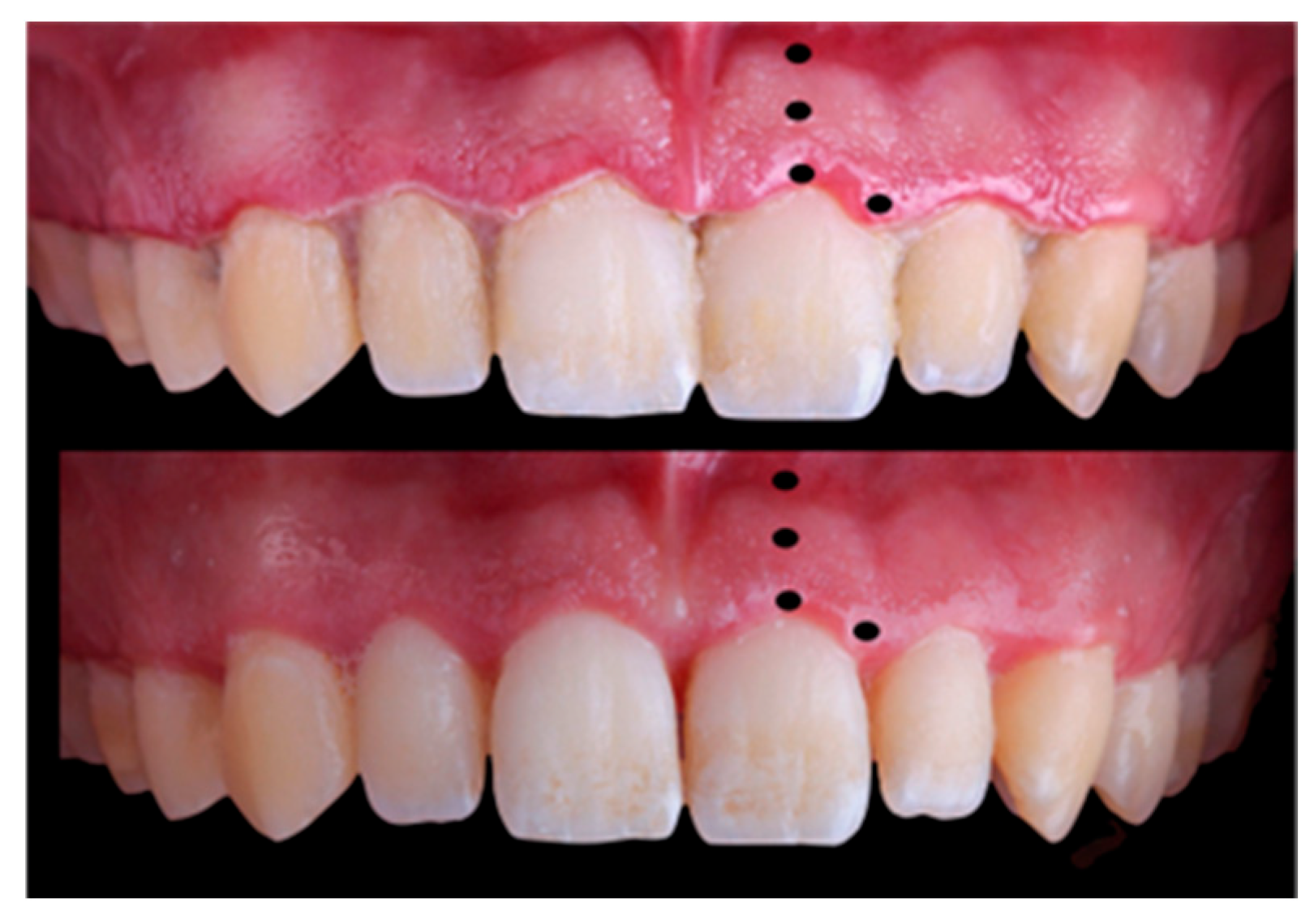
All data on periodontal, oral hygiene, and chromatic variables were collected by a single operator: a university lecturer in periodontology with over 20 years of experience (Y.G.G). Each participant sat in a standardised upright position in a chair during examination. Lip retractors were used to enable adequate illumination of the buccal cavity and clearly expose each participant’s teeth and gums. The surface of the mucosa was dried and cleaned using airflow or a gauze pad. Two photographs were taken per patient (before the initial treatment and after re-examination).
In the first appointment, the following data were recorded at maxillary central incisor 11 or 21:
- (1)
The gingival biotype, using a probe to assess the transparency of the tissue [
51];
- (2)
O’Leary’s Plaque Index [
52], which is a dichotomous index (0/1) assessing the presence or absence of bacterial plaque at three sites vestibular and one site palatal to the cervical margin of the tooth. In the present research, only the vestibular sites were taken into consideration;
- (3)
Löe and Silness’ Gingival Index [
22], using a CP-15 manual periodontal probe (Hu-Friedy, Chicago, United States). The Gingival Index developed by Löe and Silness [
22] has four grades that relate bleeding on probing and change in gingival colour to the severity of inflammation. The scale is as follows:
Grade 0: Normal gingiva, no inflammation, no change in colour, no bleeding.
Grade 1: Mild inflammation, slight change in colour, minimal superficial alterations, no bleeding.
Grade 2: Moderate inflammation, redness, oedema, bleeding on probing and application of pressure.
Grade 3: Severe inflammation, marked redness, oedema, tendency to spontaneous bleeding, potential ulceration;
- (4)
The colour coordinates obtained using spectrophotometry. After calibration of the SpectroShade Micro spectrophotometer (MHT Optic Research, Zürich, Switzerland), three colour measurements were made per participant, including both papillae and the mucogingival line at the zenith of the incisor. The colour coordinates (L*, a*, and b*) were measured in each of the four zones under study: the free gingival margin, middle zone of attached gingiva, mucogingival line, and distal papilla. The mean was used in the statistical analysis to minimise deviations. The spectrophotometer’s colour-measurement window was used to record these coordinates by tracing out a triangular shape that included the entire distal papilla and a rectangle with a scalloped edge extending in a cervical direction from the free gingival margin to the mucogingival line, where the coordinates of free gingival margin, middle zone of attached gingiva, and mucogingival line were collected. All three colour coordinates were measured at the distal papilla and in the vestibular gingiva, which was divided into three horizontal areas: the lower containing the free gingival margin, the middle area including the attached gingiva, and the upper containing the mucogingival line.
All these variables were recorded prior to treatment and then repeated four weeks after periodontal treatment [
53], except the gingival biotype (
Figure 1). In this first visit, scaling and root surface debridement was performed, including removal of calculus as well as supra- and subgingival biofilm. In addition, the principal investigator instructed participants on conventional and interdental toothbrushing, encouraging them to improve their dental hygiene. A verbal explanation was also given on the relationship between inflammation and dental plaque and how plaque can be removed through frequent and effective hygiene measures.
2.3. Statistical Analysis
The statistical analysis produced basic descriptive statistics for the quantitative variables and frequencies and percentages for the qualitative variables. Comparisons were made using
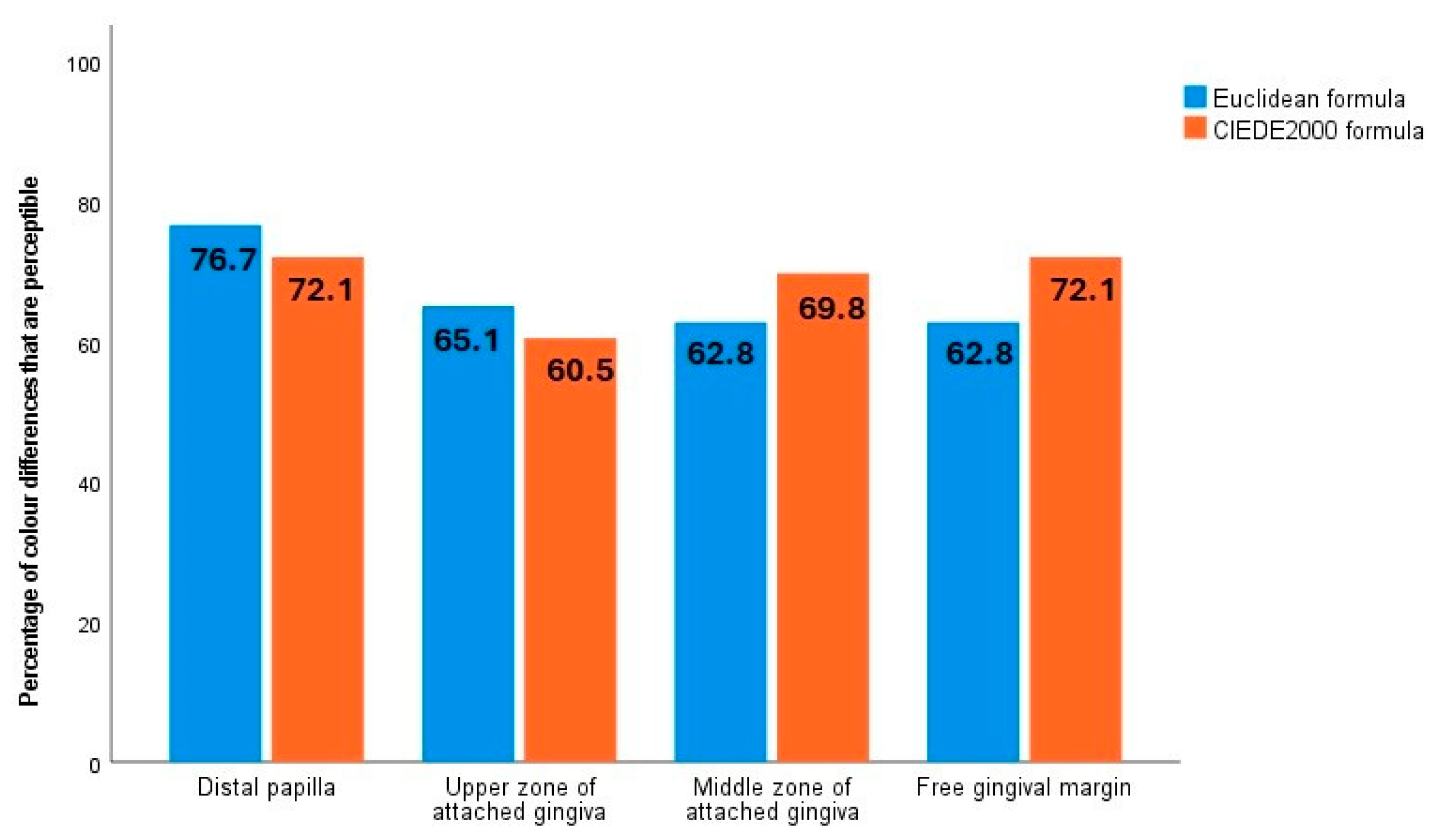
t-tests for paired and unpaired samples, the independent samples proportions test, and McNemar’s test. The effect sizes in the comparison of means tests were measured using Cohen’s d. (Cohen’s d values below 0.2 are considered negligible, values between 0.2 and 0.5 are interpreted as small, those between 0.5 and 0.8 as medium, and values above 0.8 as large effects.) The statistical significance level was set at 0.05. All analyses were performed using SPSS software (version 28). To assess the effect of the biotype and periodontal treatment on gingival colour, colour differences were calculated (using the Euclidean and CIEDE2000 formulae), then summarised using descriptive statistics, and compared with the published 50:50% perceptibility thresholds [
54].
4. Discussion
Gingivitis and periodontal disease are, together with caries, among the most common oral pathologies in humans. These infectious conditions progressively damage the periodontium, potentially leading to loss of teeth [
3,
4] and significantly reducing quality of life [
55]. Conventionally, inflammation is identified using Celso’s four cardinal signs [
19]: calor (heat), rubor (redness), tumor (swelling/oedema), and dolor (pain). Calor and rubor occur because of the vascular changes that lead to an accumulation of blood in the affected area. The healing process after root surface debridement can involve repair or regeneration, which can affect the appearance and quality of the tissue [
5,
56]. Objectively determining the colour changes resulting from inflammation and its subsequent resolution is therefore vital, but subjective evaluation of such chromatic changes continues to be standard practice. Quantifying gingival colour changes through spectrophotometry is a precise, non-invasive means to support early diagnosis of alterations to the gingival tissue [
19] and to objectively assess the chromatic response of the gingiva to periodontal treatment. This methodological improvement has yet to be studied in depth, and the definition of gingival colour is a field that continues to require further research. The objectives of the present study were designed to respond to these research gaps as well as addressing the need to quantify the colour change caused by gingival inflammation and determine its relationship to biotype, plaque, and periodontal treatment.
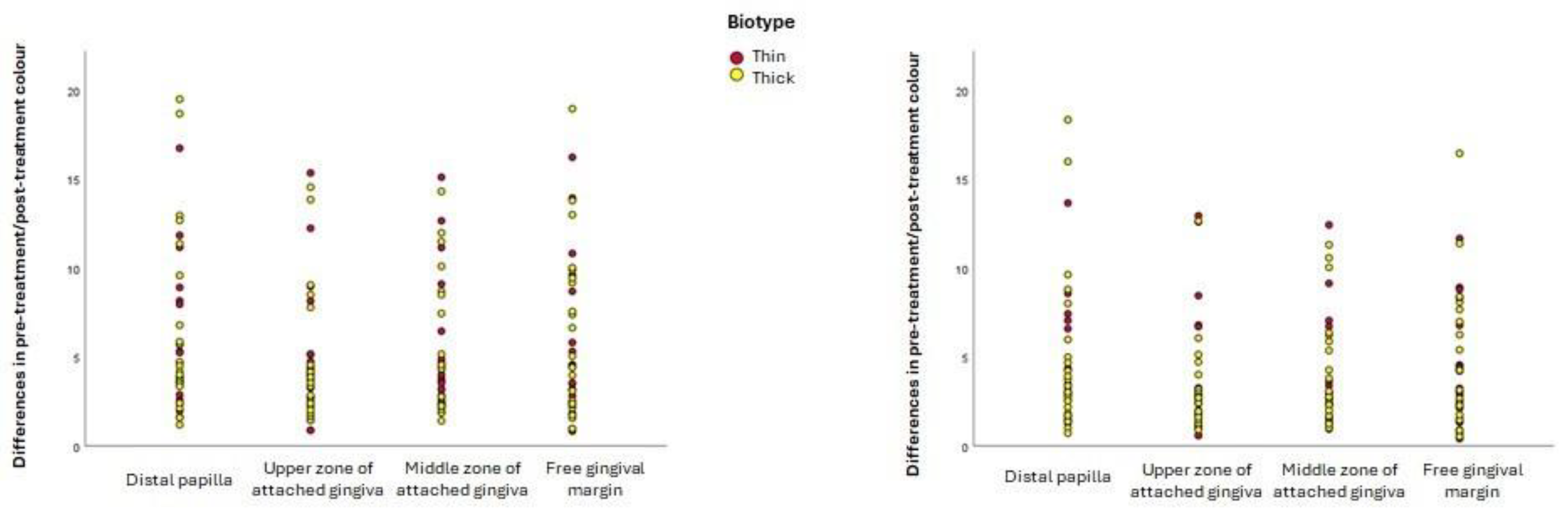
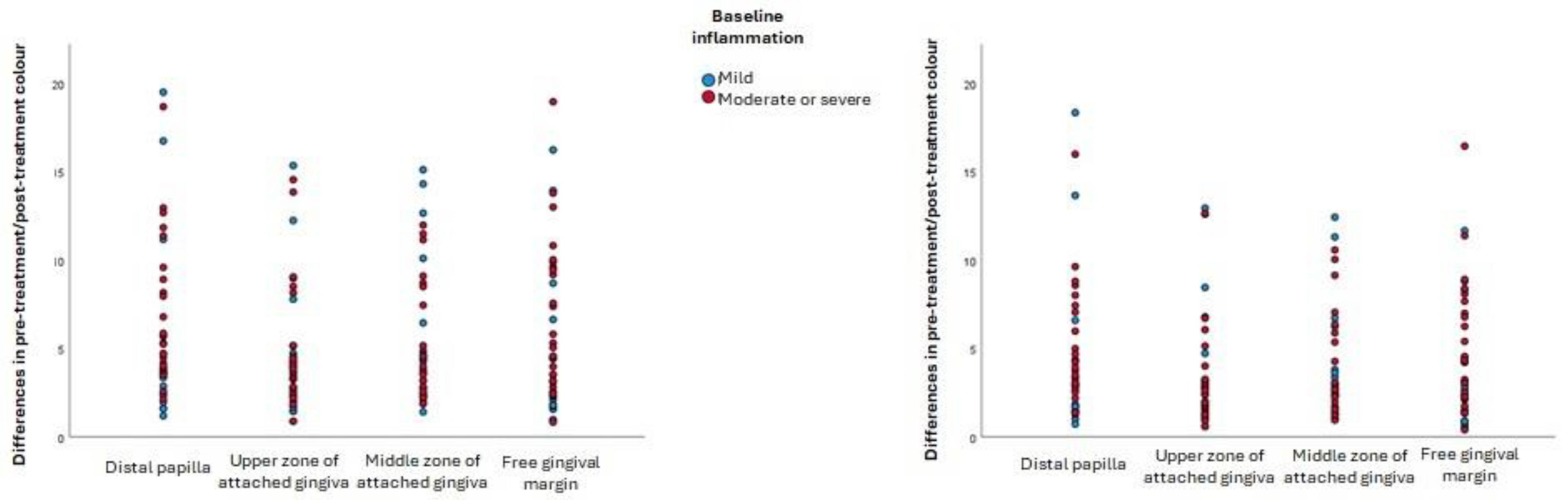
All the null hypotheses of the present research should be rejected. The first should be rejected because statistically significant differences were found between the a* coordinates of participants with thin biotypes and thick biotypes: the former had perceptibly redder inflamed gingiva than the latter. The second null hypothesis should be rejected because moderately or severely inflamed gingiva were darker than mildly inflamed gingiva in all gingival zones examined. The third null hypothesis should be rejected since debridement created a colour change in all gingival zones examined, which in most cases was perceptible: there were statistically significant changes in the L* coordinate (lightness) at the free gingival margin and the distal papilla (lightness), where the gingiva became lighter (higher L* coordinates) after inflammation was resolved. The colour change after resolution of gingival inflammation was independent of the initial severity of inflammation.
These findings are limited to a Caucasian population and need to be corroborated by further longitudinal research that includes several follow-ups over a longer time period, is applied to different racial populations [
57], and uses larger sample sizes. Other potential issues, such as the lack of blinding, intra-examiner error, short follow-up period (four weeks), or potential measurement errors with the spectrophotometer, could have influenced the results obtained. It would also be useful to explore the confidence intervals of the colour coordinates corresponding to each category in periodontal disease classification systems. Such research could help determine chromatic ranges to support diagnosis, with gingival colour data providing clinicians with information about patients’ periodontal health.
Existing periodontal indices [
58] describe colour changes using a range of narrative descriptions (such as ‘normal-coral colour’, ‘slight change of colour’, ‘redness’, and ‘marked redness’) whose utility and precision is limited by disparities between the subjective chromatic perception of distinct observers. Improving the classification and diagnosis of periodontal pathologies through standardised, objective quantification of gingival colour is not possible through the use of such instruments alone. In an attempt to eliminate subjectivity and inter- or intra-observer inconsistencies from colour selection [
59,
60], non-invasive methods for diagnosing periodontal disease based on digital technologies are starting to appear [
26]. These methods primarily measure volumetric [
61,
62,
63] or colour [
12,
30,
64] changes. More work is needed to improve diagnostic precision and to design well-targeted, reliable treatment plans [
65]. Informing patients about positive aesthetic changes resulting from treatment, perhaps by showing them the estimated colour of healthy gums after debridement, could increase motivation for treatment requests and adherence thereto. Additionally, it is widely recognised that such aesthetic improvements have positive psychosocial repercussions for patients [
66].
Strengths and Limitations of the Study
To date, the present study is the first that has used spectrophotometry to precisely quantify colour changes in gingiva during and after inflammation. The main advantages of current spectrophotometers developed for use in dentistry are their objectivity and operator independence, along with high levels of temporal stability and reliability [
31]. Most of these devices were designed to measure dental colour and provide results either in colour coordinates or standard shade-guide terminology (Vita Classical/3D Master). Only a limited number of spectrophotometer models have been used in gingival research [
19,
67], and their physical design prevents their use in posterior regions because the ‘open window screens’ used for colour measurement are too big to fit inside the mouth or to enable correct alignment with premolars or molars. For practical clinical purposes, then, the use of objective colour measurement to support diagnosis has been limited to the anterior region, and in general, spectrophotometry has yet to make a significant impact on everyday diagnostic practice.
The present study had a similar sample size to that used in related longitudinal studies on inflamed gingiva before and after periodontal treatment [
12,
30]. These earlier studies by Mayer et al. in 2017 [
30] and Ginesin et al. five years later [
12] used photographs from a digital single-lens-reflex (DSLR) camera to record colour changes after debridement. However, this necessitated conversion of the colour in the photographs to colour coordinates using Adobe Photoshop CS4 software, as CIELAB coordinates could not be obtained directly from the patients using this method. This conversion process can introduce errors and imprecision into the data. Another factor that can affect the chromatic results is the zone in which colour is measured. Mayer et al. measured a gingival band extending from the mesial to the distal papilla adjacent to the maxillary lateral incisor, including the free gingiva, keratinised gingiva, and part of the papilla proximate to this incisor [
30], whereas most of Ginesin et al.’s colour measurements were in posterior regions of the maxilla and mandible [
12]. It may be suspected that there is a homogeneous response to the resolution of inflammation across the intraoral regions, but we cannot make any such claim unequivocally in the absence of more complete spectrophotometric data. Further, the reasonable assumption that toothbrushing may be less vigorous in non-visible zones would also cast doubt on such a statement. The aforementioned authors analysed the mean colour coordinates of the whole selected area, which makes the real chromatic effect of each anatomical zone of the gingiva less clear since the singular characteristics of each region may create a distinct chromatic response. In the present research, a spectrophotometer was used to make direct colour measurements of each anatomical region [
68] in order to perform a more precise, fine-grained assessment of how the resolution of inflammation affects the gingiva adjacent to the maxillary central incisor. It should be noted that some research [
69,
70,
71] has found significant correlations between the colour measurements resulting from digital photography and from spectrophotometry. Like the present research, both of the studies described above [
12,
30] included participants with different types of periodontal diagnoses [
58,
72], thereby providing an overarching vision of the resolution of gingival inflammation.
In inflamed gingiva, the present findings show changes in the a* coordinate to be of greatest importance, with the values of this coordinate being higher in subjects with plaque than participants without plaque at the distal papilla (28.0 vs. 25.2) and the free gingival margin (28.1 vs. 24.7). The intensity of redness is therefore greater in patients with an accumulation of plaque, particularly in the zones located closest to the bacterial plaque, which is where the inflammation begins. The mean initial a* coordinate in Mayer et al. and Ginesin et al.’s studies (approximately 32.0) [
12,
30] for participants with distinct types of inflammation was greater than the mean initial values for this coordinate presented here, which could be due to the methodological differences explained above.
In all four gingival zones under study, the gingiva with the most severe initial inflammation were found to be darker (lower L* value), while no statistically significant differences in the a* coordinate were identified. This finding was also evident in the effect observed after inflammation was resolved. The main difference between the present research and similar studies [
12,
30] was that it found lightness (L*) to be the only coordinate to have undergone statistically significant changes four weeks after root surface debridement. Again, the free gingival margin (48.8 vs. 50.7) and the distal papilla (46.3 vs. 48.2) were the sites of these changes, where the gingiva became lighter. Other studies [
12,
30] have determined the a* coordinate to be responsible for most of the colour change after inflammation is resolved, with the gingiva becoming less red. Nevertheless, Ginesin et al. [
12] also found statistically significant changes in the L* coordinate before and after treatment (56.3 vs. 60.7). From another perspective, the results of all the studies discussed concur in that the colour changes presented were perceptible to the human eye [
12,
30]. However, the authors of the earlier studies only used the Euclidean formula [
12,
30] and referred to perceptibility thresholds created for the dental colour space, whereas use of thresholds designed for the gingival colour space would be preferable to ensure accurate interpretations [
54]. In the present study, the percentage of pre- and post-treatment colour differences that were perceptible was above 60% in all four zones examined, using both formulae (ΔEab > 3.1 and ΔE00 > 2.1 thresholds specific to the gingival colour space).
The fact that colour change after debridement treatment did not depend on either the initial severity of inflammation or the gingival biotype is particularly interesting. This novel finding arose from analysing inflammation and gingival biotype from a chromatic perspective since few studies have explored this topic recently with spectrophotometry [
73] or with objective methodology like intraoral scanners [
29,
74,
75].
One of these studies assessed the consistency between gingival inflammation scores obtained directly using the modified gingival index (MGI) and those derived from intraoral scans (IOS) evaluated 10 days later. Conducted on 23 healthy adults aged 18–72, the study analysed 552 gingival sites. The findings showed a 90% agreement between chairside and IOS-based MGI scores, with the most frequent discrepancies involving minor one-point differences. Scores derived from IOS were consistent across two evaluators, with 91% agreement and no variations exceeding one scale point. The results confirm that intraoral scans reliably capture gingival characteristics such as contour and colour, allowing accurate inflammation assessment. [
29]. Intraoral scanning proves to be a reliable, non-invasive tool for evaluating gingival health, offering significant potential for clinical practice and remote diagnostics through accurate data collection and analysis. [
29]. Along the same lines is a later publication in which digital colour analysis of intraoral scans showed promising results in identifying sites with bleeding on probing (BOP), ranging from acceptable to excellent performance. This technology represents a valuable tool for improving the detection and management of gingivitis [
74]. In our study, we investigated a more objective method for identifying gingival inflammation by analysing colour data from intraoral scans, reducing reliance on the subjective visual assessment typically used by clinicians. We evaluated 110 scans from 55 individuals, comparing gum colour before and after treatment. Using specific colour metrics, we were able to differentiate between healthy and inflamed tissues with up to 80.8% accuracy. These results are very interesting, but they do not use the ‘gold standard’ methodology for recording colour in dentistry, nor do they offer results based on the severity of inflammation or gingival biotype.
A systematic review suggested that although the use of artificial intelligence to detect dental plaque, diagnose gingivitis, and measure alveolar bone loss is still under development, these technologies have the potential to become valuable tools for clinical support and diagnosis [
75]. Another similar study, whose results cannot be directly compared with the previous ones, is the one published by Shrivastava et al., as it extracted colour coordinates from intraoral photographs and did not record them in vivo [
76].
Inflamed gingiva with a thin biotype were found to have a larger quantity of red at the free gingival margin and the distal papilla than inflamed gingiva with a thick biotype. Although the underlying cause remains uncertain, this may be related to the degree of keratinisation of the epithelium [
77,
78]. The colour differences in the inflamed gums of participants with distinct biotypes were clearly visible, exceeding the thresholds of clinical acceptability [
54]. It should be noted that Mayer et al.’s study [
30] did not identify significant changes after periodontal treatment in either the severe periodontitis or gingivitis group, although the samples used were not large (the maximum sample size was 19), and the signs and symptoms of gingivitis were rather variable [
28]. All this suggests that the chromatic gingival response after debridement is complex and may have several influences, including genetic and hormone-related factors, smoking, and oral hygiene habits [
19]. Further, not only can plaque accumulation can cause colour changes [
79] but also nodular gingival lesions [
80] such as pyogenic granulomas [
79] and the presence of dental implants with thin gingival biotypes [
67].
Digital colour analysis may therefore be useful as a diagnostic aid, as it is more sensitive than the human eye, enabling it to detect the minor chromatic changes related to such phenomena. One of the impediments to the incorporation of spectrophotometry into daily clinical practice is its high cost. Spectrophotometry, which would be useful in restorative dentistry, tooth whitening, caries diagnosis, material ageing, and periodontal diagnosis, is practically used for research. It would be desirable in the coming years to expand its use to optimise treatment and diagnostic results. In the future, applications using artificial intelligence to analyse large datasets representing a significant proportion of the population may support clinicians from a diagnostic and motivational (increased treatment adherence) perspective since one of the first warning signs of periodontal pathology is colour change, and gums that are darker and redder are viewed less positively.