Missed Gastroesophageal Injuries During Antireflux Surgery: Infrequent but Catastrophic Complications
Abstract
1. Introduction
2. Methods
2.1. Study Design and Settings
2.2. Study Population
2.3. Data Source and Variables
2.4. Literature Review
2.5. Statistical Analysis
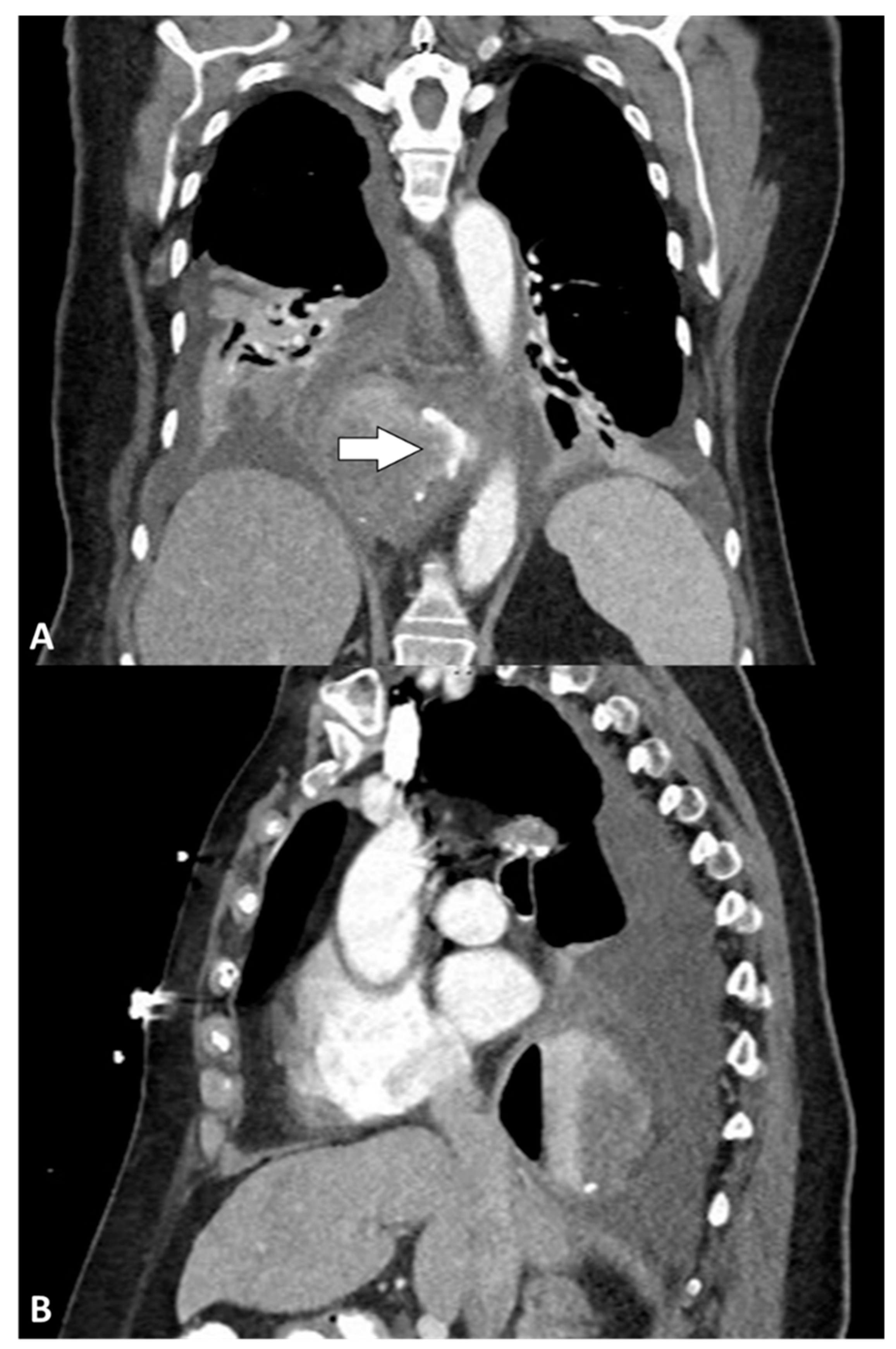
3. Results
3.1. Population Characteristics
3.2. Antireflux Surgery
3.3. Clinical Presentation and Diagnosis
3.4. Perforation Treatment
3.5. Postoperative Course
3.6. Outcome and Long-Term Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Serag, H.B.; Sweet, S.; Winchester, C.C.; Dent, J. Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut 2014, 63, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Daly, S.; Kumar, S.S.; Collings, A.T.; Hanna, N.M.; Pandya, Y.K.; Kurtz, J.; Kooragayala, K.; Barber, M.W.; Paranyak, M.; Kurian, M.; et al. SAGES guidelines for the surgical treatment of hiatal hernias. Surg. Endosc. 2024, 38, 4765–4775. [Google Scholar] [CrossRef] [PubMed]
- Slater, B.J.; Dirks, R.C.; McKinley, S.K.; Ansari, M.T.; Kohn, G.P.; Thosani, N.; Qumseya, B.; Billmeier, S.; Daly, S.; Crawford, C.; et al. SAGES guidelines for the surgical treatment of gastroesophageal reflux (GERD). Surg. Endosc. 2021, 35, 4903–4917. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Tahir, U.; Tessier, L.; Yang, K.; Hassan, T.; Dang, J.; Kroh, M.; Hong, D. Long-term outcomes following Dor, Toupet, and Nissen fundoplication: A network meta-analysis of randomized controlled trials. Surg. Endosc. 2023, 37, 5052–5064. [Google Scholar] [CrossRef]
- Robinson, B.; Dunst, C.M.; Cassera, M.A.; Reavis, K.M.; Sharata, A.; Swanstrom, L.L. 20 years later: Laparoscopic fundoplication durability. Surg. Endosc. 2015, 29, 2520–2524. [Google Scholar] [CrossRef]
- Salvador, R.; Vittori, A.; Capovilla, G.; Riccio, F.; Nezi, G.; Forattini, F.; Provenzano, L.; Nicoletti, L.; Moletta, L.; Costantini, A.; et al. Antireflux Surgery’s Lifespan: 20 Years After Laparoscopic Fundoplication. J. Gastrointest. Surg. 2023, 27, 2325–2335. [Google Scholar] [CrossRef]
- Rantanen, T.K.; Salo, J.A.; Sipponen, J.T. Fatal and life-threatening complications in antireflux surgery: Analysis of 5,502 operations. Br. J. Surg. 1999, 86, 1573–1577. [Google Scholar] [CrossRef]
- Maret-Ouda, J.; Wahlin, K.; El-Serag, H.B.; Lagergren, J. Association Between Laparoscopic Antireflux Surgery and Recurrence of Gastroesophageal Reflux. JAMA 2017, 318, 939–946. [Google Scholar] [CrossRef]
- Schauer, P.R.; Meyers, W.C.; Eubanks, S.; Norem, R.F.; Franklin, M.; Pappas, T.N. Mechanisms of gastric and esophageal perforations during laparoscopic Nissen fundoplication. Ann Surg. 1996, 223, 43–52. [Google Scholar] [CrossRef]
- Urschel, J.D. Gastroesophageal leaks after antireflux operations. Ann Thorac. Surg. 1994, 57, 1229–1232. [Google Scholar] [CrossRef]
- Mathew, G.; Sohrabi, C.; Franchi, T.; Nicola, M.; Kerwan, A.; Agha, R. Preferred Reporting of Case Series in Surgery (PROCESS) 2023 guidelines. Int. J. Surg. 2023, 109, 3760–3769. [Google Scholar] [CrossRef] [PubMed]
- McDowell, I. Measures of self-perceived well-being. J. Psychosom. Res. 2010, 69, 69–79. [Google Scholar] [CrossRef]
- Bhutta, B.S.; Alghoula, F.; Berim, I. Hypoxia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK482316/ (accessed on 22 March 2025).
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Watson, D.I.; Jamieson, G.G.; Lally, C.; Archer, S.; Bessell, J.R.; Booth, M.; Cade, R.; Cullingford, G.; Devitt, P.G.; Fletcher, D.R.; et al. Multicenter, Prospective, Double-blind, Randomized Trial of Laparoscopic Nissen vs Anterior 90o Partial Fundoplication. Arch. Surg. 2004, 139, 1160–1167. [Google Scholar] [CrossRef]
- Håkanson, B.S.; Lundell, L.; Bylund, A.; Thorell, A. Comparison of Laparoscopic 270° Posterior Partial Fundoplication vs Total Fundoplication for the Treatment of Gastroesophageal Reflux Disease: A Randomized Clinical Trial. JAMA Surg. 2019, 154, 479–486. [Google Scholar] [CrossRef]
- Latorre-Rodríguez, A.R.; Rajan, A.; Mittal, S.K. Perioperative morbidity after primary hiatal hernia repair increases as hernia size increases. Dis. Esophagus. 2025, 38, doae117. [Google Scholar] [CrossRef]
- Schlottmann, F.; Strassle, P.D.; Patti, M.G. Comparative Analysis of Perioperative Outcomes and Costs Between Laparoscopic and Open Antireflux Surgery. J. Am. Coll. Surg. 2017, 224, 327–333. [Google Scholar] [CrossRef]
- Singhal, S.; Kirkpatrick, D.R.; Masuda, T.; Gerhardt, J.; Mittal, S.K. Primary and Redo Antireflux Surgery: Outcomes and Lessons Learned. J. Gastrointest. Surg. 2018, 22, 177–186. [Google Scholar] [CrossRef]
- Trus, T.L.; Bax, T.; Richardson, W.S.; Branum, G.D.; Mauren, S.J.; Swanstrom, L.L.; Hunter, J.G. Complications of laparoscopic paraesophageal hernia repair. J. Gastrointest. Surg. 1997, 1, 221–227, discussion 228. [Google Scholar] [CrossRef]
- Pohl, D.; Eubanks, T.R.; Omelanczuk, P.E.; Pellegrini, C.A. Management and outcome of complications after laparoscopic antireflux operations. Arch. Surg. 2001, 136, 399–404. [Google Scholar] [CrossRef]
- Lindenmann, J.; Maier, A.; Fink-Neuboeck, N.; Smolle-Juettner, F.M. Fatal aortic hemorrhage after over-the-scope clipping and subsequent esophageal stenting for sealing of iatrogenic esophageal perforation. Endoscopy 2015, 47 (Suppl. S1), E280–E281. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y. Conservative Management for Persisted Leak After the Operative Repair of an Abdominal Esophageal Perforation: A Case Report. Gastroenterol. Nurs. 2016, 39, 321–323. [Google Scholar] [CrossRef]
- Pamart, W.; Majerus, B. Unusual complication after laparoscopic Nissen fundoplication. J. Surg. Case Rep. 2021, 2021, rjab023. [Google Scholar] [CrossRef] [PubMed]
- Anthony, P.; Andrawis, N. Early gastro-oesophageal junction perforation repaired using through-the-scope clips following Nissen fundoplication. J. Surg. Case Rep. 2024, 2024, rjae194. [Google Scholar] [CrossRef]
- Rantanen, T.K.; Oksala, N.K.J.; Oksala, A.K.; Salo, J.A.; Sihvo, E.I.T. Complications in antireflux surgery: National-based analysis of laparoscopic and open fundoplications. Arch. Surg. 2008, 143, 359–365, discussion 365. [Google Scholar] [CrossRef] [PubMed]
- Kassem, M.M.; Wallen, J.M. Esophageal Perforation and Tears. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK532298/ (accessed on 27 February 2025).
- van Beek, D.B.; Auyang, E.D.; Soper, N.J. A comprehensive review of laparoscopic redo fundoplication. Surg. Endosc. 2011, 25, 706–712. [Google Scholar] [CrossRef]
- Zhang, L.P.; Chang, R.; Matthews, B.D.; Awad, M.; Meyers, B.; Eagon, J.C.; Brunt, L.M. Incidence, mechanisms, and outcomes of esophageal and gastric perforation during laparoscopic foregut surgery: A retrospective review of 1223 foregut cases. Surg. Endosc. 2014, 28, 85–90. [Google Scholar] [CrossRef]
- Lampridis, S.; Mitsos, S.; Hayward, M.; Lawrence, D.; Panagiotopoulos, N. The insidious presentation and challenging management of esophageal perforation following diagnostic and therapeutic interventions. J. Thorac. Dis. 2020, 12, 2724–2734. [Google Scholar] [CrossRef]
- Nachira, D.; Sassorossi, C.; Petracca-Ciavarella, L.; Zanfrini, E.; Tabacco, D.; Pogliani, L.; Meacci, E.; Congedo, M.T.; Vita, M.L.; Chiappetta, M.; et al. Management of esophageal perforations and postoperative leaks. Ann. Esophagus 2023, 6. [Google Scholar] [CrossRef]
- Fairbairn, K.; Worrell, S.G. Esophageal Perforation: Is Surgery Still Necessary? Thorac. Surg. Clin. 2023, 33, 117–123. [Google Scholar] [CrossRef]
- Chirica, M.; Kelly, M.D.; Siboni, S.; Aiolfi, A.; Riva, C.G.; Asti, E.; Ferrari, D.; Leppäniemi, A.; Broek, R.P.G.T.; Brichon, P.Y.; et al. Esophageal emergencies: WSES guidelines. World J. Emerg. Surg. 2019, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Salehpoor, A.; Shiehmorteza, M.; Terrazas, M.; Thompson, W. Imaging in the Evaluation of Esophageal Trauma Including Surgery. Contemp. Diagn. Radiol. 2022, 45, 1–7. [Google Scholar] [CrossRef]
- Baker, M.E. Role of the Barium Esophagram in Antireflux Surgery. Gastroenterol. Hepatol. 2014, 10, 677–679. [Google Scholar]
- Tsunoda, S.; Jamieson, G.G.; Devitt, P.G.; Watson, D.I.; Thompson, S.K. Early reoperation after laparoscopic fundoplication: The importance of routine postoperative contrast studies. World J. Surg. 2010, 34, 79–84. [Google Scholar] [CrossRef]
- Contini, S.; Scarpignato, C. Early esophageal transit study after laparoscopic fundoplication: How useful is it? Am. J. Surg. 2002, 183, 226–231. [Google Scholar] [CrossRef]
- Robertson-More, C.; Prasad, S.; Gill, R.; Church, N.; Mitchell, P.; Debru, E. Early Routine Use of Upper GI Contrast Series Post Paraesophageal Hernia Repair: A Single Institution Consecutive Case Series. Surg Laparosc Endosc Percutan Tech. 2019, 29, 203–206. [Google Scholar] [CrossRef]
- Wei, C.J.; Levenson, R.B.; Lee, K.S. Diagnostic Utility of CT and Fluoroscopic Esophagography for Suspected Esophageal Perforation in the Emergency Department. AJR Am. J. Roentgenol. 2020, 215, 631–638. [Google Scholar] [CrossRef]
- Norton-Gregory, A.A.; Kulkarni, N.M.; O’Connor, S.D.; Budovec, J.J.; Zorn, A.P.; Desouches, S.L. CT Esophagography for Evaluation of Esophageal Perforation. Radiographics. 2021, 41, 447–461. [Google Scholar] [CrossRef]
- Grewal, J.; Gillaspie, E.A. Pneumomediastinum. Thorac. Surg. Clin. 2024, 34, 309–319. [Google Scholar] [CrossRef]
- Loftus, I.A.; Umana, E.E.; Scholtz, I.P.; McElwee, D. Mackler’s Triad: An Evolving Case of Boerhaave Syndrome in the Emergency Department. Cureus 2023, 15, e37978. [Google Scholar] [CrossRef]
- Montminy, E.M.; Jones, B.; Heller, J.C.; Attwell, A. Endoscopic iatrogenic esophageal perforation and management: A retrospective outcome analysis in the modern era. BMC Gastroenterol. 2023, 23, 371. [Google Scholar] [CrossRef] [PubMed]
- Sepesi, B.; Raymond, D.P.; Peters, J.H. Esophageal perforation: Surgical, endoscopic and medical management strategies. Curr. Opin. Gastroenterol. 2010, 26, 379–383. [Google Scholar] [CrossRef] [PubMed]
- De Pasqual, C.A.; Mengardo, V.; Tomba, F.; Veltri, A.; Sacco, M.; Giacopuzzi, S.; Weindelmayer, J.; de Manzoni, G. Effectiveness of endoscopic vacuum therapy as rescue treatment in refractory leaks after gastro-esophageal surgery. Updat. Surg. 2021, 73, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, P.; Zumblick, M.; Wächter, S.; Schiffmann, L.; Gress, T.M.; Bartsch, D.; Seitz, G.; Denzer, U.W. Endoscopic vacuum therapy (EVT) for acute esophageal perforation: Could it replace surgery? Endosc. Int. Open. 2022, 10, E686–E693. [Google Scholar] [CrossRef]
- Persson, S.; Rouvelas, I.; Irino, T.; Lundell, L. Outcomes following the main treatment options in patients with a leaking esophagus: A systematic literature review. Dis. Esophagus. 2017, 30, 1–10. [Google Scholar] [CrossRef]
- Brunt, L.M.; Deziel, D.J.; Telem, D.A.; Strasberg, S.M.; Aggarwal, R.; Asbun, H.; Bonjer, J.; McDonald, M.; Alseidi, A.; Ujiki, M.; et al. Safe cholecystectomy multi-society practice guideline and state-of-the-art consensus conference on prevention of bile duct injury during cholecystectomy. Surg. Endosc. 2020, 34, 2827–2855. [Google Scholar] [CrossRef]
- Garg, R.; Lakhan, S.E.; Dhanasekaran, A.K. How to review a case report. J. Med. Case Rep. 2016, 10, 88. [Google Scholar] [CrossRef][Green Version]
- Varghese, B.T. The Dilemma of Defining and Interpreting Case Series. Indian J. Surg. Oncol. 2023, 14, 707. [Google Scholar] [CrossRef]

| Demographic and Clinical Characteristics | Study Population (N = 5) |
|---|---|
| Age, years | 73 (67–74) |
| Gender | |
| Female | 4 (80) |
| Male | 1 (20) |
| BMI, kg/m2 | 31.60 (30.30–36.11) |
| Antireflux surgery | |
| Surgery indication | |
| PEH | 3 (60) |
| GERD and PEH | 2 (40) |
| Redo surgery | 0 |
| Approach | |
| Laparoscopic | 3 (60) |
| Robot-assisted | 2 (40) |
| Fundoplication type | |
| Nissen | 3 (60) |
| Toupet | 1 (20) |
| Watson | 1 (20) |
| Mesh used | 4 (80) |
| OR time, minutes | 210 (170–235) |
| Clinical Presentation | Study Population (N = 5) |
|---|---|
| Time from LARS to clinical presentation, days | 2 (1–8) |
| Shortness of breath | 5 (100) |
| Pain | 3 (60) |
| Abdominal | 1 (33.3) |
| Thoracic | 1 (33.3) |
| Thoracic–midscapular | 1 (33.3) |
| Hypoxia | 5 (100) |
| Septic shock | 4 (80) |
| Emergent intubation | 4 (80) |
| Atrial fibrillation | 1 (20) |
| Acute kidney injury | 3 (60) |
| Subcutaneous emphysema * | 3 (60) |
| WBC count, × 109/L | 14.5 (13.8–16.5) |
| Imaging | |
| Chest X-ray | 4 (80) |
| Pleural effusion | 4 (100) |
| Pneumothorax | 3 (75) |
| Pulmonary consolidation | 2 (50) |
| Esophagram | 3 (60) |
| Perforation | 1 (33.3) |
| No perforation | 2 (66.7) |
| Computed tomography | 5 (100) |
| Contrast extravasation ** | 4 (80) |
| Pleural effusion | 5 (100) |
| Pneumomediastinum | 5 (100) |
| Pneumothorax | 3 (80) |
| Hiatal hernia recurrence | 2 (40) |
| Treatment and Postoperative Course | Study Population (N = 5) |
|---|---|
| Time from clinical presentation to NTI consult | 2 (0–3) |
| Perforation location | |
| Distal esophagus | 4 (80) |
| Middle thoracic esophagus | 1 (20) |
| Primary repair * | 2 (40) |
| With intercostal muscle buttressing and esophageal stent | 1 (50) |
| With pleural flap | 1 (50) |
| Esophagectomy | 3 (60) |
| Proximal gastrectomy | 3 (60) |
| Gastrostomy or gastrojejunostomy tube | 5 (100) |
| Jejunostomy tube | 1 (20) |
| Esophagostomy | 3 (60) |
| Complications | 5 (100) |
| Pneumonia | 1 (20) |
| Seizure | 1 (20) |
| Altered mental status | 3 (60) |
| Deep vein thrombosis | 1 (20) |
| PICC line thrombosis | 1 (20) |
| Ventilator dependence requiring tracheostomy | 3 (60) |
| No. of surgeries ** | 2 (1–2) |
| Hospital stay, days | 58 (34–59) |
| Follow-up, months | 14 (6–28) |
| Author, Year | N | Indication | Procedure Type | Symptoms | Perforation Location | Treatment | Postoperative Course | LOS (Days) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Urschel et al., 1994 [10] | 11 | GERD (3 redo) | Modified Nissen (n = 9); Belsey (n = 1); intrathoracic Nissen (n = 1) | Fever (n = 10) | Chest (n = 6); abdomen (n = 5) | Abdominal: nonoperative (n = 4); esophageal repair (n = 1). Chest: nonoperative (n = 1); thoracotomy drainage (n = 2); transthoracic esophageal repair (n = 2); gastrectomy (n = 1). | NR | NR | 2 deaths (sepsis), 1 after thoracotomy drainage and 1 after transthoracic esophageal repair. Follow-up time not reported. |
| Schauer et al., 1996 [9] | 6 | GERD | Laparoscopic Nissen | Abdominal pain (n = 4); chest pain (n = 1); respiratory distress (n = 1) | NR | Wrap takedown, primary perforation closure and rewrap (n = 5); one of them had also omental patch and J-tube. Wrap takedown, primary closure, excision of the necrotic stomach, G-tube, duodenal feeding tube, and abdominal drainage (n = 1). | NR | 14 (range, 10–32) | 17% mortality at 11-months of follow-up |
| Pohl et al., 2001 [21] | 1 | GERD | NR | NR | Esophagus | Surgical drainage and redo Nissen | NR | NR | NR |
| Lindenmann et al., 2015 [22] | 1 | NR | NR | NR | 3 mm esophageal leak proximal to the fundoplication cuff | Endoscopic fixation using OTSC and self-expandable, fully covered removable stent | Stent dislocation, repositioning using purse string; sepsis on day 5; hematemesis from esophageal-aortic fistula at clip site on day 6 | NR | NR |
| Wang et al., 2016 [23] | 1 | Hiatal hernia | Toupet | Fever on POD4, acute chest pain and respiratory distress on POD7 | 2.5 cm distal esophageal perforation | Primary repaired with 4-0 Prolene continuous sutures | 10 days after repair, suture disruption at EGD; paraoesophageal tube pulled into the esophagus endoscopically + NGT positioning | 57 | Oral nutrition was tolerated without sequelae; well at 6-month follow-up |
| Pamart et al., 2021 [24] | 1 | GERD (redo) | Nissen | POD4: abdominal pain with rebound tenderness, nausea, dysphagia, fever, sepsis | Gastric perforation on the wrap | Nissen dismantling and primary suture | Subphrenic abscess resolved with antibiotics | 22 | NR |
| Anthony et al., 2024 [25] | 1 | NR | Nissen, Posterior cardiopexy + anterior gastropexy | POD7: tachypnea, tachycardia, rebound tenderness | EGJ | Exploratory laparotomy, endoscopic clips; AbThera dressing; definitive closure 48 h later | Fever, ileus | 13 | Resumed regular diet, no further complications. Follow-up time not reported. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vittori, A.; Latorre-Rodríguez, A.R.; Keogan, A.; Huang, J.; Schaheen, L.; Bremner, R.M.; Mittal, S.K. Missed Gastroesophageal Injuries During Antireflux Surgery: Infrequent but Catastrophic Complications. J. Clin. Med. 2025, 14, 4577. https://doi.org/10.3390/jcm14134577
Vittori A, Latorre-Rodríguez AR, Keogan A, Huang J, Schaheen L, Bremner RM, Mittal SK. Missed Gastroesophageal Injuries During Antireflux Surgery: Infrequent but Catastrophic Complications. Journal of Clinical Medicine. 2025; 14(13):4577. https://doi.org/10.3390/jcm14134577
Chicago/Turabian StyleVittori, Arianna, Andrés R. Latorre-Rodríguez, Andrew Keogan, Jasmine Huang, Lara Schaheen, Ross M. Bremner, and Sumeet K. Mittal. 2025. "Missed Gastroesophageal Injuries During Antireflux Surgery: Infrequent but Catastrophic Complications" Journal of Clinical Medicine 14, no. 13: 4577. https://doi.org/10.3390/jcm14134577
APA StyleVittori, A., Latorre-Rodríguez, A. R., Keogan, A., Huang, J., Schaheen, L., Bremner, R. M., & Mittal, S. K. (2025). Missed Gastroesophageal Injuries During Antireflux Surgery: Infrequent but Catastrophic Complications. Journal of Clinical Medicine, 14(13), 4577. https://doi.org/10.3390/jcm14134577







