COVID-19 and Myocarditis: Trends, Clinical Characteristics, and Future Directions
Abstract
1. Introduction
2. Materials and Methods
3. Epidemiology
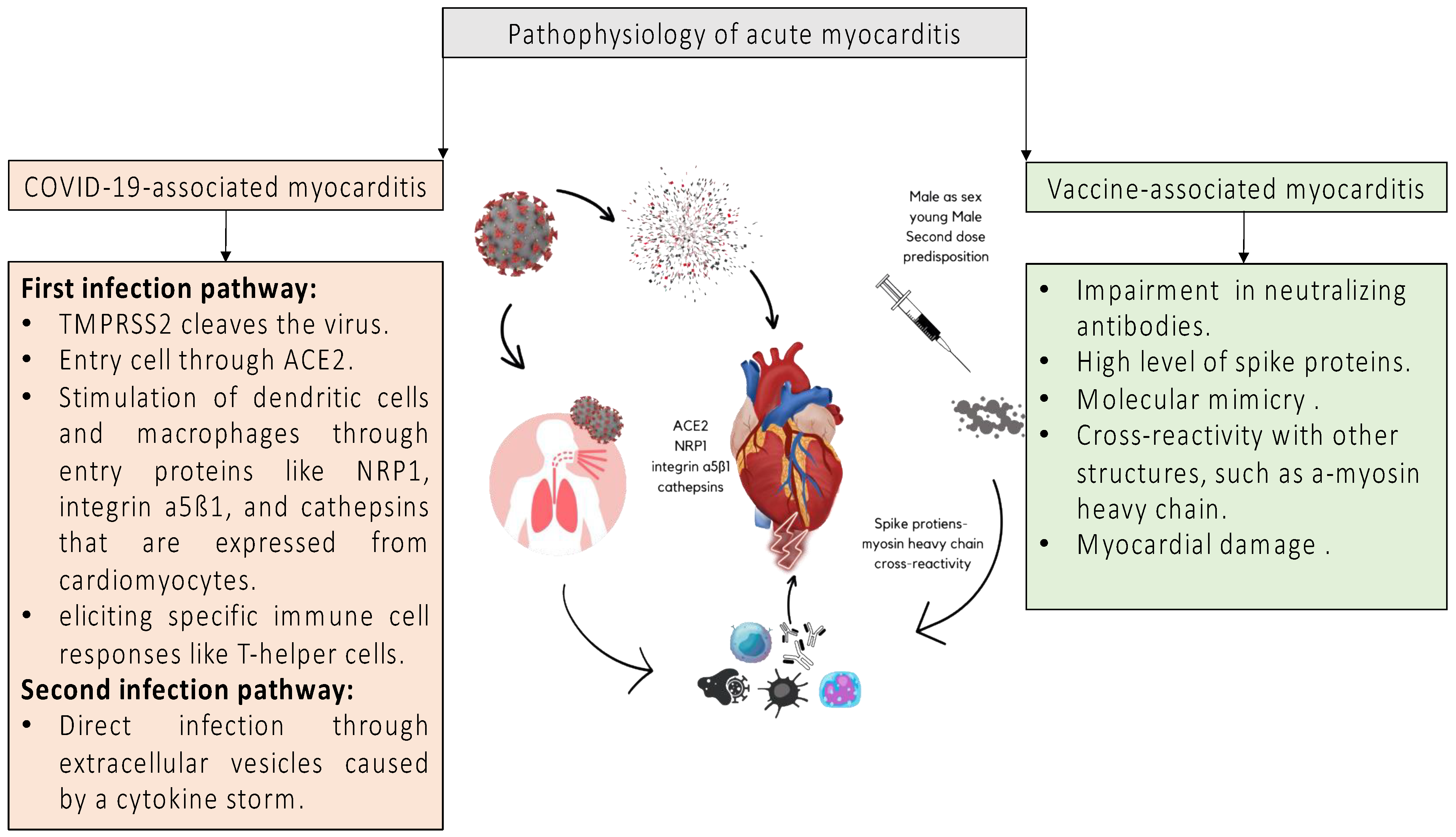
4. Pathophysiology
5. Clinical Presentation and Diagnosis of COVID-19-Associated Acute Myocarditis
6. Management of COVID-19-Associated Acute Myocarditis
7. Myocarditis After SARS-CoV-2-Vaccination; Should Vaccination Be Omitted?
| Vaccine-Associated Acute Myocarditis | ||||
|---|---|---|---|---|
| Country | Vaccine | Time Period | Incidence | References |
| US | BioNTech and Moderna | December 2020–June 2021 | 1226/300 million doses | [103] |
| Israel | BioNTech | December 2020–May 2021 | 117/10.2 million doses | [104] |
| Denmark | BioNTech and Moderna | October 2020–October 2021 | 269/4.1 million doses | [106] |
| US and Canada | BioNTech and Moderna | February–May 2021 | 20/3.5 million doses | [107] |
| Clinical Presentation | Vaccine | Prevalence | References |
|---|---|---|---|
| Chest pain | Pfizer-BioNTech/Moderna–P/M/J/AZD/C * | 90.1–100% | [108,109,110] |
| Syncope | P/M/J/AZD/C * | 0.3% | [110] |
| Palpitations | P/M/J/AZD/C * | 6.1% | [110] |
| Dyspnea | Pfizer-BioNTech/Moderna–P/M/J/AZD/C * | 25–25.7% | [109,110] |
| Cough | P/M/J/AZD/C * | 0.4% | [110] |
| Fever | Pfizer-BioNTech/Moderna–P/M/J/AZD/C * | 11.9–32% | [109,110] |
| Myalgia | Pfizer-BioNTech/Moderna | 6% | [109] |
| Gastrointestinal symptoms | Pfizer-BioNTech/Moderna–P/M/J/AZD/C * | 5.7–11% | [109,110] |
| ECG | |||
| ST-segment elevation | Pfizer-BioNTech/Moderna–P/M/J/AZD/C * | 34.9–67.3% | [109,110] |
| Other abnormal ST changes | Pfizer-BioNTech/Moderna | 9.6% | [109] |
| Laboratory parameters | |||
| Troponin | Pfizer-BioNTech/Moderna–P/M/J/AZD/C * | 97.6–98.1% | [109,110] |
| NT-proBNP | Pfizer-BioNTech/Moderna | 69.6% | [109] |
| Echocardiographic findings | |||
| LV dysfunction | Pfizer-BioNTech/Moderna–P/M/J/AZD/C * | 23.2–44% | [109,110] |
| Wall motion abnormalities | Pfizer-BioNTech/Moderna | 34% | [109] |
| Pericardial effusion | Pfizer-BioNTech/Moderna P/M/J/AZD/C * | 1.8–22% | [109,110] |
| cMRI | |||
| T2 elevation | Pfizer-BioNTech/Moderna P/M/J/AZD/C * | 61.9–63.3% | [109,110] |
| Late gadolinium enhancement | Pfizer-BioNTech/Moderna P/M/J/AZD/C * | 80.7–95.2% | [109,110] |
| Pericardial effusion | P/M/J/AZD/C * | 4.2% | [110] |
8. Outcomes and Long-Term Cardiac Complications Following COVID-19-Associated Myocarditis
9. Current Evidence and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, J.J.; Dong, X.; Liu, G.H.; Gao, Y.D. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin. Rev. Allergy Immunol. 2023, 64, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S.; Lane, H.C.; Redfield, R.R. COVID-19—Navigating the Uncharted. N. Engl. J. Med. 2020, 382, 1268–1269. [Google Scholar] [CrossRef]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe COVID-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, J.; Wang, F.; Zhou, N.; Veronese, G.; Moslehi, J.J.; Ammirati, E.; Wang, D.W. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J. Mol. Cell. Cardiol. 2020, 147, 74–87. [Google Scholar] [CrossRef]
- Alvarez-Garcia, J.; Jaladanki, S.; Rivas-Lasarte, M.; Cagliostro, M.; Gupta, A.; Joshi, A.; Ting, P.; Mitter, S.S.; Bagiella, E.; Mancini, D. New Heart Failure Diagnoses Among Patients Hospitalized for COVID-19. J. Am. Coll. Cardiol. 2021, 77, 2260–2262. [Google Scholar] [CrossRef]
- Castiello, T.; Georgiopoulos, G.; Finocchiaro, G.; Claudia, M.; Gianatti, A.; Delialis, D.; Aimo, A.; Prasad, S. COVID-19 and myocarditis: A systematic review and overview of current challenges. Heart Fail. Rev. 2022, 27, 251–261. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Chen, M.; Feng, Y.; Xiong, C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020, 116, 1097–1100. [Google Scholar] [CrossRef]
- Akhmerov, A.; Marban, E. COVID-19 and the Heart. Circ. Res. 2020, 126, 1443–1455. [Google Scholar] [CrossRef]
- Lindner, D.; Fitzek, A.; Brauninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.P. Association of Cardiac Infection with SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020, 5, 1281–1285. [Google Scholar] [CrossRef]
- Bearse, M.; Hung, Y.P.; Krauson, A.J.; Bonanno, L.; Boyraz, B.; Harris, C.K.; Helland, T.L.; Hilburn, C.F.; Hutchison, B.; Jobbagy, S.; et al. Factors associated with myocardial SARS-CoV-2 infection, myocarditis, and cardiac inflammation in patients with COVID-19. Mod. Pathol. 2021, 34, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Scally, C.; Abbas, H.; Ahearn, T.; Srinivasan, J.; Mezincescu, A.; Rudd, A.; Spath, N.; Yucel-Finn, A.; Yuecel, R.; Oldroyd, K.; et al. Myocardial and Systemic Inflammation in Acute Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2019, 139, 1581–1592. [Google Scholar] [CrossRef]
- Bader, F.; Manla, Y.; Atallah, B.; Starling, R.C. Heart failure and COVID-19. Heart Fail. Rev. 2021, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Kontogeorgos, S.; Thunstrom, E.; Zverkova Sandstrom, T.; Kroon, C.; Bollano, E.; Schaufelberger, M.; Rosengren, A. Trends in myocarditis incidence, complications and mortality in Sweden from 2000 to 2014. Sci. Rep. 2022, 12, 1810. [Google Scholar] [CrossRef]
- Pahuja, M.; Adegbala, O.; Mishra, T.; Akintoye, E.; Chehab, O.; Mony, S.; Singh, M.; Ando, T.; Abubaker, H.; Yassin, A.; et al. Trends in the Incidence of In-Hospital Mortality, Cardiogenic Shock, and Utilization of Mechanical Circulatory Support Devices in Myocarditis (Analysis of National Inpatient Sample Data, 2005–2014). J. Card. Fail. 2019, 25, 457–467. [Google Scholar] [CrossRef]
- Ammirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Friedrich, M.G.; Klingel, K.; Lehtonen, J.; Moslehi, J.J.; et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document. Circ. Heart Fail. 2020, 13, e007405. [Google Scholar] [CrossRef]
- Tschope, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- Nguyen, L.S.; Cooper, L.T.; Kerneis, M.; Funck-Brentano, C.; Silvain, J.; Brechot, N.; Hekimian, G.; Ammirati, E.; Ben M’barek, B.; Redheuil, A.; et al. Systematic analysis of drug-associated myocarditis reported in the World Health Organization pharmacovigilance database. Nat. Commun. 2022, 13, 25. [Google Scholar] [CrossRef]
- Khan, A.A.; Ashraf, A.; Baker, D.; Al-Omary, M.S.; Savage, L.; Ekmejian, A.; Singh, R.S.H.; Brienesse, S.; Majeed, T.; Gordon, T.; et al. Clozapine and incidence of myocarditis and sudden death—Long term Australian experience. Int. J. Cardiol. 2017, 238, 136–139. [Google Scholar] [CrossRef]
- Harmon, K.G.; Drezner, J.A.; Maleszewski, J.J.; Lopez-Anderson, M.; Owens, D.; Prutkin, J.M.; Asif, I.M.; Klossner, D.; Ackerman, M.J. Pathogeneses of sudden cardiac death in national collegiate athletic association athletes. Circ. Arrhythm. Electrophysiol. 2014, 7, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, E.B.; Hutchins, G.M.; Herskowitz, A.; Rose, N.R.; Baughman, K.L. Clinicopathologic description of myocarditis. J. Am. Coll. Cardiol. 1991, 18, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee; Gluckman, T.J.; Bhave, N.M.; Allen, L.A.; Chung, E.H.; Spatz, E.S.; Ammirati, E.; Baggish, A.L.; Bozkurt, B.; Cornwell, W.K.; et al. 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: Myocarditis and Other Myocardial Involvement, Post-Acute Sequelae of SARS-CoV-2 Infection, and Return to Play: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 79, 1717–1756. [Google Scholar]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Caceres-Acosta, M.F.; Idrobo, B.D.; Urbano Alban, D.C. SARS-CoV-2 dilated cardiomyopathy. Br. J. Cardiol. 2022, 29, 30. [Google Scholar]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. Corrigendum to: 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 4901. [Google Scholar]
- Ho, J.S.; Sia, C.H.; Chan, M.Y.; Lin, W.; Wong, R.C. Coronavirus-induced myocarditis: A meta-summary of cases. Heart Lung 2020, 49, 681–685. [Google Scholar] [CrossRef]
- Hu, H.; Ma, F.; Wei, X.; Fang, Y. Corrigendum to: Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur. Heart J. 2021, 42, 191. [Google Scholar] [CrossRef]
- Huyut, M.A. Novel Coronavirus Pneumonia and Cardiomyopathy: A Case Report. Arq. Bras. Cardiol. 2020, 114, 843–845. [Google Scholar] [CrossRef]
- Zeng, J.H.; Liu, Y.X.; Yuan, J.; Wang, F.X.; Wu, W.B.; Li, J.X.; Wang, L.F.; Gao, H.; Wang, Y.; Dong, C.F.; et al. First case of COVID-19 complicated with fulminant myocarditis: A case report and insights. Infection 2020, 48, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Mir, M.; Sanchez, P.; Beg, M.; Peters, J.; Enriquez, O.; Gilbert, A. COVID-19 in a Hispanic Woman. Arch. Pathol. Lab. Med. 2020, 144, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Newton-Cheh, C.; Zlotoff, D.A.; Hung, J.; Rupasov, A.; Crowley, J.C.; Funamoto, M. Case 24-2020: A 44-Year-Old Woman with Chest Pain, Dyspnea, and Shock. N. Engl. J. Med. 2020, 383, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.; Ryan, M.; Engler, R.; Hoffman, D.; McClenathan, B.; Collins, L.; Loran, D.; Hrncir, D.; Herring, K.; Platzer, M.; et al. Myocarditis Following Immunization with mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021, 6, 1202–1206. [Google Scholar] [CrossRef]
- Kim, H.W.; Jenista, E.R.; Wendell, D.C.; Azevedo, C.F.; Campbell, M.J.; Darty, S.N.; Parker, M.A.; Kim, R.J. Patients with Acute Myocarditis Following mRNA COVID-19 Vaccination. JAMA Cardiol. 2021, 6, 1196–1201. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T.; Chahal, C.A.A. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020, 17, 1463–1471. [Google Scholar] [CrossRef]
- Daniels, C.J.; Rajpal, S.; Greenshields, J.T.; Rosenthal, G.L.; Chung, E.H.; Terrin, M.; Jeudy, J.; Mattson, S.E.; Law, I.H.; Borchers, J.; et al. Prevalence of Clinical and Subclinical Myocarditis in Competitive Athletes with Recent SARS-CoV-2 Infection: Results From the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021, 6, 1078–1087. [Google Scholar] [CrossRef]
- Buckley, B.J.R.; Harrison, S.L.; Fazio-Eynullayeva, E.; Underhill, P.; Lane, D.A.; Lip, G.Y.H. Prevalence and clinical outcomes of myocarditis and pericarditis in 718,365 COVID-19 patients. Eur. J. Clin. Investig. 2021, 51, e13679. [Google Scholar] [CrossRef]
- Ammirati, E.; Lupi, L.; Palazzini, M.; Hendren, N.S.; Grodin, J.L.; Cannistraci, C.V.; Schmidt, M.; Hekimian, G.; Peretto, G.; Bochaton, T.; et al. Prevalence, Characteristics, and Outcomes of COVID-19-Associated Acute Myocarditis. Circulation 2022, 145, 1123–1139. [Google Scholar] [CrossRef]
- Basso, C.; Leone, O.; Rizzo, S.; De Gaspari, M.; van der Wal, A.C.; Aubry, M.C.; Bois, M.C.; Lin, P.T.; Maleszewski, J.J.; Stone, J.R. Pathological features of COVID-19-associated myocardial injury: A multicentre cardiovascular pathology study. Eur. Heart J. 2020, 41, 3827–3835. [Google Scholar] [CrossRef] [PubMed]
- Halushka, M.K.; Vander Heide, R.S. Myocarditis is rare in COVID-19 autopsies: Cardiovascular findings across 277 postmortem examinations. Cardiovasc. Pathol. 2021, 50, 107300. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Heberto, A.B.; Carlos, P.C.J.; Antonio, C.R.J.; Patricia, P.P.; Enrique, T.R.; Danira, M.P.J.; Benito, G.Á.E.; Alfredo, M.R.J. Implications of myocardial injury in Mexican hospitalized patients with coronavirus disease 2019 (COVID-19). Int. J. Cardiol. Heart Vasc. 2020, 30, 100638. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280 e8. [Google Scholar] [CrossRef]
- Bailey, A.L.; Dmytrenko, O.; Greenberg, L.; Bredemeyer, A.L.; Ma, P.; Liu, J.; Penna, V.; Winkler, E.S.; Sviben, S.; Brooks, E.; et al. SARS-CoV-2 Infects Human Engineered Heart Tissues and Models COVID-19 Myocarditis. JACC Basic Transl. Sci. 2021, 6, 331–345. [Google Scholar] [CrossRef]
- Theoharides, T.C. Potential association of mast cells with coronavirus disease 2019. Ann. Allergy Asthma Immunol. 2021, 126, 217–218. [Google Scholar] [CrossRef]
- Hikmet, F.; Mear, L.; Edvinsson, A.; Micke, P.; Uhlen, M.; Lindskog, C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020, 16, e9610. [Google Scholar] [CrossRef]
- Bojkova, D.; Wagner, J.U.G.; Shumliakivska, M.; Aslan, G.S.; Saleem, U.; Hansen, A.; Luxán, G.; Günther, S.; Pham, M.D.; Krishnan, J.; et al. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc. Res. 2020, 116, 2207–2215. [Google Scholar] [CrossRef]
- Robinson, E.L.; Alkass, K.; Bergmann, O.; Maguire, J.J.; Roderick, H.L.; Davenport, A.P. Genes encoding ACE2, TMPRSS2 and related proteins mediating SARS-CoV-2 viral entry are upregulated with age in human cardiomyocytes. J. Mol. Cell. Cardiol. 2020, 147, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Hu, W.; Yu, H.; Zhao, L.; Zhao, Y.; Zhao, X.; Xue, H.; Zhao, Y. Little to no expression of angiotensin-converting enzyme-2 on most human peripheral blood immune cells but highly expressed on tissue macrophages. Cytom. Part A 2023, 103, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Angileri, F.; Legare, S.; Gammazza, A.M.; de Macario, E.C.; Macario, A.J.; Cappello, F. Molecular mimicry may explain multi-organ damage in COVID-19. Autoimmun. Rev. 2020, 19, 102591. [Google Scholar] [CrossRef]
- Fairweather, D.; Beetler, D.J.; Di Florio, D.N.; Musigk, N.; Heidecker, B.; Cooper, L.T., Jr. COVID-19, Myocarditis and Pericarditis. Circ. Res. 2023, 132, 1302–1319. [Google Scholar] [CrossRef] [PubMed]
- Ryabkova, V.A.; Churilov, L.P.; Shoenfeld, Y. Influenza infection, SARS, MERS and COVID-19: Cytokine storm-The common denominator and the lessons to be learned. Clin. Immunol. 2021, 223, 108652. [Google Scholar] [CrossRef]
- Fairweather, D.; Kaya, Z.; Shellam, G.R.; Lawson, C.M.; Rose, N.R. From infection to autoimmunity. J. Autoimmun. 2001, 16, 175–186. [Google Scholar] [CrossRef]
- Kim, I.C.; Kim, J.Y.; Kim, H.A.; Han, S. COVID-19-related myocarditis in a 21-year-old female patient. Eur. Heart J. 2020, 41, 1859. [Google Scholar] [CrossRef]
- Sala, S.; Peretto, G.; Gramegna, M.; Palmisano, A.; Villatore, A.; Vignale, D.; De Cobelli, F.; Tresoldi, M.; Cappelletti, A.M.; Basso, C.; et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur. Heart J. 2020, 41, 1861–1862. [Google Scholar] [CrossRef]
- Younis, A.; Matetzky, S.; Mulla, W.; Masalha, E.; Afel, Y.; Chernomordik, F.; Fardman, A.; Goitein, O.; Ben-Zekry, S.; Peled, Y.; et al. Epidemiology Characteristics and Outcome of Patients with Clinically Diagnosed Acute Myocarditis. Am. J. Med. 2020, 133, 492–499. [Google Scholar] [CrossRef]
- Ammirati, E.; Cipriani, M.; Moro, C.; Raineri, C.; Pini, D.; Sormani, P.; Mantovani, R.; Varrenti, M.; Pedrotti, P.; Conca, C.; et al. Clinical Presentation and Outcome in a Contemporary Cohort of Patients with Acute Myocarditis: Multicenter Lombardy Registry. Circulation 2018, 138, 1088–1099. [Google Scholar] [CrossRef]
- White, J.A.; Hansen, R.; Abdelhaleem, A.; Mikami, Y.; Peng, M.; Rivest, S.; Satriano, A.; Dykstra, S.; Flewitt, J.; Heydari, B.; et al. Natural History of Myocardial Injury and Chamber Remodeling in Acute Myocarditis. Circ. Cardiovasc. Imaging. 2019, 12, e008614. [Google Scholar] [CrossRef] [PubMed]
- Moulson, N.; Petek, B.J.; Drezner, J.A.; Harmon, K.G.; Kliethermes, S.A.; Patel, M.R.; Baggish, A.L. SARS-CoV-2 Cardiac Involvement in Young Competitive Athletes. Circulation 2021, 144, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Rigatelli, G.; Bilato, C.; Porcari, A.; Merlo, M.; Roncon, L.; Sinagra, G. One-Year Risk of Myocarditis After COVID-19 Infection: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2023, 39, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819–824. [Google Scholar] [CrossRef]
- Freund, O.; Eviatar, T.; Bornstein, G. Concurrent myopathy and inflammatory cardiac disease in COVID-19 patients: A case series and literature review. Rheumatol. Int. 2022, 42, 905–912. [Google Scholar] [CrossRef]
- Susca, N.; Solimando, A.G.; Borrelli, P.; Marziliano, D.; Monitillo, F.; Raimondo, P.; Vestito, D.; Lopizzo, A.; Brindicci, G.; Abumayyaleh, M.; et al. Electrocardiographic Pathological Findings Caused by the SARS-CoV-2 Virus Infection: Evidence from a Retrospective Multicenter International Cohort Longitudinal Pilot Study of 548 Subjects. J. Cardiovasc. Dev. Dis. 2023, 10, 58. [Google Scholar] [CrossRef]
- Santoro, F.; Monitillo, F.; Raimondo, P.; Lopizzo, A.; Brindicci, G.; Gilio, M.; Musaico, F.; Mazzola, M.; Vestito, D.; Di Benedetto, R.; et al. QTc Interval Prolongation and Life-Threatening Arrhythmias During Hospitalization in Patients with Coronavirus Disease 2019 (COVID-19): Results From a Multicenter Prospective Registry. Clin. Infect. Dis. 2021, 73, e4031–e4038. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Lovell, J.P.; Cihakova, D.; Gilotra, N.A. COVID-19 and Myocarditis: Review of Clinical Presentations, Pathogenesis and Management. Heart Int. 2022, 16, 20–27. [Google Scholar] [CrossRef]
- Berg, J.; Kottwitz, J.; Baltensperger, N.; Kissel, C.K.; Lovrinovic, M.; Mehra, T.; Scherff, F.; Schmied, C.; Templin, C.; Lüscher, T.F.; et al. Cardiac Magnetic Resonance Imaging in Myocarditis Reveals Persistent Disease Activity Despite Normalization of Cardiac Enzymes and Inflammatory Parameters at 3-Month Follow-Up. Circ. Heart Fail. 2017, 10, e004262. [Google Scholar] [CrossRef] [PubMed]
- Gilotra, N.A.; Minkove, N.; Bennett, M.K.; Tedford, R.J.; Steenbergen, C.; Judge, D.P.; Halushka, M.K.; Russell, S.D. Lack of Relationship Between Serum Cardiac Troponin I Level and Giant Cell Myocarditis Diagnosis and Outcomes. J. Card. Fail. 2016, 22, 583–585. [Google Scholar] [CrossRef]
- Peretto, G.; Sala, S.; Caforio, A.L.P. Acute myocardial injury, MINOCA, or myocarditis? Improving characterization of coronavirus-associated myocardial involvement. Eur. Heart J. 2020, 41, 2124–2125. [Google Scholar] [CrossRef]
- Rout, A.; Suri, S.; Vorla, M.; Kalra, D.K. Myocarditis associated with COVID-19 and its vaccines—A systematic review. Prog. Cardiovasc. Dis. 2022, 74, 111–121. [Google Scholar] [CrossRef]
- Sawalha, K.; Abozenah, M.; Kadado, A.J.; Battisha, A.; Al-Akchar, M.; Salerno, C.; Hernandez-Montfort, J.; Islam, A.M. Systematic Review of COVID-19 Related Myocarditis: Insights on Management and Outcome. Cardiovasc. Revasc. Med. 2021, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Theetha Kariyanna, P.; Sabih, A.; Sutarjono, B.; Shah, K.; Vargas Pelaez, A.; Lewis, J.; Yu, R.; Grewal, E.S.; Jayarangaiah, A.; Das, S.; et al. A Systematic Review of COVID-19 and Pericarditis. Cureus 2022, 14, e27948. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, S.; Ma, P.; Yang, B.; Si, D.; Liu, G.; Liu, L.; Ding, M.; Yang, W.; Li, J.; et al. Cardiac injury is associated with inflammation in geriatric COVID-19 patients. J. Clin. Lab. Anal. 2021, 35, e23654. [Google Scholar] [CrossRef]
- Rathore, S.S.; Rojas, G.A.; Sondhi, M.; Pothuru, S.; Pydi, R.; Kancherla, N.; Singh, R.; Ahmed, N.K.; Shah, J.; Tousif, S.; et al. Myocarditis associated with COVID-19 disease: A systematic review of published case reports and case series. Int. J. Clin. Pract. 2021, 75, e14470. [Google Scholar] [CrossRef]
- Starekova, J.; Bluemke, D.A.; Bradham, W.S.; Eckhardt, L.L.; Grist, T.M.; Kusmirek, J.E.; Purtell, C.S.; Schiebler, M.L.; Reeder, S.B. Evaluation for Myocarditis in Competitive Student Athletes Recovering From Coronavirus Disease 2019 with Cardiac Magnetic Resonance Imaging. JAMA Cardiol. 2021, 6, 945–950. [Google Scholar] [CrossRef]
- Lagana, N.; Cei, M.; Evangelista, I.; Cerutti, S.; Colombo, A.; Conte, L.; Mormina, E.; Rotiroti, G.; Versace, A.G.; Porta, C.; et al. Suspected myocarditis in patients with COVID-19: A multicenter case series. Medicine 2021, 100, e24552. [Google Scholar] [CrossRef]
- Dweck, M.R.; Bularga, A.; Hahn, R.T.; Bing, R.; Lee, K.K.; Chapman, A.R.; White, A.; Di Salvo, G.; Sade, L.E.; Pearce, K.; et al. Global evaluation of echocardiography in patients with COVID-19. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 949–958. [Google Scholar] [CrossRef]
- Ammirati, E.; Veronese, G.; Brambatti, M.; Merlo, M.; Cipriani, M.; Potena, L.; Sormani, P.; Aoki, T.; Sugimura, K.; Sawamura, A.; et al. Fulminant Versus Acute Nonfulminant Myocarditis in Patients with Left Ventricular Systolic Dysfunction. J. Am. Coll. Cardiol. 2019, 74, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Kelle, S.; Bucciarelli-Ducci, C.; Judd, R.M.; Kwong, R.Y.; Simonetti, O.; Plein, S.; Raimondi, F.; Weinsaft, J.W.; Wong, T.C.; Carr, J. Society for Cardiovascular Magnetic Resonance (SCMR) recommended CMR protocols for scanning patients with active or convalescent phase COVID-19 infection. J. Cardiovasc. Magn. Reson. 2020, 22, 61. [Google Scholar] [CrossRef]
- Clark, D.E.; Aggarwal, S.K.; Phillips, N.J.; Soslow, J.H.; Dendy, J.M.; Hughes, S.G. Cardiac Magnetic Resonance in the Evaluation of COVID-19. Card. Fail. Rev. 2022, 8, e09. [Google Scholar] [CrossRef] [PubMed]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Puntmann, V.O.; Martin, S.; Shchendrygina, A.; Hoffmann, J.; Ka, M.M.; Giokoglu, E.; Vanchin, B.; Holm, N.; Karyou, A.; Laux, G.S.; et al. Long-term cardiac pathology in individuals with mild initial COVID-19 illness. Nat. Med. 2022, 28, 2117–2123. [Google Scholar] [CrossRef]
- Aretz, H.T. Myocarditis: The Dallas criteria. Hum. Pathol. 1987, 18, 619–624. [Google Scholar] [CrossRef]
- Haussner, W.; DeRosa, A.P.; Haussner, D.; Tran, J.; Torres-Lavoro, J.; Kamler, J.; Shah, K. COVID-19 associated myocarditis: A systematic review. Am. J. Emerg. Med. 2022, 51, 150–155. [Google Scholar] [CrossRef]
- Bennett, M.K.; Gilotra, N.A.; Harrington, C.; Rao, S.; Dunn, J.M.; Freitag, T.B.; Halushka, M.K.; Russell, S.D. Evaluation of the role of endomyocardial biopsy in 851 patients with unexplained heart failure from 2000-2009. Circ. Heart Fail. 2013, 6, 676–684. [Google Scholar] [CrossRef]
- Singh, V.; Mendirichaga, R.; Savani, G.T.; Rodriguez, A.; Blumer, V.; Elmariah, S.; Inglessis-Azuaje, I.; Palacios, I. Comparison of Utilization Trends, Indications, and Complications of Endomyocardial Biopsy in Native Versus Donor Hearts (from the Nationwide Inpatient Sample 2002 to 2014). Am. J. Cardiol. 2018, 121, 356–363. [Google Scholar] [CrossRef]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J. Am. Coll. Cardiol. 2007, 50, 1914–1931. [Google Scholar]
- Chimenti, C.; Frustaci, A. Contribution and risks of left ventricular endomyocardial biopsy in patients with cardiomyopathies: A retrospective study over a 28-year period. Circulation 2013, 128, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Kociol, R.D.; Cooper, L.T.; Fang, J.C.; Moslehi, J.J.; Pang, P.S.; Sabe, M.A.; Shah, R.V.; Sims, D.B.; Thiene, G.; Vardeny, O.; et al. Recognition and Initial Management of Fulminant Myocarditis: A Scientific Statement From the American Heart Association. Circulation 2020, 141, e69–e92. [Google Scholar] [CrossRef] [PubMed]
- Sarda, L.; Colin, P.; Boccara, F.; Daou, D.; Lebtahi, R.; Faraggi, M.; Nguyen, C.; Cohen, A.; Slama, M.S.; Steg, P.G.; et al. Myocarditis in patients with clinical presentation of myocardial infarction and normal coronary angiograms. J. Am. Coll. Cardiol. 2001, 37, 786–792. [Google Scholar] [CrossRef]
- Phelan, D.; Kim, J.H.; Chung, E.H. A game plan for the resumption of sport and exercise after coronavirus disease 2019 (COVID-19) Infection. JAMA Cardiol. 2020, 5, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Cecchi, E.; Demichelis, B.; Ierna, S.; Demarie, D.; Ghisio, A.; Pomari, F.; Coda, L.; Belli, R.; Trinchero, R. Indicators of poor prognosis of acute pericarditis. Circulation 2007, 115, 2739–2744. [Google Scholar] [CrossRef]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Baron-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar]
- Tscholl, V.; Wielander, D.; Kelch, F.; Stroux, A.; Attanasio, P.; Tschope, C.; Landmesser, U.; Roser, M.; Huemer, M.; Heidecker, B.; et al. Benefit of a wearable cardioverter defibrillator for detection and therapy of arrhythmias in patients with myocarditis. ESC Heart Fail. 2021, 8, 2428–2437. [Google Scholar] [CrossRef]
- El-Battrawy, I.; Koepsel, K.; Tenbrink, D.; Kovacs, B.; Dreher, T.C.; Blockhaus, C.; Gotzmann, M.; Klein, N.; Kuntz, T.; Shin, D.; et al. Use of the Wearable Cardioverter-Defibrillator Among Patients with Myocarditis and Reduced Ejection Fraction or Ventricular Tachyarrhythmia: Data From a Multicenter Registry. J. Am. Heart Assoc. 2023, 12, e030615. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Chau, V.Q.; Giustino, G.; Mahmood, K.; Oliveros, E.; Neibart, E.; Oloomi, M.; Moss, N.; Mitter, S.S.; Contreras, J.P.; Croft, L.; et al. Cardiogenic Shock and Hyperinflammatory Syndrome in Young Males with COVID-19. Circ. Heart Fail. 2020, 13, e007485. [Google Scholar] [CrossRef] [PubMed]
- Su, J.R.; McNeil, M.M.; Welsh, K.J.; Marquez, P.L.; Ng, C.; Yan, M.; Cano, M.V. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990–2018. Vaccine 2021, 39, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Wallace, M.; Hadler, S.C.; Langley, G.; Su, J.R.; Oster, M.E.; Broder, K.R.; Gee, J.; Weintraub, E.; Shimabukuro, T.; et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices-United States, June 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 977–982. [Google Scholar] [CrossRef]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis after BNT162b2 mRNA Vaccine against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Husby, A.; Hansen, J.V.; Fosbol, E.; Thiesson, E.M.; Madsen, M.; Thomsen, R.W.; Sørensen, H.T.; Andersen, M.; Wohlfahrt, J.; Gislason, G.; et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: Population based cohort study. BMJ 2021, 375, 068665. [Google Scholar] [CrossRef]
- Diaz, G.A.; Parsons, G.T.; Gering, S.K.; Meier, A.R.; Hutchinson, I.V.; Robicsek, A. Myocarditis and Pericarditis After Vaccination for COVID-19. JAMA 2021, 326, 1210–1212. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis with COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Behers, B.J.; Patrick, G.A.; Jones, J.M.; Carr, R.A.; Behers, B.M.; Melchor, J.; Rahl, D.E.; Guerriero, T.D.; Zhang, H.; Ozkardes, C.; et al. Myocarditis Following COVID-19 Vaccination: A Systematic Review of Case Reports. Yale J. Biol. Med. 2022, 95, 237–247. [Google Scholar]
- Goyal, M.; Ray, I.; Mascarenhas, D.; Kunal, S.; Sachdeva, R.A.; Ish, P. Myocarditis post-SARS-CoV-2 vaccination: A systematic review. QJM 2023, 116, 7–25. [Google Scholar] [CrossRef]
- Kariko, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Burns, M.D.; Kane, A.; Boribong, B.P.; Davis, J.P.; Loiselle, M.; Novak, T.; Senussi, Y.; et al. Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis. Circulation 2023, 147, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.J.; Longo, D.L. A Possible Role for Anti-idiotype Antibodies in SARS-CoV-2 Infection and Vaccination. N. Engl. J. Med. 2022, 386, 394–396. [Google Scholar] [CrossRef]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef] [PubMed]
- Kanduc, D.; Shoenfeld, Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: Implications for the vaccine. Immunol. Res. 2020, 68, 310–313. [Google Scholar] [CrossRef]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 2022, 28, 410–422. [Google Scholar] [CrossRef]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.; et al. Risk of Myocarditis After Sequential Doses of COVID-19 Vaccine and SARS-CoV-2 Infection by Age and Sex. Circulation 2022, 146, 743–754. [Google Scholar] [CrossRef]
- Woo, W.; Kim, A.Y.; Yon, D.K.; Lee, S.W.; Hwang, J.; Jacob, L.; Koyanagi, A.; Kim, M.S.; Moon, D.H.; Jung, J.W.; et al. Clinical characteristics and prognostic factors of myocarditis associated with the mRNA COVID-19 vaccine. J. Med. Virol. 2022, 94, 1566–1580. [Google Scholar] [CrossRef]
- Shiyovich, A.; Witberg, G.; Aviv, Y.; Eisen, A.; Orvin, K.; Wiessman, M.; Grinberg, T.; Porter, A.; Kornowski, R.; Hamdan, A. Myocarditis following COVID-19 vaccination: Magnetic resonance imaging study. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1075–1082. [Google Scholar] [CrossRef]
- Kim, S.G.; Lee, J.Y.; Jeong, W.G.; Lee, J.E.; Kim, Y.H. Cardiac Magnetic Resonance Imaging Findings and Clinical Features of COVID-19 Vaccine-Associated Myocarditis, Compared with Those of Other Types of Myocarditis. J. Korean Med. Sci. 2024, 39, e42. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; A Blom, N.; A de Boer, R.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA 2022, 327, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, S.E.; Guo, Y.; Heath, K.; Dasmarinas, M.C.; Jubilo, K.G.; Samranvedhya, J.; Lipsitch, M.; Cohen, K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ 2021, 373, 1098. [Google Scholar] [CrossRef]
- Bemtgen, X.; Kaier, K.; Rilinger, J.; Rottmann, F.; Supady, A.; von Zur Muhlen, C.; Westermann, D.; Wengenmayer, T.; Staudacher, D.L. Myocarditis mortality with and without COVID-19: Insights from a national registry. Clin. Res. Cardiol. 2024, 113, 216–222. [Google Scholar] [CrossRef]
- Hulscher, N.; Hodkinson, R.; Makis, W.; McCullough, P.A. Autopsy findings in cases of fatal COVID-19 vaccine-induced myocarditis. ESC Heart Fail. 2024, 11, 2467–2468. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Conti, N.; Palazzini, M.; Rocchetti, M.; Spangaro, A.; Garascia, A.; Lupi, L.; Cereda, A. Fulminant Myocarditis Temporally Associated with COVID-19 Vaccination. Curr. Cardiol. Rep. 2024, 26, 97–112. [Google Scholar] [CrossRef]
- Bouchlarhem, A.; Boulouiz, S.; Bazid, Z.; Ismaili, N.; El Ouafi, N. Is There a Causal Link Between Acute Myocarditis and COVID-19 Vaccination: An Umbrella Review of Published Systematic Reviews and Meta-Analyses. Clin. Med. Insights Cardiol. 2024, 18, 11795468231221406. [Google Scholar] [CrossRef] [PubMed]
- Salah, H.M.; Fudim, M.; O’Neil, S.T.; Manna, A.; Chute, C.G.; Caughey, M.C. Post-recovery COVID-19 and incident heart failure in the National COVID Cohort Collaborative (N3C) study. Nat. Commun. 2022, 13, 4117. [Google Scholar] [CrossRef] [PubMed]
- Fatuyi, M.; Amoah, J.; Egbuchiem, H.; Antia, A.; Akinti, S.; Mararenko, A.; Alzamara, M.; Bhatia, A. Impact of COVID-19 Infection on Clinical Outcomes Among Patients with Acute Decompensated Heart Failure: A Nationwide Analysis. Curr. Probl. Cardiol. 2023, 48, 101908. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.Y.; Wang, S.I.; Wei, J.C. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: A retrospective cohort study from the TriNetX US collaborative networks. eClinicalMedicine 2022, 53, 101619. [Google Scholar] [CrossRef]
- Minhas, A.M.K.; Fudim, M.; Abramov, D. The Pandemic That Never Left. J. Card. Fail. 2024, 30, 1179–1180. [Google Scholar] [CrossRef]
- Zuin, M.; Rigatelli, G.; Roncon, L.; Pasquetto, G.; Bilato, C. Risk of incident heart failure after COVID-19 recovery: A systematic review and meta-analysis. Heart Fail. Rev. 2023, 28, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Manla, Y.; Badarin, F.A.; Bader, N.; Lee-St John, T.; Mehra, M.R.; Bader, F. Worldwide and Country-Specific Impact of the COVID-19 Pandemic on Heart Transplantation Volumes: A Longitudinal Analysis of 2020 and 2021. Curr. Probl. Cardiol. 2023, 48, 101870. [Google Scholar] [CrossRef]
- Nunez-Gil, I.J.; Fernandez-Perez, C.; Estrada, V.; Becerra-Munoz, V.M.; El-Battrawy, I.; Uribarri, A.; Fernández-Rozas, I.; Feltes, G.; Viana-Llamas, M.C.; Trabattoni, D.; et al. Mortality risk assessment in Spain and Italy, insights of the HOPE COVID-19 registry. Intern. Emerg. Med. 2021, 16, 957–966. [Google Scholar] [CrossRef] [PubMed]
- El-Battrawy, I.; Nunez-Gil, I.J.; Abumayyaleh, M.; Estrada, V.; Manuel Becerra-Munoz, V.; Uribarri, A.; Fernández-Rozas, I.; Feltes, G.; Arroyo-Espliguero, R.; Trabattoni, D.; et al. COVID-19 and the impact of arterial hypertension-An analysis of the international HOPE COVID-19 Registry (Italy-Spain-Germany). Eur. J. Clin. Investig. 2021, 51, e13582. [Google Scholar] [CrossRef] [PubMed]
- Abumayyaleh, M.; Nunez-Gil, I.J.; El-Battrawy, I.; Estrada, V.; Becerra-Munoz, V.M.; Uribarri, A.; Fernández-Rozas, I.; Feltes, G.; Arroyo-Espliguero, R.; Trabattoni, D.; et al. Sepsis of Patients Infected by SARS-CoV-2: Real-World Experience from the International HOPE-COVID-19-Registry and Validation of HOPE Sepsis Score. Front Med. 2021, 8, 728102. [Google Scholar] [CrossRef]
- Abumayyaleh, M.; Nunez Gil, I.J.; El-Battrawy, I.; Estrada, V.; Becerra-Munoz, V.M.; Aparisi, A.; Fernández-Rozas, I.; Feltes, G.; Arroyo-Espliguero, R.; Trabattoni, D.; et al. Does there exist an obesity paradox in COVID-19? Insights of the international HOPE-COVID-19-registry. Obes. Res. Clin. Pract. 2021, 15, 275–280. [Google Scholar] [CrossRef]
- Mensah, G.A.; Fuster, V.; Murray, C.J.L.; Roth, G.A. Global Burden of Cardiovascular D, Risks C. Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef]
- Petersen, M.; Mehrad, B.; Keeley, E.C. Acute myocarditis during the COVID-19 pandemic: A single center experience. Am. Heart J. Plus 2021, 5, 100030. [Google Scholar] [CrossRef]
| COVID-19-Associated Acute Myocarditis | ||||
|---|---|---|---|---|
| Country | Time Period | Sex Male | Prevalence | References |
| US | March 2020–December 2020 | 60.4% | 2.3% * | [38] |
| US | January 2021–June 2020 | 43.5% | 5% Ω | [39] |
| US and Europe | February 2020–April 2021 | 61.1% | 2.4–4.1 per 1000 h.Ω | [40] |
| International | until September 2020 | 62% | 2% π | [42] |
| Clinical Presentation | Prevalence | References |
|---|---|---|
| Chest pain | 55.5–60% | [40,74,75,76] |
| Syncope | 6% | [40] |
| Palpitations | 11.1% | [40] |
| Dyspnea | 53.7–80% | [40,74,75,76] |
| Cough | 39–67% | [40,75,76] |
| Fever | 57–82.4% | [77,78] |
| Myalgia | 12% | [76] |
| Gastrointestinal symptoms | 33–44.4% | [40,75] |
| Asymptomatic | 1.5–5% | [63,79] |
| ECG | ||
| ST-segment elevation | 25.9–28% | [40,75] |
| Other abnormal ST changes | 13–24% | [40,76] |
| QTc prolongation | 25% | [80] |
| Laboratory parameters | ||
| Troponin | 86–90% | [75,78] |
| NT-proBNP | 50–87% | [75,78] |
| Radiographic findings | ||
| Cardiomegaly in x-ray | 31% | [76] |
| Bilateral infiltrates in x-ray | 31% | [76] |
| Ground-glass opacities in CT | 50% | [76] |
| Echocardiographic findings | ||
| LV dysfunction | 20% | [76] |
| Wall motion abnormalities | 20% | [76] |
| Pericardial effusion | 46–67% | [40] |
| cMRI | ||
| T2 elevation | 100% | [38] |
| Late gadolinium enhancement | 40.7–50% | [38,76] |
| EMB | ||
| Positive results for myocarditis | 82.3% | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abumayyaleh, M.; Schupp, T.; Behnes, M.; El-Battrawy, I.; Hamdani, N.; Akin, I. COVID-19 and Myocarditis: Trends, Clinical Characteristics, and Future Directions. J. Clin. Med. 2025, 14, 4560. https://doi.org/10.3390/jcm14134560
Abumayyaleh M, Schupp T, Behnes M, El-Battrawy I, Hamdani N, Akin I. COVID-19 and Myocarditis: Trends, Clinical Characteristics, and Future Directions. Journal of Clinical Medicine. 2025; 14(13):4560. https://doi.org/10.3390/jcm14134560
Chicago/Turabian StyleAbumayyaleh, Mohammad, Tobias Schupp, Michael Behnes, Ibrahim El-Battrawy, Nazha Hamdani, and Ibrahim Akin. 2025. "COVID-19 and Myocarditis: Trends, Clinical Characteristics, and Future Directions" Journal of Clinical Medicine 14, no. 13: 4560. https://doi.org/10.3390/jcm14134560
APA StyleAbumayyaleh, M., Schupp, T., Behnes, M., El-Battrawy, I., Hamdani, N., & Akin, I. (2025). COVID-19 and Myocarditis: Trends, Clinical Characteristics, and Future Directions. Journal of Clinical Medicine, 14(13), 4560. https://doi.org/10.3390/jcm14134560







