Impact of a Multimodal Intervention Combining Manual Therapy, Exercise, Reduced Methylxanthine Intake, and Nocturnal Light Avoidance on Inflammatory and Metabolic Profiles, Pain, Functionality, and Sleep Quality in Patients with Frozen Shoulder: A Single-Blind Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
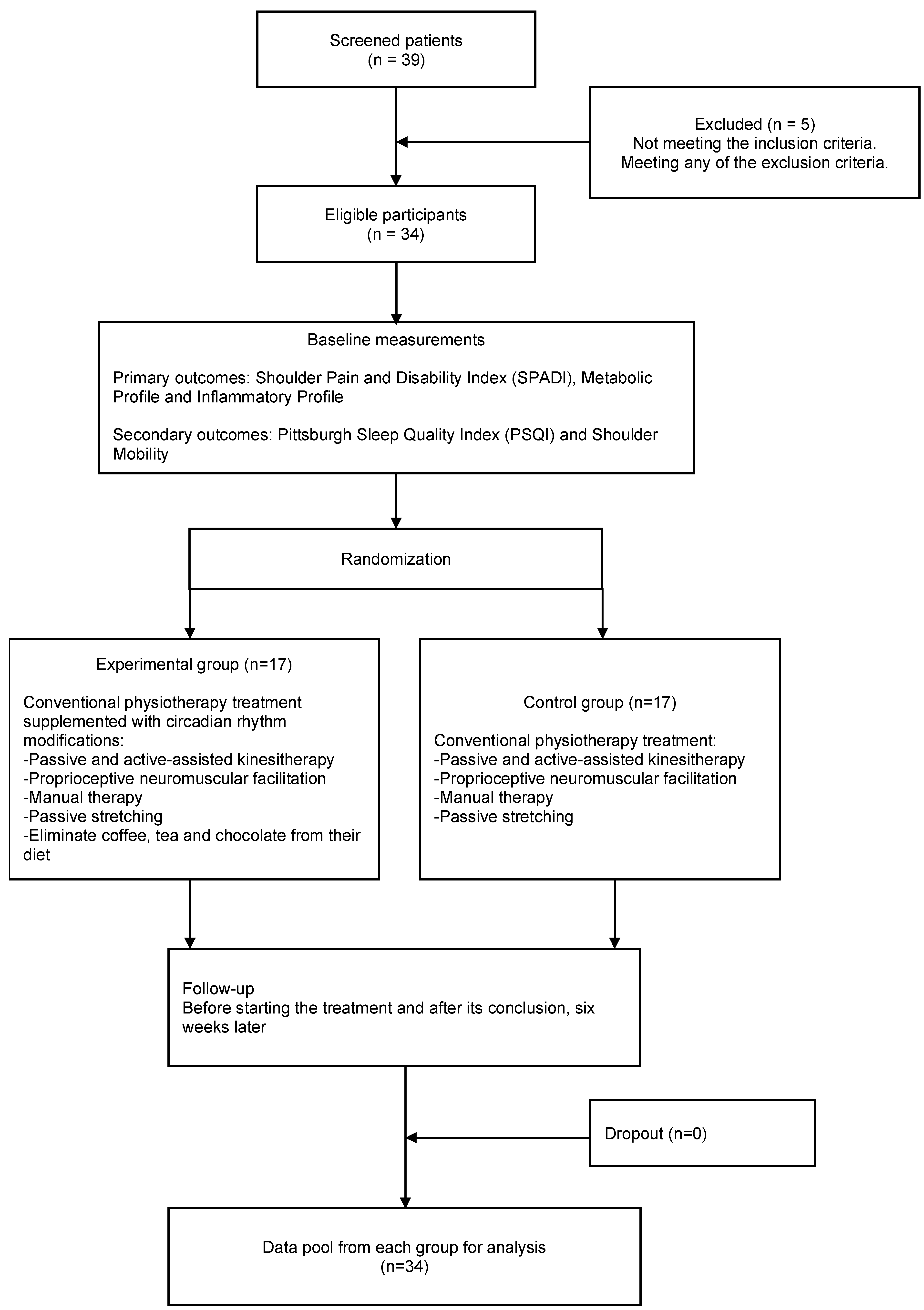
2.2. Patient Cohort
2.3. Procedures
2.4. Evaluations
2.4.1. Primary Outcomes
2.4.2. Secondary Outcomes
2.4.3. Sample Size
2.5. Statistical Analyses
3. Results
3.1. Intra-Group Changes
3.2. Inter-Group Comparisons (n = 17 per Group)
4. Discussion
4.1. Strengths and Weaknesses
4.2. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maria Carmignano, S. Frozen Shoulder: Symptoms, Causes, Diagnosis, and Treatment. In Shoulder Surgery for RC Pathology, Arthropathy and Tumors; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Tamai, K.; Akutsu, M.; Yano, Y. Primary frozen shoulder: Brief review of pathology and imaging abnormalities. J. Orthop. Sci. 2014, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Roubal, P.; Dobritt, D.; Placzek, D. Glenohumeral Gliding Manipulation Following lnterscalene Brachial Plexus Block in Patients with Adhesive Capsulitis. J. Orthop. Sports Phys. Ther. 1996, 24, 66–77. [Google Scholar] [CrossRef]
- Toprak, M.; Erden, M. Sleep quality, pain, anxiety, depression and quality of life in patients with frozen shoulder. J. Back. Musculoskelet. Rehabil. 2019, 32, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.R. Correlation between functional disability and quality of life in patients with adhesive capsulitis. Acta Ortop. Bras. 2015, 23, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Sarasua, S.M.; Floyd, S.; Bridges, W.C.; Pill, S.G. The epidemiology and etiology of adhesive capsulitis in the U.S. Medicare population. BMC Musculoskelet. Disord. 2021, 22, 828. [Google Scholar] [CrossRef]
- Ramirez, J. Adhesive capsulitis: Diagnosis and management. Am. Fam. Physician 2019, 99, 297–300. [Google Scholar]
- Le, H.V.; Lee, S.J.; Nazarian, A.; Rodriguez, E.K. Adhesive capsulitis of the shoulder: Review of pathophysiology and current clinical treatments. Shoulder Elb. 2017, 9, 75–84. [Google Scholar] [CrossRef]
- Brindisino, F.; Turgut, E.; Struyf, F. Frozen shoulder: Exploration of terminology and classification. Front. Rehabil. Sci. 2024, 5, 1498263. [Google Scholar] [CrossRef]
- de la Serna, D.; Navarro-Ledesma, S.; Alayón, F.; López, E.; Pruimboom, L. A Comprehensive View of Frozen Shoulder: A Mystery Syndrome. Front. Med. 2021, 8, 663703. [Google Scholar] [CrossRef]
- Date, A.; Rahman, L. Frozen shoulder: Overview of clinical presentation and review of the current evidence base for management strategies. Future Sci. OA 2020, 6, FSO647. [Google Scholar] [CrossRef]
- Navarro-Ledesma, S.; Hamed-Hamed, D.; Pruimboom, L. A new perspective of frozen shoulder pathology; the interplay between the brain and the immune system. Front. Physiol. 2024, 15, 1248612. [Google Scholar] [CrossRef] [PubMed]
- Kabbabe, B.; Ramkumar, S.; Richardson, M. Cytogenetic analysis of the pathology of frozen shoulder. Int. J. Shoulder Surg. 2010, 4, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Ryan, V.; Brown, H.; Minns Lowe, C.J.; Lewis, J.S. The pathophysiology associated with primary (idiopathic) frozen shoulder: A systematic review. BMC Musculoskelet. Disord. 2016, 17, 340. [Google Scholar] [CrossRef]
- Bunker, T.D.; Reilly, J.; Baird, K.S.; Hamblen, D.L. Expression of growth factors, cytokines and matrix metalloproteinases in frozen shoulder. J. Bone Jt. Surg. Br. 2000, 82, 768–773. [Google Scholar] [CrossRef]
- Hamed-Hamed, D.; Rodríguez-Pérez, C.; Pruimboom, L.; Navarro-Ledesma, S. Influence of the metabolic and inflammatory profile in patients with frozen shoulder—Systematic review and meta-analysis. BMC Musculoskelet. Disord. 2025, 26, 475. [Google Scholar] [CrossRef] [PubMed]
- Scalf, R.E.; Wenger, D.E.; Frick, M.A.; Mandrekar, J.N.; Adkins, M.C. MRI findings of 26 patients with Parsonage-Turner syndrome. Am. J. Roentgenol. 2007, 189, 251. [Google Scholar] [CrossRef]
- Koreki, A.; Sado, M.; Mitsukura, Y.; Tachimori, H.; Kubota, A.; Kanamori, Y.; Uchibori, M.; Usune, S.; Ninomiya, A.; Shirahama, R.; et al. The association between salivary IL-6 and poor sleep quality assessed using Apple watches in stressed workers in Japan. Sci. Rep. 2024, 14, 22620. [Google Scholar] [CrossRef]
- Krueger, J.M. Living Legends in Sleep Research Tripping on the edge of consciousness. SLEEP Adv. 2023, 4, zpad039. [Google Scholar] [CrossRef]
- Tekeoglu, I.; Ediz, L.; Hiz, O.; Toprak, M.; Yazmalar, L.; Karaaslan, G. The relationship between shoulder impingement syndrome and sleep quality. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 370–374. [Google Scholar]
- Litaker, D.; Pioro, M.; Bilbeisi HEl Brems, J. Returning to the bedside: Using the history and physical examination to identify rotator cuff tears. J. Am. Geriatr. Soc. 2000, 48, 1633–1637. [Google Scholar] [CrossRef]
- Cho, C.H.; Jung, S.W.; Park, J.Y.; Song, K.S.; Yu, K.I. Is shoulder pain for three months or longer correlated with depression, anxiety, and sleep disturbance? J. Shoulder Elb. Surg. 2013, 22, 222–228. [Google Scholar] [CrossRef]
- Wesensten, N.J. Legitimacy of concerns about caffeine and energy drink consumption. Nutr. Rev. 2014, 72, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, S.; Daria, P.; Sani, G.; Aromatario, M. Caffeine: Cognitive and Physical Performance Enhancer or Psychoactive Drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Laaboub, N.; Gholam, M.; Sibailly, G.; Sjaarda, J.; Delacrétaz, A.; Dubath, C.; Grosu, C.; Piras, M.; Ansermot, N.; Crettol, S.; et al. Associations Between High Plasma Methylxanthine Levels, Sleep Disorders and Polygenic Risk Scores of Caffeine Consumption or Sleep Duration in a Swiss Psychiatric Cohort. Front. Psychiatry 2021, 12, 756403. [Google Scholar] [CrossRef]
- Takahashi, J.S. Molecular architecture of the circadian clock in mammals. In A Time for Metabolism and Hormones; Sassone-Corsi, P., Christen, Y., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [PubMed]
- Ohta, H.; Yamazaki, S.; McMahon, D.G. Constant light desynchronizes mammalian clock neurons. Nat. Neurosci. 2005, 8, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Pazienza, V.; Vinciguerra, M. Clock genes and clock-controlled genes in the regulation of metabolic rhythms. Chronobiol. Int. 2012, 29, 227–251. [Google Scholar] [CrossRef]
- Randjelović, P.; Stojanović, N.; Ilić, I.; Vučković, D. The effect of reducing blue light from smartphone screen on subjective quality of sleep among students. Chronobiol. Int. 2023, 40, 335–342. [Google Scholar] [CrossRef]
- Kirker, K.; O’Connell, M.; Bradley, L.; Torres-Panchame, R.E.; Masaracchio, M. Manual therapy and exercise for adhesive capsulitis: A systematic review with meta-analysis. J. Man. Manip. Ther. 2023, 31, 311–327. [Google Scholar] [CrossRef]
- Mertens, M.G.; Meert, L.; Struyf, F.; Schwank, A.; Meeus, M. Exercise Therapy Is Effective for Improvement in Range of Motion, Function, and Pain in Patients with Frozen Shoulder: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2022, 103, 998–1012.e14. [Google Scholar] [CrossRef]
- Kelley, M.J.; Mcclure, P.W.; Leggin, B.G. Frozen shoulder: Evidence and a proposed model guiding rehabilitation. J. Orthop. Sports Phys. Ther. 2009, 39, 135–148. [Google Scholar] [CrossRef]
- Navarro-Ledesma, S.; Hamed-Hamed, D.; Gonzalez-Muñoz, A.; Pruimboom, L. Impact of physical therapy techniques and common interventions on sleep quality in patients with chronic pain: A systematic review. Sleep Med. Rev. 2024, 76, 101937. [Google Scholar] [CrossRef] [PubMed]
- Butcher, N.J.; Monsour, A.; Mew, E.J.; Chan, A.W.; Moher, D.; Mayo-Wilson, E.; Terwee, C.B.; Chee-A-Tow, A.; Baba, A.; Gavin, F.; et al. Guidelines for Reporting Outcomes in Trial Reports: The CONSORT-Outcomes 2022 Extension. JAMA 2022, 328, 2252–2264. [Google Scholar] [CrossRef] [PubMed]
- Hanchard, N.C.A.; Goodchild, L.; Thompson, J.; O’Brien, T.; Davison, D.; Richardson, C. Evidence-based clinical guidelines for the diagnosis, assessment and physiotherapy management of contracted (frozen) shoulder: Quick reference summary. Physiotherapy 2012, 98, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Noten, S.; Meeus, M.; Stassijns, G.; Van Glabbeek, F.; Verborgt, O.; Struyf, F. Efficacy of Different Types of Mobilization Techniques in Patients with Primary Adhesive Capsulitis of the Shoulder: A Systematic Review. Arch. Phys. Med. Rehabil. 2016, 97, 815–825. [Google Scholar] [CrossRef]
- Lin, P.; Yang, M.; Huang, D.; Lin, H.; Wang, J.; Zhong, C.; Guan, L. Effect of proprioceptive neuromuscular facilitation technique on the treatment of frozen shoulder: A pilot randomized controlled trial. BMC Musculoskelet. Disord. 2022, 23, 367. [Google Scholar] [CrossRef]
- Kelley, M.J.; Shaffer, M.A.; Kuhn, J.E.; Michener, L.A.; Seitz, A.L.; Uhl, T.L.; Godges, J.J.; McClure, P.W. Shoulder pain and mobility deficits: Adhesive capsulitis. J. Orthop. Sports Phys. Ther. 2013, 43, A1–A31. [Google Scholar] [CrossRef]
- Chaudhary, N.S.; Taylor, B.V.; Grandner, M.A.; Troxel, W.M.; Chakravorty, S. The effects of caffeinated products on sleep and functioning in the military population: A focused review. Pharmacol. Biochem. Behav. 2021, 206, 173206. [Google Scholar] [CrossRef]
- Drake, C.; Roehrs, T.; Shambroom, J.; Roth, T. Caffeine effects on sleep taken 0, 3, or 6 hours before going to bed. J. Clin. Sleep Med. 2013, 9, 1195–1200. [Google Scholar] [CrossRef]
- Gardiner, C.; Weakley, J.; Burke, L.M.; Roach, G.D.; Sargent, C.; Maniar, N.; Townshend, A.; Halson, S.L. The effect of caffeine on subsequent sleep: A systematic review and meta-analysis. Sleep Med. Rev. 2023, 69, 101764. [Google Scholar] [CrossRef]
- Abdelmissih, S.; Hosny, S.A.; Elwi, H.M.; Sayed, W.M.; Eshra, M.A.; Shaker, O.G.; Samir, N.F. Chronic Caffeine Consumption, Alone or Combined with Agomelatine or Quetiapine, Reduces the Maximum EEG Peak, as Linked to Cortical Neurodegeneration, Ovarian Estrogen Receptor Alpha, and Melatonin Receptor 2. Psychopharmacology 2024, 241, 2073–2101. [Google Scholar] [CrossRef]
- Härtter, S.; Korhonen, T.; Lundgren, S.; Rane, A.; Tolonen, A.; Turpeinen, M.; Laine, K. Effect of caffeine intake 12 or 24 hours prior to melatonin intake and CYP1A2*1F polymorphism on CYP1A2 phenotyping by melatonin. Basic Clin. Pharmacol. Toxicol. 2006, 99, 300–304. [Google Scholar] [CrossRef] [PubMed]
- JeongBin, P.; JiWon, H.; JuRi, L.; SeonJeong, B.; SeungWan, S.; Tae, K.; InYoung, Y.; KiWoong, K. Lifetime Coffee Consumption, Pineal Gland Volume, and Sleep Quality in Late Life. Sleep 2018, 41, zsy127. [Google Scholar]
- Tout, A.F.; Tang, N.K.Y.; Sletten, T.L.; Toro, C.T.; Kershaw, C.; Meyer, C.; Rajaratnam, S.M.W.; Moukhtarian, T.R. Current sleep interventions for shift workers: A mini review to shape a new preventative, multicomponent sleep management programme. Front. Sleep 2024, 3, 1343393. [Google Scholar] [CrossRef]
- Redeker, N.S.; Caruso, C.C.; Hashmi, S.D.; Mullington, J.M.; Grandner, M.; Morgenthaler, T.I. Workplace interventions to promote sleep health and an alert, healthy workforce. J. Clin. Sleep Med. 2019, 15, 649–657. [Google Scholar] [CrossRef]
- Roach, K.B.M.E.S.N.L.Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991, 4, 143–149. [Google Scholar] [CrossRef]
- Membrilla-Mesa, M.D.; Cuesta-Vargas, A.I.; Pozuelo-Calvo, R.; Tejero-Fernández, V.; Martín-Martín, L.; Arroyo-Morales, M. Shoulder pain and disability index: Cross cultural validation and evaluation of psychometric properties of the Spanish version. Health Qual. Life Outcomes 2015, 13, 200. [Google Scholar] [CrossRef]
- National, S.; Network, B.; Red Nacional de Biobancos. May 2011. Available online: www.redbiobancos.es (accessed on 1 March 2024).
- Buysse Charles FReynolds Ill, D.J.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–195. [Google Scholar] [CrossRef]
- Royuela, A.M.J. Propiedades clinimetricas de la versión castellana del cuestionario de Pittsburgh. Vigilia-Sueño 1997, 9, 81–94. Available online: https://www.researchgate.net/publication/258705863 (accessed on 26 May 2025).
- Tveitå, E.K.; Ekeberg, O.M.; Juel, N.G.; Bautz-Holter, E. Range of shoulder motion in patients with adhesive capsulitis; Intra-tester reproducibility is acceptable for group comparisons. BMC Musculoskelet. Disord. 2008, 9, 49. [Google Scholar] [CrossRef]
- Tveitå, E.K.; Ekeberg, O.M.; Juel, N.G.; Bautz-Holter, E. Responsiveness of the Shoulder Pain and Disability Index in patients with adhesive capsulitis. BMC Musculoskelet. Disord. 2008, 9, 161. [Google Scholar] [CrossRef]
- Navarro-Ledesma, S. Frozen Shoulder as a Metabolic and Immune Disorder: Potential Roles of Leptin Resistance, JAK-STAT Dysregulation, and Fibrosis. J. Clin. Med. 2025, 14, 1780. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.T.H.; Borst, R.; Gacaferi, H.; Davidson, S.; Ackerman, J.E.; Johnson, P.A.; Machado, C.C.; Reekie, I.; Attar, M.; Windell, D.; et al. A single cell atlas of frozen shoulder capsule identifies features associated with inflammatory fibrosis resolution. Nat. Commun. 2024, 15, 1394. [Google Scholar] [CrossRef]
- Akbar, M.; Crowe, L.A.N.; Mclean, M.; Garcia-Melchor, E.; Macdonald, L.; Carter, K.; Fazzi, U.G.; Martin, D.; Arthur, A.; Reilly, J.H.; et al. Translational targeting of inflammation and fibrosis in frozen shoulder: Molecular dissection of the T cell/ IL-17A axis. Proc. Natl. Acad. Sci. USA 2021, 118, e2102715118. [Google Scholar] [CrossRef]
- da Silva, M.D.; Bobinski, F.; Sato, K.L.; Kolker, S.J.; Sluka, K.A.; Santos, A.R.S. IL-10 Cytokine Released from M2 Macrophages Is Crucial for Analgesic and Anti-inflammatory Effects of Acupuncture in a Model of Inflammatory Muscle Pain. Mol. Neurobiol. 2015, 51, 19–31. [Google Scholar] [CrossRef]
- Miki, S.; Suzuki Jichiro Takashima, M.; Ishida, M.; Kokubo, H.; Yoshizumi, M. S-1-Propenylcysteine promotes IL-10-induced M2c macrophage polarization through prolonged activation of IL-10R/STAT3 signaling. Sci. Rep. 2021, 11, 22469. [Google Scholar] [CrossRef] [PubMed]
- Mertens, M.G.; Struyf, F.; Verborgt, O.; Dueñas, L.; Balasch-Bernat, M.; Navarro-Ledesma, S.; Fernandez-Sanchez, M.; Luque-Suarez, A.; Girbes, E.L.; Meeus, M. Exploration of the clinical course and longitudinal correlations in frozen shoulder: The role of autonomic function, central pain processing, and psychological variables. A longitudinal multicenter prospective observational study. Musculoskelet. Sci. Pract. 2023, 67, 102857. [Google Scholar] [CrossRef] [PubMed]
- Mertens, M.G.; Meeus, M.; Verborgt, O.; Girbes, E.L.; Horno, S.M.D.; Aguilar-Rodriguez, M.; Dueñas, L.; Navarro-Ledesma, S.; Fernandez-Sanchez, M.; Luque-Suarez, A.; et al. Exploration of the clinical course of frozen shoulder: A longitudinal multicenter prospective study of functional impairments. Braz. J. Phys. Ther. 2023, 27, 100539. [Google Scholar] [CrossRef]
- Mertens, M.G.C.A.M.; Meeus, M.; Noten, S.; Verborgt, O.; Fransen, E.; Girbés, E.L.; Rodríguez, M.A.; Navarro-Ledesma, S.; Fernandez-Sanchez, M.; Luque-Suarez, A.; et al. Understanding the clinical profile of patients with frozen shoulder: A longitudinal multicentre observational study. BMJ Open 2022, 12, e056563. [Google Scholar] [CrossRef]
- Zeng, Y.; Guo, Z.; Wu, M.; Chen, F.; Chen, L. Circadian rhythm regulates the function of immune cells and participates in the development of tumors. Cell Death Discov. 2024, 10, 199. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef]
- Luo, M.; Zhao, F.; Cheng, H.; Su, M.; Wang, Y. Macrophage polarization: An important role in inflammatory diseases. Front. Immunol. 2024, 15, 1352946. [Google Scholar] [CrossRef] [PubMed]
- Carlini, V.; Noonan, D.M.; Abdalalem, E.; Goletti, D.; Sansone, C.; Calabrone, L.; Albini, A. The multifaceted nature of IL-10: Regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 2023, 14, 1161067. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef] [PubMed]
- Ballesio, A. Inflammatory hypotheses of sleep disturbance—Depression link: Update and research agenda. Brain Behav. Immun. Health 2023, 31, 100647. [Google Scholar] [CrossRef]
- Taraz, M.; Khatami, M.R.; Hajiseyedjavadi, M.; Farrokhian, A.; Amini, M.; Khalili, H.; Abdollahi, A.; Dashti-Khavidaki, S. Association between antiinflammatory cytokine, IL-10, and sleep quality in patients on maintenance hemodialysis. Hemodial. Int. 2013, 17, 382–390. [Google Scholar] [CrossRef]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef]
- Shivshankar, P.; Fekry, B.; Eckel-Mahan, K.; Wetsel, R.A. Circadian Clock and Complement Immune System—Complementary Control of Physiology and Pathology? Front. Cell. Infect. Microbiol. 2020, 10, 418. [Google Scholar] [CrossRef]
- Zhang, J.J.; Rizk, R.; Li, X.; Lee, B.G.; Matthies, M.L.; Bietz, K.A.; Kim, K.; Huard, J.; Wang, Y.; Chen, W.C.W. Interleukin-10 exhibit dose-dependent effects on macrophage phenotypes and cardiac remodeling after myocardial infarction. Front. Physiol. 2024, 15, 1481460. [Google Scholar] [CrossRef]
- Steen, E.H.; Wang, X.; Balaji, S.; Butte, M.J.; Bollyky, P.L.; Keswani, S.G. The Role of the Anti-Inflammatory Cytokine Interleukin-10 in Tissue Fibrosis. Adv. Wound Care 2020, 9, 184–198. [Google Scholar] [CrossRef]
- York, A.G.; Skadow, M.H.; Oh, J.; Qu, R.; Zhou, Q.D.; Hsieh, W.Y.; Mowel, W.K.; Brewer, J.R.; Kaffe, E.; Williams, K.J.; et al. IL-10 constrains sphingolipid metabolism to limit inflammation. Nature 2024, 627, 628–635. [Google Scholar] [CrossRef]
- Parisi, L.; Gini, E.; Baci, D.; Tremolati, M.; Fanuli, M.; Bassani, B.; Farronato, G.; Bruno, A.; Mortara, L. Macrophage Polarization in Chronic Inflammatory Diseases: Killers or Builders? J. Immunol. Res. 2018, 2018, 8917804. [Google Scholar] [CrossRef] [PubMed]
- Subramanian Iyer, S.; Cheng, G. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit. Rev. Immunol. 2012, 32, 23. [Google Scholar] [CrossRef]
- Timmons, G.A.; O’Siorain, J.R.; Kennedy, O.D.; Curtis, A.M.; Early, J.O. Innate Rhythms: Clocks at the Center of Monocyte and Macrophage Function. Front. Immunol. 2020, 11, 1743. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Fentress, S.J.; Qiu, Y.; Yun, K.; Cox, J.S.; Chawla, A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6Chi inflammatory monocytes. Science 2013, 341, 1483–1488. [Google Scholar] [CrossRef]
- Keller, M.; Mazuch, J.; Abraham, U.; Eom, G.D.; Herzog, E.D.; Volk, H.D.; Kramer, A.; Maier, B. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. USA 2009, 106, 21407–21412. [Google Scholar] [CrossRef]
- Zielinski, M.R.; McKenna, J.T.; McCarley, R.W. Functions and mechanisms of sleep. AIMS Neurosci. 2016, 3, 67–104. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Opp, M.R. Sleep Health: Reciprocal Regulation of Sleep and Innate Immunity. Neuropsychopharmacology 2017, 42, 129–155. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and immune function. Pflugers Arch. 2012, 463, 121–137. [Google Scholar] [CrossRef]
- Shirato, K.; Sato, S. Macrophage Meets the Circadian Clock: Implication of the Circadian Clock in the Role of Macrophages in Acute Lower Respiratory Tract Infection. Front. Cell. Infect. Microbiol. 2022, 12, 826738. [Google Scholar] [CrossRef]
- Moratalla, R. Neurobiología de las metilxantinas. Trastor. Adict. 2008, 10, 201–208. [Google Scholar] [CrossRef]
- Addicott, M.A. Caffeine Use Disorder: A Review of the Evidence and Future Implications. Curr. Addict. Rep. 2014, 1, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Banks, N.F.; Tomko, P.M.; Colquhoun, R.J.; Muddle, T.W.D.; Emerson, S.R.; Jenkins, N.D.M. Genetic Polymorphisms in ADORA2A and CYP1A2 Influence Caffeine’s Effect on Postprandial Glycaemia. Sci. Rep. 2019, 9, 10532. [Google Scholar] [CrossRef]
- Windred, D.P.; Burns, A.C.; Lane, J.M.; Olivier, P.; Rutter, M.K.; Saxena, R.; Phillips, A.J.; Cain, S.W. Brighter nights and darker days predict higher mortality risk: A prospective analysis of personal light exposure in >88,000 individuals. Proc. Natl. Acad. Sci. USA 2024, 121, e2405924121. [Google Scholar] [CrossRef]
- Garcia-Saenz, A.; Sánchez de Miguel, A.; Espinosa, A.; Costas, L.; Aragonés, N.; Tonne, C.; Moreno, V.; Pérez-Gómez, B.; Valentin, A.; Pollán, M.; et al. Association Between Outdoor Light-at-night Exposure and Colorectal Cancer in Spain. Epidemiology 2020, 31, 718–727. [Google Scholar] [CrossRef]
- Blume, C.; Garbazza, C.; Spitschan, M. Effects of light on human circadian rhythms, sleep and mood. Somnologie 2019, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Gooley, J.J.; Chamberlain, K.; Smith, K.A.; Khalsa, S.B.S.; Rajaratnam, S.M.W.; Van Reen, E.; Zeitzer, J.M.; Czeisler, C.A.; Lockley, S.W. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. Metab. 2011, 96, E463–E472. [Google Scholar] [CrossRef]
- Satpute, K.; Reid, S.; Mitchell, T.; Mackay, G.; Hall, T. Efficacy of mobilization with movement (MWM) for shoulder conditions: A systematic review and meta-analysis. J. Man. Manip. Ther. 2022, 30, 13–32. [Google Scholar] [CrossRef]
- Pérez-Montilla, J.J.; Guzmán-García, R.; Pruimboom, L.; Navarro-Ledesma, S. Does Leptin and Insulin Levels Influence Pain and Disability in Subjects with Frozen Shoulder? A Cross-Sectional Study. Eur. J. Pain 2025, 29, e70007. [Google Scholar] [CrossRef]
| Experimental Group (n = 17) | Control Group (n = 17) | Shapiro–Wilk | |
|---|---|---|---|
| Count/Mean-SD | Count/Mean-SD | p | |
| Age | 50.2–10.2 | 52.1–12.5 | 0.373 |
| Weight (kg) | 69.4–10.8 | 67.8–12.0 | 0.308 |
| Height (cm) | 171–4.69 | 169–6.11 | 0.612 |
| BMI | 23.8–3.00 | 23.9–4.10 | 0.093 |
| Sex | |||
| Female | 14 | 11 | |
| Male | 3 | 6 | |
| Comorbidities | |||
| Diabetes type 2 | 4 | 2 | |
| Hypertension | 5 | 0 | |
| Chondromalacia patellae | 3 | 0 | |
| Hypothyroidism | 1 | 3 | |
| Obstructive sleep apnea | 0 | 2 |
| Experimental Group (n = 17) | Control Group (n = 17) | ||
|---|---|---|---|
| Mean/Media–SD/IQR | Mean/Median–SD/IQR | p | |
| Basal insulin (mU/L) | 18.00-10.90 | 12.00–11.90 | 0.026 *–0.163 † |
| HOMA | 4.840–3.440 | 2.370–2.470 | 0.002 *–0.148 † |
| IL-1 (pg/mL) | 8.300–3.600 | 5.300–2.150 | 0.001 *–0.076 † |
| IL-6 (pg/mL) | 4.070–3.740 | 1.960–1.830 | <0.001 *–0.048 † |
| IL-10 (pg/mL) | 1.170–1.030 | 1.140–0.700 | <0.001 *–0.469 † |
| IL-17 (pg/mL) | 1.210–1.030 | 1.200–0.440 | <0.001 *–0.654 † |
| IL-33 (pg/mL) | 123.0–124.0 | 23.40–114.0 | <0.001 *–0.278 † |
| TNF (pg/mL) | 15.43–4.795 | 14.14–3.777 | 0.353 *–0.392 |
| HMBG1 (ug/L) | 1.100–0.850 | 0.830–0.780 | 0.016 *–0.158 † |
| Leptin (ng/mL) | 8.300–8.070 | 6.300–6.000 | <0.001 *–0.134 † |
| Glucose (mg/dL) | 108.82–16.353 | 100.0–23.06 | 0.107 *–0.207 |
| Triglycerides (mg/dL) | 132.35–50.241 | 122.7–48.11 | 0.081 *–0.574 |
| Total cholesterol (mg/dL) | 231.2–31.40 | 233.4–29.00 | 0.028 *–0.428 † |
| HDL cholesterol (mg/dL) | 51.76–12.770 | 57.84–15.40 | 0.139 *–0.219 |
| LDL cholesterol (mg/dL) | 156.59–26.686 | 155.8–27.68 | 0.310 *–0.939 |
| Uric acid (mg/dL) | 4.400–2.700 | 4.300–1.700 | 0.035 *–0.904 † |
| C-reactive protein (mg/L) | 5.500–2.800 | 4.100–2.680 | <0.001 *–0.148 † |
| Total PITT | 7.000–6.00 | 7.000–4.00 | 0.021 *–0.808 † |
| SPADI total pain | 48.50–8.590 | 47.76–8.930 | 0.050 *–0.816 |
| SPADI total disc | 68.10–11.70 | 64.70–13.50 | 0.526 *–0.445 |
| ROM FLEX | 130–35.0 | 100–60.0 | 0.024 *–0.214 † |
| ROM EXT | 40.0–15.0 | 40.0–20.0 | 0.010*–0.333† |
| ROM ABD | 122–34.2 | 100–30.1 | 0.105*–0.061 |
| ROM ADD | 20.0–5.00 | 20.0–15.0 | 0.001 *–0.703 † |
| ROM EXT ROT | 50.0–25.0 | 50.0–15.0 | 0.004 *–0.889 † |
| ROM INTER ROT | 50.0–15.0 | 50.0–15.0 | 0.005 *–0.661 † |
| Experimental Group (n = 17) | Control Group (n = 17) | |||||||
|---|---|---|---|---|---|---|---|---|
| p | Mean Differences | 95% CI | Cohen’s D Test | p | Mean Differences | 95% CI | Cohen’s D Test | |
| Basal insulin (mU/L) | <0.001 | 2.9471 | 1.5139/4.380 | 1.0572 | 0.105 | 2.4394 | −0.5698/5.449 | 0.4168 |
| HOMA | <0.001 | 1.1459 | 0.5808/1.711 | 1.0427 | 0.149 | 0.8171 | −0.3252/1.959 | 0.3678 |
| IL-1 (pg/mL) | 0.003 | 2.4700 | 0.9599/3.980 | 0.8410 | 0.126 | 0.7224 | −0.2261/1.671 | 0.3916 |
| IL-6 (pg/mL) | <0.001 | 1.5735 | 0.8628/2.284 | 1.1383 | 0.078 | 0.5688 | −0.0713/1.209 | 0.4569 |
| IL-10 (pg/mL) | 0.117 | −0.5053 | −1.1526/0.142 | −0.4013 | 0.740 | −0.0406 | −0.2950/0.214 | −0.0820 |
| IL-17 (pg/mL) | 0.224 | 0.1682 | −0.1139/0.450 | 0.3066 | 0.217 | 0.1300 | −0.0846/0.345 | 0.3114 |
| IL-33 (pg/mL) | 0.080 | 14.4794 | −1.9350/30.894 | 0.4535 | 0.198 | 13.9294 | −8.0787/35.938 | 0.3254 |
| TNF (pg/mL) | 0.003 | 2.4700 | 0.9526/3.987 | 0.8369 | 0.174 | 1.1647 | −0.5687/2.898 | 0.3455 |
| HMBG1 (ug/L) | 0.105 | 0.1735 | −0.0403/0.387 | 0.4173 | 0.340 | 0.1400 | −0.1615/0.442 | 0.2387 |
| Leptin (ng/mL) | <0.001 | 2.4894 | 1.4746/3.504 | 1.2612 | 0.273 | 1.1194 | −0.9707/3.210 | 0.2754 |
| Glucose (mg/dL) | 0.009 | 6.2941 | 1.8201/10.768 | 0.7233 | 0.884 | 0.4706 | −6.2310/7.172 | 0.0361 |
| Triglycerides (mg/dL) | 0.256 | 4.6471 | −3.7147/13.009 | 0.2857 | 0.955 | 0.2941 | −10.6908/11.279 | 0.0138 |
| Total cholesterol (mg/dL) | 0.597 | 1.7176 | −5.0352/8.470 | 0.1308 | 0.217 | 5.4306 | −3.5289/14.390 | 0.3116 |
| HDL cholesterol (mg/dL) | 0.007 | −3.8588 | −6.5237/−1.194 | −0.7445 | 0.869 | 0.3353 | −3.8949/4.565 | 0.0408 |
| LDL cholesterol (mg/dL) | 0.144 | 4.2335 | −1.6016/10.069 | 0.3730 | 0.276 | 4.7353 | −4.1745/13.645 | 0.2733 |
| Uric acid (mg/dL) | 0.954 | 0.0153 | −0.5390/0.570 | 0.0142 | 0.906 | 0.0388 | −0.6487/0.726 | 0.0290 |
| C-reactive protein (mg/L) | <0.001 | 2.0118 | 0.9554/3.068 | 0.9792 | 0.023 | 1.3335 | 0.2068/2.460 | 0.6085 |
| Total PITT | 0.006 | 1.7647 | 0.5933/2.936 | 0.7745 | 0.332 | −0.1765 | −0.5506/0.198 | −0.2425 |
| SPADI total pain | <0.001 | 8.5294 | 6.9023/10.156 | 2.6953 | <0.001 | 5.7059 | 4.1876/7.224 | 1.9322 |
| SPADI total disc | <0.001 | 16.5294 | 14.0561/19.003 | 3.4362 | <0.001 | 13.2941 | 10.8910/15.697 | 2.8443 |
| ROM FLEX | <0.001 | −12.3529 | −15.2440/−9.462 | −2.1969 | <0.001 | −4.4118 | −6.4201/−2.403 | −1.1295 |
| ROM EXT | <0.001 | −6.7647 | −9.7711/−3.758 | −1.1569 | 0.005 | −3.8235 | −6.3175/−1.330 | −0.7882 |
| ROM ABD | 0.505 | −4.4118 | −18.1323/9.309 | −0.1653 | <0.001 | −5.0000 | −6.5743/−3.426 | −1.6330 |
| ROM ADD | <0.001 | −4.1176 | −5.9882/−2.247 | −1.1318 | <0.001 | −4.4118 | −6.2026/−2.621 | −1.2666 |
| ROM EXT ROT | <0.001 | −10.2941 | −13.7516/−6.837 | −1.5308 | 0.020 | −7.9412 | −14.4358/−1.447 | −0.6287 |
| ROM INTER ROT | <0.001 | −9.4118 | −12.9181/−5.905 | −1.3801 | <0.001 | −4.4118 | −6.4201/−2.403 | −1.1295 |
| p | Mean Differences | Cohen’s D Test | |
|---|---|---|---|
| Basal insulin (mU/L) | 0.174 † | −2.71588 | −0.39709 |
| HOMA | 0.099 † | −0.61588 | −0.27536 |
| IL-1 (pg/mL) | 0.163 † | −0.65824 | −0.20035 |
| IL-6 (pg/mL) | 0.058 † | −0.70706 | −0.39293 |
| IL-10 (pg/mL) | <0.001 † | −0.88353 | −1.36375 |
| IL-17 (pg/mL) | 0.796 † | 0.05941 | 0.07385 |
| IL-33 (pg/mL) | 0.168 † | −33.48706 | −0.61773 |
| TNF (pg/mL) | 0.985 | 0.02118 | 0.00664 |
| HMBG1 (ug/L) | 0.262 † | −0.11765 | −0.27389 |
| Leptin (ng/mL) | 0.196 † | −0.93000 | −0.11987 |
| Glucose (mg/dL) | 0.550 | −3.00000 | −0.20717 |
| Triglycerides (mg/dL) | 0.741 | −5.23529 | −0.11432 |
| Total cholesterol (mg/dL) | 0.931 † | −0.74824 | −0.02893 |
| HDL cholesterol (mg/dL) | 0.645 | 1.88824 | 0.15954 |
| LDL cholesterol (mg/dL) | 0.881 | −1.21941 | −0.05179 |
| Uric acid (mg/dL) | 0.945 † | −0.10941 | −0.09972 |
| C-reactive protein (mg/L) | 0.945 † | −0.00294 | −0.00137 |
| Total PITT | 0.376 † | 1.52941 | 0.40731 |
| SPADI total pain | 0.518 | 2.11765 | 0.22432 |
| SPADI total disc | 0.980 | −0.11765 | −0.00868 |
| ROM FLEX | 0.060 † | −22.64706 | −0.68478 |
| ROM EXT | 0.114 † | −7.35294 | −0.54853 |
| ROM ABD | 0.063 | −20.88235 | −0.66191 |
| ROM ADD | 0.883 † | −0.29412 | −0.05092 |
| ROM EXT ROT | 0.414 † | −2.64706 | −0.23825 |
| ROM INTER ROT | 0.462 † | −3.52941 | −0.31838 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-García, R.; Pérez-Montalbán, M.; Pruimboom, L.; Navarro-Ledesma, S. Impact of a Multimodal Intervention Combining Manual Therapy, Exercise, Reduced Methylxanthine Intake, and Nocturnal Light Avoidance on Inflammatory and Metabolic Profiles, Pain, Functionality, and Sleep Quality in Patients with Frozen Shoulder: A Single-Blind Randomized Controlled Trial. J. Clin. Med. 2025, 14, 4539. https://doi.org/10.3390/jcm14134539
Guzmán-García R, Pérez-Montalbán M, Pruimboom L, Navarro-Ledesma S. Impact of a Multimodal Intervention Combining Manual Therapy, Exercise, Reduced Methylxanthine Intake, and Nocturnal Light Avoidance on Inflammatory and Metabolic Profiles, Pain, Functionality, and Sleep Quality in Patients with Frozen Shoulder: A Single-Blind Randomized Controlled Trial. Journal of Clinical Medicine. 2025; 14(13):4539. https://doi.org/10.3390/jcm14134539
Chicago/Turabian StyleGuzmán-García, Rafael, María Pérez-Montalbán, Leo Pruimboom, and Santiago Navarro-Ledesma. 2025. "Impact of a Multimodal Intervention Combining Manual Therapy, Exercise, Reduced Methylxanthine Intake, and Nocturnal Light Avoidance on Inflammatory and Metabolic Profiles, Pain, Functionality, and Sleep Quality in Patients with Frozen Shoulder: A Single-Blind Randomized Controlled Trial" Journal of Clinical Medicine 14, no. 13: 4539. https://doi.org/10.3390/jcm14134539
APA StyleGuzmán-García, R., Pérez-Montalbán, M., Pruimboom, L., & Navarro-Ledesma, S. (2025). Impact of a Multimodal Intervention Combining Manual Therapy, Exercise, Reduced Methylxanthine Intake, and Nocturnal Light Avoidance on Inflammatory and Metabolic Profiles, Pain, Functionality, and Sleep Quality in Patients with Frozen Shoulder: A Single-Blind Randomized Controlled Trial. Journal of Clinical Medicine, 14(13), 4539. https://doi.org/10.3390/jcm14134539









