Mortality Risk Factors and Survival Outcomes in Infants with Persistent Pulmonary Hypertension of the Newborn
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Population
2.2. Data Collection
2.3. Definitions
2.4. Management for PPHN
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bendapudi, P.; Rao, G.G.; Greenough, A. Diagnosis and management of persistent pulmonary hypertension of the newborn. Paediatr. Respir. Rev. 2015, 16, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Steinhorn, R.H. Advances in neonatal pulmonary hypertension. Neonatology 2016, 109, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Walsh-Sukys, M.C.; Tyson, J.E.; Wright, L.L.; Bauer, C.R.; Korones, S.B.; Stevenson, D.K.; Verter, J.; Stoll, B.J.; Lemons, J.A.; Papile, L.A.; et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: Practice variation and outcomes. Pediatrics 2000, 105, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Konduri, G.G.; Kim, U.O. Advances in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatr. Clin. N. Am. 2009, 56, 579–600. [Google Scholar] [CrossRef]
- Lakshminrusimha, S.; Keszler, M. Persistent pulmonary hypertension of the newborn. Neoreviews 2015, 16, e680–e692. [Google Scholar] [CrossRef]
- Steurer, M.A.; Jelliffe-Pawlowski, L.L.; Baer, R.J.; Partridge, J.C.; Rogers, E.E.; Keller, R.L. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics 2017, 139, e20161165. [Google Scholar] [CrossRef]
- Sharma, V.; Berkelhamer, S.; Lakshminrusimha, S. Persistent pulmonary hypertension of the newborn. Matern. Health Neonatol. Perinatol. 2015, 1, 14. [Google Scholar] [CrossRef]
- Thatrimontrichai, A. Evidence-based neonatal care. J. Health Sci. Med. Res. 2019, 37, 163–169. [Google Scholar] [CrossRef]
- Walsh, M.C.; Stork, E.K. Persistent pulmonary hypertension of the newborn. Rational therapy based on pathophysiology. Clin. Perinatol. 2001, 28, 609–627. [Google Scholar] [CrossRef]
- Roofthooft, M.T.; Elema, A.; Bergman, K.A.; Berger, R.M. Patient characteristics in persistent pulmonary hypertension of the newborn. Pulm. Med. 2011, 2011, 858154. [Google Scholar] [CrossRef]
- Nakwan, N.; Jain, S.; Kumar, K.; Hosono, S.; Hammoud, M.; Elsayed, Y.Y.; Ariff, S.; Hasan, B.; Khowaja, W.; Poon, W.B. An Asian multicenter retrospective study on persistent pulmonary hypertension of the newborn: Incidence, etiology, diagnosis, treatment and outcome. J. Matern. Fetal Neonatal Med. 2020, 33, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Chuaikaew, K.; Maneenil, G.; Thatrimontrichai, A.; Janjindamai, W.; Dissaneevate, S. Survival of Infants with Persistent Pulmonary Hypertension of The Newborn: 12-year Experience. In Proceedings of the 9th Congress of the European Academy of Pediatric Societies (EAPS 2022) Conference, Barcelona, Spain, 7–11 October 2022. [Google Scholar]
- Maneenil, G.; Premprat, N.; Janjindamai, W.; Dissaneevate, S.; Phatigomet, M.; Thatrimontrichai, A. Correlation and prediction of oxygen index from oxygen saturation index in neonates with acute respiratory failure. Am. J. Perinatol. 2024, 41, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Musick, M.A.; Loftis, L.L.; Kennedy, C.E. Comparing vasoactive-inotropic score reporting strategies in the PICU relative to mortality risk. Pediatr. Crit. Care Med. 2018, 19, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.K.; Corcoran, J.D.; Escobar, G.J.; Lee, S.K. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J. Pediatr. 2001, 138, 92–100. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N. KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef]
- Maneenil, G.; Thatrimontrichai, A.; Janjindamai, W.; Dissaneevate, S. Effect of bosentan therapy in persistent pulmonary hypertension of the newborn. Pediatr. Neonatol. 2018, 59, 58–64. [Google Scholar] [CrossRef]
- Pakhathirathien, P.; Maneenil, G.; Thatrimontrichai, A.; Dissaneevate, S.; Praditaukrit, M. Mortality prediction in newborns with persistent pulmonary hypertension: A comparison of four illness severity scores. Pediatr. Pulmonol. 2025, 60, e27484. [Google Scholar] [CrossRef]
- Steurer, M.A.; Baer, R.J.; Oltman, S.; Ryckman, K.K.; Feuer, S.K.; Rogers, E.; Keller, R.L.; Jelliffe-Pawlowski, L.L. Morbidity of persistent pulmonary hypertension of the newborn in the first year of life. J. Pediatr. 2019, 213, 58–65. [Google Scholar] [CrossRef]
- Rozance, P.J.; Seedorf, G.J.; Brown, A.; Roe, G.; O’Meara, M.G.; Gien, J.; Tang, J.R.; Abman, S.H. Intrauterine growth restriction decreases pulmonary alveolar and vessel growth and causes pulmonary artery endothelial cell dysfunction in vitro in fetal sheep. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 301, L860–L871. [Google Scholar] [CrossRef]
- Kim, Y.J.; Shin, S.H.; Park, H.W.; Kim, E.K.; Kim, H.S. Risk factors of early pulmonary hypertension and its clinical outcomes in preterm infants: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 14186. [Google Scholar] [CrossRef]
- Kim, H.H.; Sung, S.I.; Yang, M.S.; Han, Y.S.; Kim, H.S.; Ahn, S.Y.; Jeon, G.W.; Chang, Y.S.; Park, W.S. Early pulmonary hypertension is a risk factor for bronchopulmonary dysplasia-associated late pulmonary hypertension in extremely preterm infants. Sci. Rep. 2021, 11, 11206. [Google Scholar] [CrossRef] [PubMed]
- Jetton, J.G.; Boohaker, L.J.; Sethi, S.K.; Wazir, S.; Rohatgi, S.; Soranno, D.E.; Chishti, S.S.; Woroniecki, R.; Mammen, C.; Swanson, J.R. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): A multicentre, multinational, observational cohort study. Lancet Child. Adolesc. Health 2017, 1, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Ustun, N.; Ovali, F. Risk factors and outcomes of acute kidney injury in neonates with persistent pulmonary hypertension of the newborn. Medeni. Med. J. 2021, 36, 193–200. [Google Scholar]
- Sheta, M.M.; Al-Khalafawi, A.I.; Raza, S.M.; Gad, S.S.; Hesham, M.A. Acute kidney injury in term babies with persistent pulmonary hypertension of the newborn. Glob. J. Health Sci. 2019, 11, 60–66. [Google Scholar] [CrossRef]
- Kamolvisit, W.; Jaroensri, S.; Ratchatapantanakorn, B.; Nakwan, N. Factors and outcomes of persistent pulmonary hypertension of the newborn associated with acute kidney injury in Thai neonates. Am. J. Perinatol. 2018, 35, 298–304. [Google Scholar] [CrossRef]
- Jaroensri, S.; Kamolvisit, W.; Nakwan, N. Risk factor analysis of pneumothorax associated with persistent pulmonary hypertension of the newborn in Thai neonates. J. Matern. Fetal Neonatal Med. 2020, 33, 4090–4095. [Google Scholar] [CrossRef]
- Lakshminrusimha, S.; Konduri, G.G.; Steinhorn, R.H. Considerations in the management of hypoxemic respiratory failure and persistent pulmonary hypertension in term and late preterm neonates. J. Perinatol. 2016, 36, S12–S19. [Google Scholar] [CrossRef]
- Morse, S.; Groer, M.; Shelton, M.M.; Maguire, D.; Ashmeade, T. A systematic review: The utility of the revised version of the score for neonatal acute physiology among critically ill neonates. J. Perinat. Neonatal Nurs. 2015, 29, 315–344. [Google Scholar] [CrossRef]
- Skarsgard, E.D.; MacNab, Y.C.; Qiu, Z.; Little, R.; Lee, S.K. Canadian Neonatal Network. SNAP-II predicts mortality among infants with congenital diaphragmatic hernia. J. Perinatol. 2005, 25, 315–319. [Google Scholar] [CrossRef]
- Kumar, D.; Super, D.M.; Fajardo, R.A.; Stork, E.E.; Moore, J.J.; Saker, F.A. Predicting outcome in neonatal hypoxic respiratory failure with the score for neonatal acute physiology (SNAP) and highest oxygen index (OI) in the first 24 hours of admission. J. Perinatol. 2004, 24, 376–381. [Google Scholar] [CrossRef]
- Nakwan, N.; Nakwan, N.; Wannaro, J. Predicting mortality in infants with persistent pulmonary hypertension of the newborn with the Score for Neonatal Acute Physiology-Version II (SNAP-II) in Thai neonates. J. Perinat. Med. 2011, 39, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Demirhan, S.; Topcuoglu, S.; Karadag, N.; Ozalkaya, E.; Karatekin, G. Vasoactive inotropic score as a predictor of mortality in neonatal septic shock. J. Trop. Pediatr. 2022, 68, fmac100. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.B.; Lavilla, O.C.; Wynn, J.L.; Lure, A.C.; Gipson, D.; de la Cruz, D. Maximum vasoactive-inotropic score and mortality in extremely premature, extremely low birth weight infants. J. Perinatol. 2021, 41, 2337–2344. [Google Scholar] [CrossRef]
- Davis, J.M.; Spitzer, A.R.; Cox, C.; Fox, W.W. Predicting survival in infants with persistent pulmonary hypertension of the newborn. Pediatr. Pulmonol. 1988, 5, 6–9. [Google Scholar] [CrossRef]
- Qian, A.M.; Zhu, W.; Yang, Y.; Lu, K.Y.; Wang, J.L.; Chen, X.; Guo, C.C.; Lu, Y.D.; Rong, H.; Chneg, R. [Early risk factors for death in neonates with persistent pulmonary hypertension of the newborn treated with inhaled nitric oxide]. Zhongguo Dang Dai Er Ke Za Zhi 2022, 24, 507–513. [Google Scholar]
- Nakwan, N.; Pithaklimnuwong, S. Acute kidney injury and pneumothorax are risk factors for mortality in persistent pulmonary hypertension of the newborn in Thai neonates. J. Matern. Fetal Neonatal Med. 2016, 29, 1741–1746. [Google Scholar] [CrossRef]
- Mat Bah, M.N.; Tan, R.Y.H.; Razak, H.; Abdullah, N.; Alias, E.Y. Survival and associated risk factors for mortality among infants with persistent pulmonary hypertension of the newborn in Malaysia. J. Perinatol. 2021, 41, 786–793. [Google Scholar] [CrossRef]
- Sardar, S.; Pal, S.; Mishra, R. A retrospective study on the profile of persistent pulmonary hypertension of newborn in a tertiary care unit of Eastern India. J. Clin. Neonatol. 2020, 9, 18–26. [Google Scholar]
- Jastania, E.; Alqarni, M.S.; Abukhodair, A.W.; Bukhari, Z.M.; Bukhari, R.A.; Khatrawi, S.; Alsomali, N.; Waggass, R. Risk factors of persistent pulmonary hypertension in neonate in a tertiary care referral center. Cureus 2022, 14, e22416. [Google Scholar] [CrossRef]
- Rosenberg, A.A.; Kennaugh, J.M.; Moreland, S.G.; Fashaw, L.M.; Hale, K.A.; Torielli, F.M.; Abman, S.H.; Kinsella, J.P. Longitudinal follow-up of a cohort of newborn infants treated with inhaled nitric oxide for persistent pulmonary hypertension. J. Pediatr. 1997, 131, 70–75. [Google Scholar] [CrossRef]
- Mohsen, A.A.H.; Amin, A.S. Risk factors and outcomes of persistent pulmonary hypertension of the newborn in neonatal intensive care unit of Al-Minya University Hospital in Egypt. J. Clin. Neonatol. 2013, 2, 78–82. [Google Scholar] [CrossRef] [PubMed]
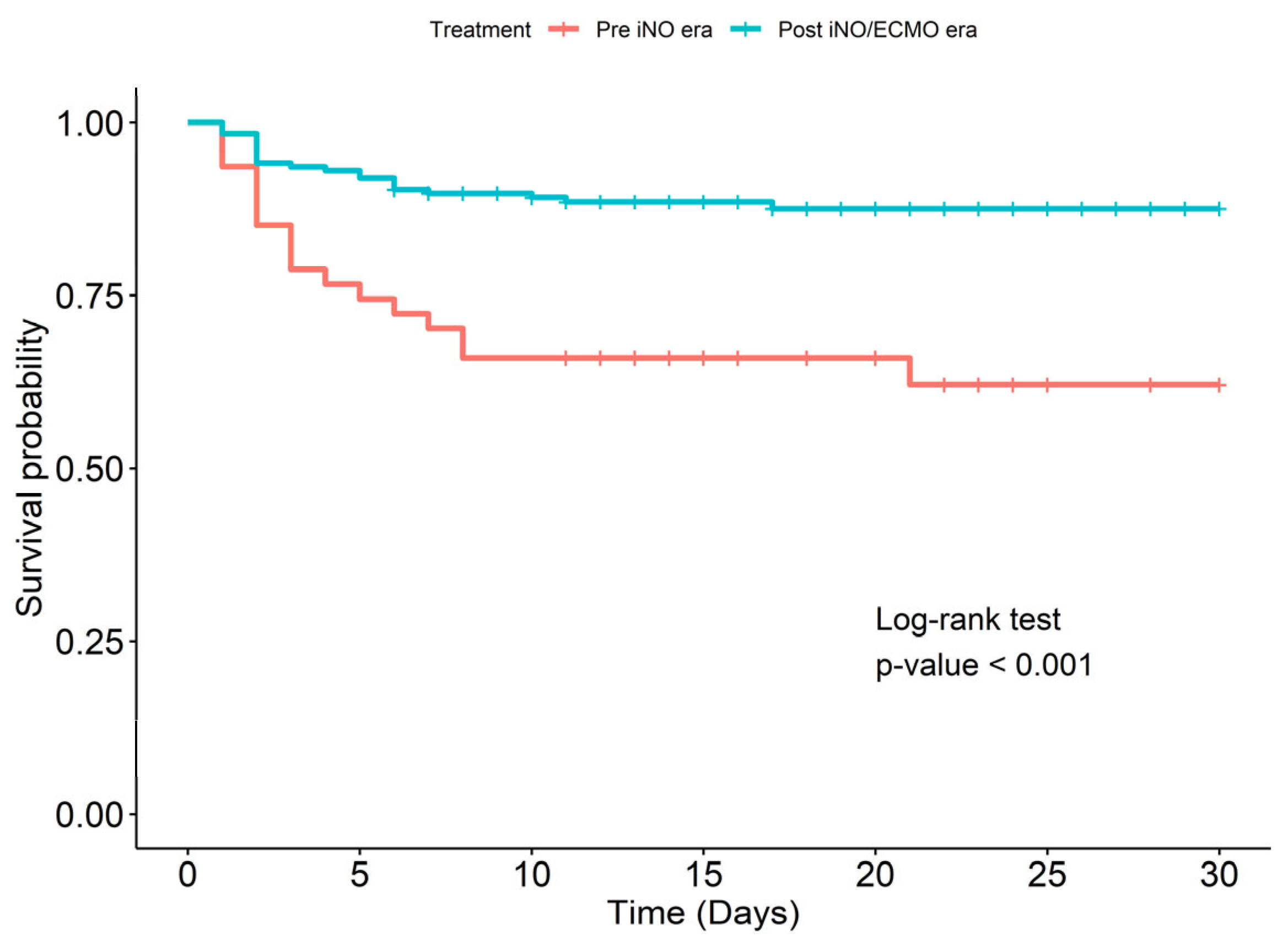
| Baseline Characteristics | Non-Survivors (n = 41) | Survivors (n = 192) | p-Value |
|---|---|---|---|
| GA, weeks | 37 (32, 38) | 37 (35, 39) | 0.047 |
| GA < 28 weeks | 10 (24.4) | 5 (2.6) | <0.001 |
| GA < 34 weeks | 13 (31.7) | 16 (8.3) | <0.001 |
| Birth weight, g | 2530 (1360, 3040) | 2948 (2579, 3348) | 0.002 |
| SGA | 7 (17.1) | 12 (6.2) | 0.052 |
| 1 min Apgar score | 6 (3, 9) | 8 (7, 9) | 0.036 |
| 5 min Apgar score | 8 (5, 9) | 9 (8, 9) | <0.001 |
| Age at PPHN diagnosis, h | 14 (7, 24) | 18 (9, 29.5) | 0.243 |
| RVSP, mmHg | 55 (45, 65) | 51.5 (40, 65) | 0.511 |
| OI at baseline | 47.3 (33.1, 71.1) | 26.9 (14.1, 42.2) | <0.001 |
| MAP at baseline, cmH2O | 15 (13.8, 17) | 13 (11, 15) | <0.001 |
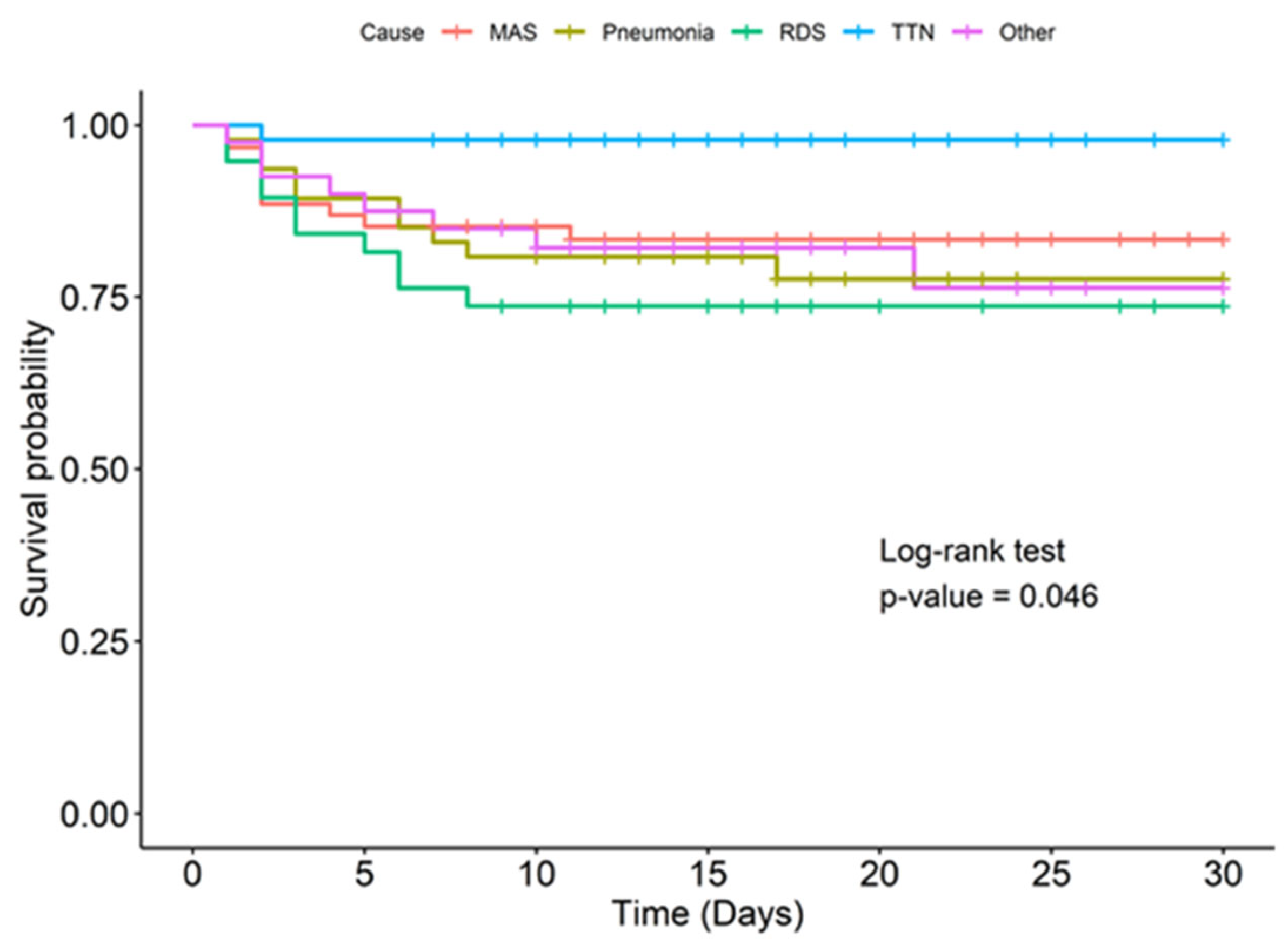
| Cause of PPHN, n (%) | |||
| MAS | 10 (24.4) | 51 (26.5) | 0.927 |
| Pneumonia/sepsis | 12 (29.3) | 38 (19.8) | 0.346 |
| TTN | 1 (2.4) | 46 (24) | 0.004 |
| RDS | 11 (26.8) | 27 (14.1) | 0.076 |
| Others | 7 (17.1) | 30 (15.6) | 0.99 |
| SNAP-II at age 12–24 h | 31 (16, 37) | 16 (5, 21) | <0.001 |
| SNAP-II at age 24–48 h | 31.5 (28, 41.8) | 16 (5, 23) | <0.001 |
| VIS at age 24 h | 172 (22, 330) | 65 (15, 219) | 0.019 |
| VIS at age 48 h | 430 (230, 440) | 149.5 (53.8, 315) | <0.001 |
| Acute kidney injury, n (%) | 19 (46.3) | 34 (17.7) | <0.001 |
| Pneumothorax, n (%) | 15 (36.6) | 37 (19.3) | 0.027 |
| OI < 20 Without iNO (n = 56) | OI > 20 withoEut iNO (n = 83) | OI > 20 with iNO (n = 87) | OI > 40 with iNO and ECMO (n = 7) | p-Value | |
|---|---|---|---|---|---|
| Survival rate, n (%) | 54, (96.4) | 60 (72.3) | 72 (82.7) | 6, (85.7) | 0.004 |
| Cumulative survival rates at the day of life, % (95% CI) | 0.042 | ||||
| 5th day | 96.5 (91.3–100) | 81.9 (74.0–90.5) | 90.8 (84.1–96.9) | 100 (100–100) | |
| 10th day | 95.7 (90.1–100) | 77.8 (68.8–86.5) | 83.9 (76.6–92.8) | 100 (100–100) | |
| 30th day | 95.7 (90.1–100) | 74.9 (65.8–85.3) | 82.8 (74.8–91.7) | 80 (51.6–100) | |
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | Adjusted HR (95% CI) | p-Value | |
| GA < 28 weeks | 5.54 (2.62–11.7) | <0.001 | 5.46 (2.25–13.24) | <0.001 |
| GA < 34 weeks | 3.54 (1.79–6.99) | <0.001 | – | – |
| BW < 2500 g | 3.18 (1.69–5.96) | <0.001 | – | – |
| SGA | 2.72 (1.2–6.16) | 0.032 | 2.93 (1.24–6.92) | 0.026 |
| 5 min Apgar score < 5 | 4.21 (2–8.9) | 0.001 | – | – |
| OI ≥ 40 | 3.87 (2–7.49) | <0.001 | – | – |
| Acute kidney injury | 3.98 (2.08–7.60) | <0.001 | 2.48 (1.27–4.84) | 0.01 |
| Pneumothorax | 2.13 (1.11–4.1) | 0.03 | 3.03 (1.48–6.21) | 0.003 |
| SNAP-II at age 12–24 h ≥ 43 | 4.82 (1.98–11.73) | 0.003 | – | – |
| SNAP-II at age 24–48 h ≥ 43 | 12.76 (5.62–28.97) | <0.001 | 4.03 (1.66–9.77) | 0.005 |
| VIS at age 24 h | 1.003 (1.001–1.005) | 0.004 | 1.0026 (1.0004–1.005) | 0.026 |
| VIS at age 48 h | 1.005 (1.003–1.008) | <0.001 | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuaikaew, K.; Maneenil, G.; Thatrimontrichai, A.; Dissaneevate, S.; Praditaukrit, M. Mortality Risk Factors and Survival Outcomes in Infants with Persistent Pulmonary Hypertension of the Newborn. J. Clin. Med. 2025, 14, 4502. https://doi.org/10.3390/jcm14134502
Chuaikaew K, Maneenil G, Thatrimontrichai A, Dissaneevate S, Praditaukrit M. Mortality Risk Factors and Survival Outcomes in Infants with Persistent Pulmonary Hypertension of the Newborn. Journal of Clinical Medicine. 2025; 14(13):4502. https://doi.org/10.3390/jcm14134502
Chicago/Turabian StyleChuaikaew, Kokaew, Gunlawadee Maneenil, Anucha Thatrimontrichai, Supaporn Dissaneevate, and Manapat Praditaukrit. 2025. "Mortality Risk Factors and Survival Outcomes in Infants with Persistent Pulmonary Hypertension of the Newborn" Journal of Clinical Medicine 14, no. 13: 4502. https://doi.org/10.3390/jcm14134502
APA StyleChuaikaew, K., Maneenil, G., Thatrimontrichai, A., Dissaneevate, S., & Praditaukrit, M. (2025). Mortality Risk Factors and Survival Outcomes in Infants with Persistent Pulmonary Hypertension of the Newborn. Journal of Clinical Medicine, 14(13), 4502. https://doi.org/10.3390/jcm14134502






