Prevalence of Cardiovascular Functional Anomalies in Large-for-Gestational-Age (LGA) Fetuses by Fetal Echocardiography
Abstract
1. Introduction
2. Materials and Methods
- Fetal echocardiography performed between 28 + 0 and 39 + 0 weeks of gestation;
- Fetuses classified as having normal heart anatomy (NHA) during fetal echocardiography at our center;
- Singleton pregnancies;
- Full-term birth, defined as≥ 37 + 6 weeks of gestation;
- The availability of postnatal follow-up.
- Fetal echocardiography performed before 28 + 0 weeks of gestation;
- Fetuses diagnosed with congenital heart defects (CHDs) during fetal echocardiography at our center;
- Extracardiac malformations (ECMs) diagnosed during fetal ultrasound;
- Fetuses with prenatally or postnatally confirmed genetic abnormalities (e.g., trisomy 21);
- Multiple pregnancies;
- Preterm birth;
- A lack of postnatal follow-up.
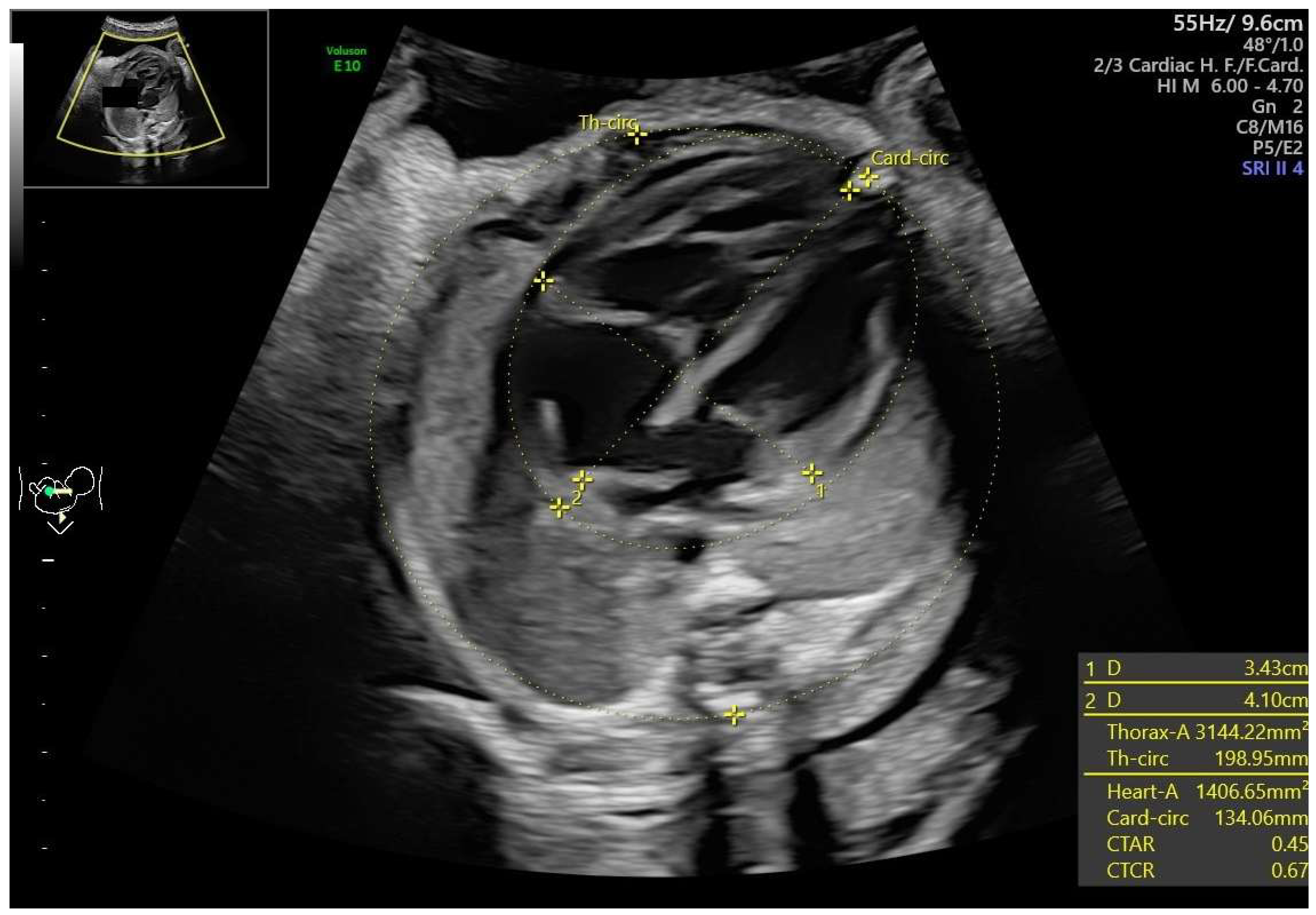
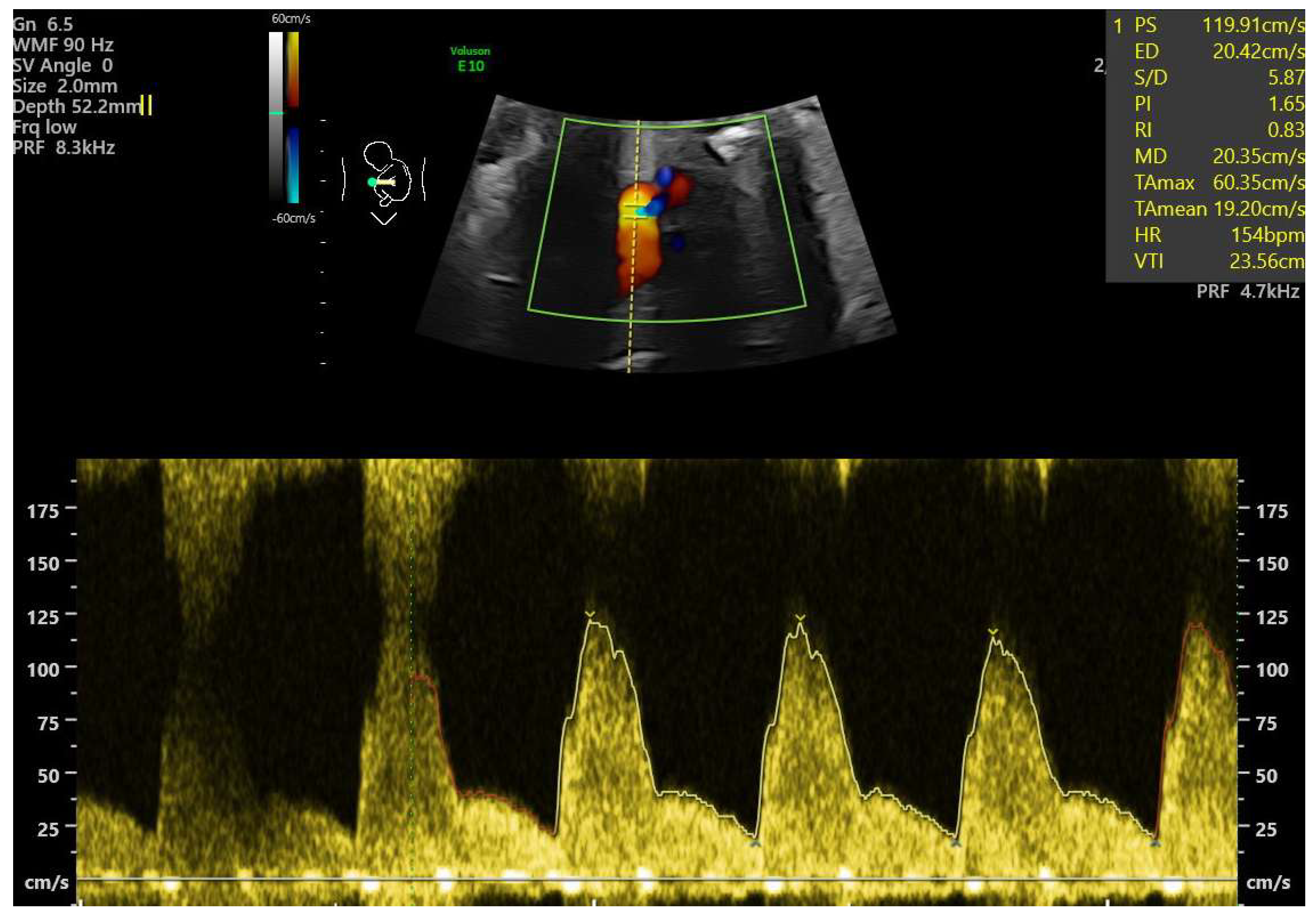
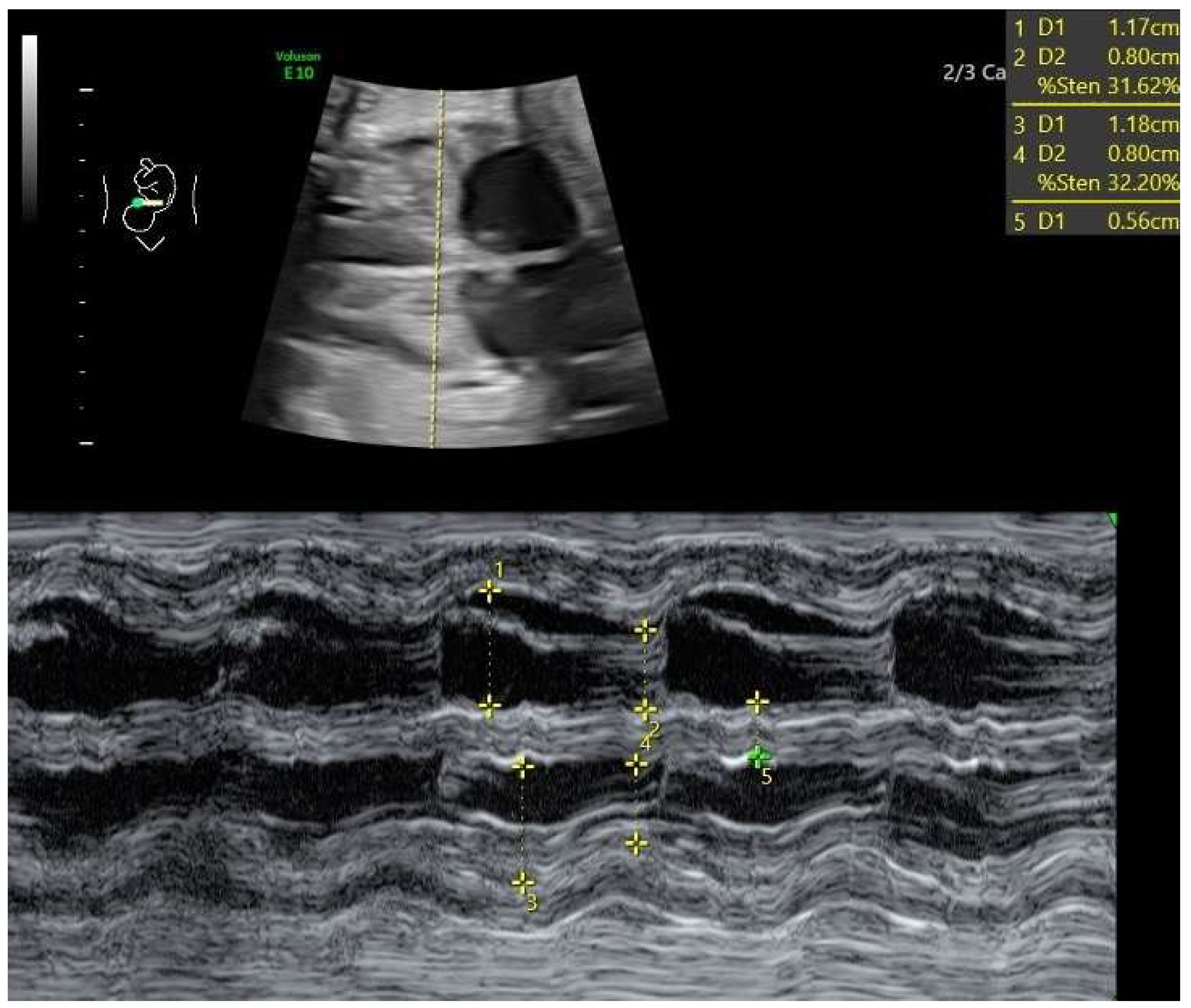
- Ductal constriction (DC), defined as a peak systolic velocity (PSV) > 1.4 m/s or >95th percentile for gestational age, a peak diastolic velocity > 35 cm/s or >95th percentile, or a pulsatility index (PI) < 1.9 [8,9]. The measurement was taken by visualizing the DA at the 12th or 6th “hour” (+/− 30 degrees), which was performed by seeing the ductal arch in the long-axis view. Fetal slow movements or hiccups were accounted for and if they occurred the measurement was taken more times during the examination (Figure 2).
- Tricuspid regurgitation (TR), detected via color or pulsed-wave Doppler, with a peak systolic velocity > 1.5 m/s and a duration > 80 ms [11].
- Aortic regurgitation (AR), detected at the left ventricular outflow tract (LVOT) [13].
- Mitral regurgitation (MR), detected at the mitral valve [13].
- Pericardial effusion, defined as fluid > 3 mm [13].
- Disproportion in the four-chamber view (4CV), defined as a ≥2 mm disparity between atria, ventricles, or both [13].
- A bright spot in the left or right ventricle [13].
- Fetal arrhythmia, detected via M-mode or color Doppler [13].
- Tricuspid monophasic flow, defined by the absence of distinguishable E and A waves [13].
- Foramen ovale (FO) abnormalities, such as atypical flow (bilateral or left-to-right in the third trimester) or the presence of a “spinnaker” FO (long, supple FO valve) [13].
- Umbilical cord around the neck, noted due to its potential to mimic arrhythmia or disproportion in 4CV [13].
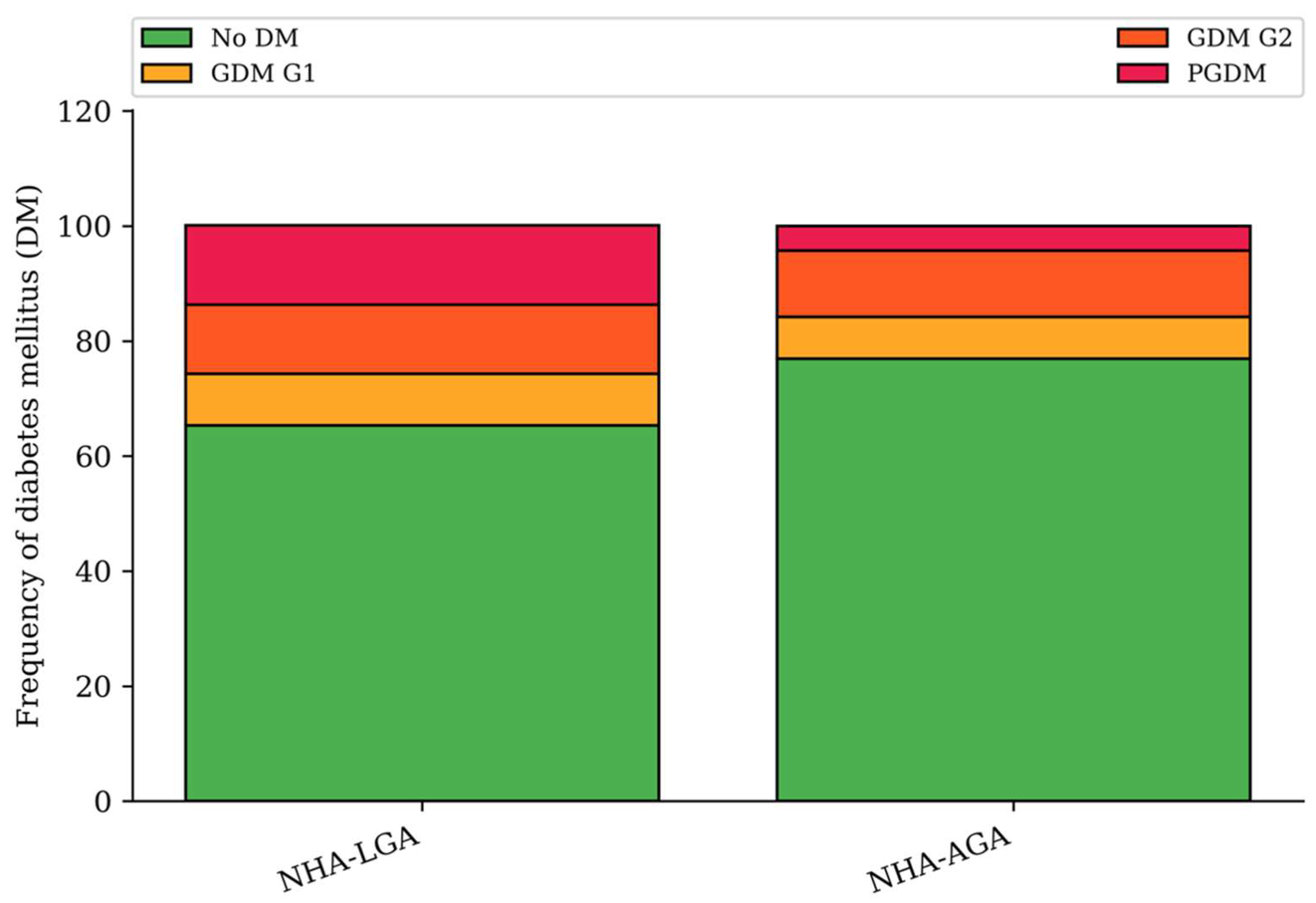
- Pregestational diabetes mellitus (PGDM);
- Gestational diabetes mellitus (GDM):
- ∘
- G1: Diet-controlled;
- ∘
- G2: Requiring insulin therapy.
- Birth weight;
- Gestational age and mode of delivery;
- Apgar score;
- A need for phototherapy due to hyperbilirubinemia;
- Neonatal sex.
- A macrosomic neonate was defined as one with a birth weight > 4000 g.
3. Results
3.1. Maternal Abnormalities
3.2. Functional Abnormalities
3.3. Neonatal Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LGA | Large for gestational age |
| NHA | Normal heart anatomy |
| DC | Ductal constriction |
| EFW | Estimated fetal weight |
| SGA | Small for gestational age |
| AGA | Appropriate for gestational age |
| FGR | Fetal growth restriction |
| AC | Abdominal circumference |
| PGDM | Pregestational diabetes |
| CHD | Congenital heart defect |
| ECM | Extracardiac malformation |
| TR | Tricuspid regurgitation |
| PR | Pulmonary regurgitation |
| RVOT | Right ventricular outflow tract |
| AR | Aortic regurgitation |
| LVOT | Left ventricular outlet tract |
| MR | Mitral regurgitation |
| 4CV | Four-chamber view |
| FO | Foramen ovale |
| DM | Diabetes mellitus |
| GDM | Gestational diabetes |
| PE | Pericardial effusion |
| IVST | interventricular septal thickness |
| CVPS | Cardiovascular Profile Score |
| ECHO | Echocardiography |
References
- Salomon, L.J.; Alfirevic, Z.; Da Silva Costa, F.; Deter, R.L.; Figueras, F.; Ghi, T.; Glanc, P.; Khalil, A.; Lee, W.; Napolitano, R.; et al. ISUOG Practice Guidelines: Ultrasound assessment of fetal biometry and growth. Ultrasound Obstet. Gynecol. 2019, 53, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Spellacy, W.N.; Miller, S.; Winegar, A.; Peterson, P.Q. Macrosomia--maternal characteristics and infant complications. Obstet. Gynecol. 1985, 66, 158–161. [Google Scholar]
- Liu, C.H.; Yang, S.T.; Wang, P.H. Maternal factors associated with fetal macrosomia. J. Chin. Med. Assoc. JCMA 2023, 86, 455–456. [Google Scholar] [CrossRef]
- Ehrenberg, H.M.; Mercer, B.M.; Catalano, P.M. The influence of obesity and diabetes on the prevalence of macrosomia. Am. J. Obstet. Gynecol. 2004, 191, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Beta, J.; Khan, N.; Khalil, A.; Fiolna, M.; Ramadan, G.; Akolekar, R. Maternal and neonatal complications of fetal macrosomia: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2019, 54, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Sylwestrzak, O.; Respondek-Liberska, M. Echocardiographic Methods of Fetal Heart Size Assessmentheart to Chest Area Ratio and Transversal Heart Diameter. Prenat. Cardiol. 2018, 8, 20–23. [Google Scholar] [CrossRef]
- Respondek, M.; Respondek, A.; Huhta, J.C.; Wilczynski, J. 2D echocardiographic assessment of the fetal heart size in the 2nd and 3rd trimester of uncomplicated pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1992, 44, 185–188. [Google Scholar] [CrossRef]
- Alvarez, S.G.V.; McBrien, A. Ductus arteriosus and fetal echocardiography: Implications for practice. Semin. Fetal Neonatal Med. 2018, 23, 285–291. [Google Scholar] [CrossRef]
- Chugh, B.D.; Makam, A. Diagnosis and Management of Fetal Ductus Arteriosus Constriction. J. Fetal Med. 2020, 07, 235–242. [Google Scholar] [CrossRef]
- Szmyd, B.; Biedrzycka, M.; Karuga, F.F.; Rogut, M.; Strzelecka, I.; Respondek-Liberska, M. Interventricular Septal Thickness as a Diagnostic Marker of Fetal Macrosomia. J. Clin. Med. 2021, 10, 949. [Google Scholar] [CrossRef]
- Respondek, M.L.; Kammermeier, M.; Ludomirsky, A.; Weil, S.R.; Huhta, J.C. The prevalence and clinical significance of fetal tricuspid valve regurgitation with normal heart anatomy. Am. J. Obstet. Gynecol. 1994, 171, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Smrcek, J.M.; Germer, U.; Gembruch, U. Functional pulmonary valve regurgitation in the fetus. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 1998, 12, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Respondek-Liberska, M. Diagnostyka Prenatalna USG, 1st ed.; PZWL Wydawnictwo Lekarskie: Warszawa, Poland, 2019. [Google Scholar]
- Sokołowski, Ł.; Słodki, M.; Murlewska, J.; Strzelecka, I.; Kordjalik, P.; Blitek, M.; Węgrzynowski, J.; Grzesiak, M.; Krekora, M.; Czajkowski, K.; et al. Fetal echocardiography in the 3rd trimester of pregnancy as an essential element of modern prenatal diagnostics and perinatal care—Recommendations of Polish Society of Prenatal Cardiology 2020. Prenat. Cardiol. 2020, 2020, 5–12. [Google Scholar] [CrossRef]
- Khan, N.; Ciobanu, A.; Karampitsakos, T.; Akolekar, R.; Nicolaides, K.H. Prediction of large-for-gestational-age neonate by routine third-trimester ultrasound. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2019, 54, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Huhta, J.C. Guidelines for the Evaluation of Heart Failure in the Fetus With or Without Hydrops. Pediatr. Cardiol. 2004, 25, 274–286. [Google Scholar] [CrossRef]
- Zielinsky, P.; Manica, J.L.L.; Piccoli, A.L.; Nicoloso, L.H.S.; Barra, M.; Alievi, M.M.; Vian, I.; Zilio, A.; Pizzato, P.E.; Silva, J.S.; et al. Fetal ductal constriction caused by maternal ingestion of green tea in late pregnancy: An experimental study. Prenat. Diagn. 2012, 32, 921–926. [Google Scholar] [CrossRef]
- Zielinsky, P. Constriction of fetal ductus arteriosus and maternal intake of polyphenol-rich foods. Pediatr. Cardiol. 2014, 4, 6–18. [Google Scholar] [CrossRef]
- Zielinsky, P.; Busato, S. Prenatal effects of maternal consumption of polyphenol-rich foods in late pregnancy upon fetal ductus arteriosus. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 256–274. [Google Scholar] [CrossRef]
- Zielinsky, P.; Piccoli, A.L.; Manica, J.L.; Nicoloso, L.H.; Menezes, H.; Busato, A.; Moraes, M.R.; Silva, J.; Bender, L.; Pizzato, P.; et al. Maternal consumption of polyphenol-rich foods in late pregnancy and fetal ductus arteriosus flow dynamics. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2010, 30, 17–21. [Google Scholar] [CrossRef]
- Zielinsky, P.; Piccoli, A.L.; Manica, J.L.L.; Nicoloso, L.H.S. New insights on fetal ductal constriction: Role of maternal ingestion of polyphenol-rich foods. Expert Rev. Cardiovasc. Ther. 2010, 8, 291–298. [Google Scholar] [CrossRef]
- Paladini, D.; Marasini, M.; Volpe, P. Severe ductal constriction in the third-trimester fetus following maternal self-medication with nimesulide. Ultrasound Obstet. Gynecol. 2005, 25, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Auer, M.; Brezinka, C.; Eller, P.; Luze, K.; Schweigmann, U.; Schwärzler, P. Prenatal diagnosis of intrauterine premature closure of the ductus arteriosus following maternal diclofenac application. Ultrasound Obstet. Gynecol. 2004, 23, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Schiessl, B.; Schneider, K.T.; Zimmermann, A.; Kainer, F.; Friese, K.; Oberhoffer, R. Prenatal Constriction of the Fetal Ductus Arteriosus—Related to Maternal Pain Medication? Z. Für Geburtshilfe Neonatol. 2005, 209, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, K.; Takeda, A.; Imamura, S.; Nakanishi, T.; Momma, K. Constriction of the ductus arteriosus by selective inhibition of cyclooxygenase-1 and -2 in near-term and preterm fetal rats. Prostaglandins Other Lipid Mediat. 2006, 79, 34–42. [Google Scholar] [CrossRef]
- Norton, M.E. Teratogen update: Fetal effects of indomethacin administration during pregnancy. Teratology 1997, 56, 282–292. [Google Scholar] [CrossRef]
- Sharpe, G.L.; Larsson, K.S.; Thalme, B. Studies on closure of the ductus arteriosus. XII. In utero effect of indomethacin and sodium salicylate in rats and rabbits. Prostaglandins 1975, 9, 585–596. [Google Scholar] [CrossRef]
- Respondek, M.; Weil, S.R.; Huhta, J.C. Fetal echocardiography during indomethacin treatment. Ultrasound Obstet. Gynecol. 1995, 5, 86–89. [Google Scholar] [CrossRef]
- Räsänen, J.; Jouppila, P. Fetal cardiac function and ductus arteriosus during indomethacin and sulindac therapy for threatened preterm labor: A randomized study. Am. J. Obstet. Gynecol. 1995, 173, 20–25. [Google Scholar] [CrossRef]
- Coceani, F.; Baragatti, B. Mechanisms for Ductus Arteriosus Closure. Semin. Perinatol. 2012, 36, 92–97. [Google Scholar] [CrossRef]
- Hung, Y.C.; Yeh, J.L.; Hsu, J.H. Molecular Mechanisms for Regulating Postnatal Ductus Arteriosus Closure. Int. J. Mol. Sci. 2018, 19, 1861. [Google Scholar] [CrossRef]
- Biały, Ł.H.; Talar, T.; Gulczyńska, E.; Strzelecka, I.; Respondek-Liberska, M. Prenatal Diagnosis of Ductal Constriction in Normal Heart Anatomy—Are There Any Neonatal Consequences? J. Clin. Med. 2025, 14, 3388. [Google Scholar] [CrossRef] [PubMed]
- Wilczynski, J.; Respondek, M.; Pertynski, T. Fetal Echocardiography (2D and M-mode) in Pregnant Women with Insulin Dependent Diabetes in the Second Half of Pregnancy. Int. J. Prenat. Perinat. Psychol. Med. 1993, 5, 27–32. [Google Scholar]
- Depla, A.L.; De Wit, L.; Steenhuis, T.J.; Slieker, M.G.; Voormolen, D.N.; Scheffer, P.G.; De Heus, R.; Van Rijn, B.B.; Bekker, M.N. Effect of maternal diabetes on fetal heart function on echocardiography: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2021, 57, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Suda-Całus, M.; Dąbrowska, K.; Gulczyńska, E. Infant of a diabetic mother: Clinical presentation, diagnosis and treatment. Pediatr. Endocrinol. Diabetes Metab. 2024, 30, 36–41. [Google Scholar] [CrossRef]
- Schwartz, R.; Gruppuso, P.A.; Petzold, K.; Brambilla, D.; Hiilesmaa, V.; Teramo, K.A. Hyperinsulinemia and Macrosomia in the Fetus of the Diabetic Mother. Diabetes Care 1994, 17, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, N.K.; Moon, J.H. Gestational Diabetes Mellitus: Mechanisms Underlying Maternal and Fetal Complications. Endocrinol. Metab. 2025, 40, 10–25. [Google Scholar] [CrossRef]
- Shang, M.; Wen, Z. Increased placental IGF-1/mTOR activity in macrosomia born to women with gestational diabetes. Diabetes Res. Clin. Pract. 2018, 146, 211–219. [Google Scholar] [CrossRef]
- Respondek-Liberska, M.; Sylwestrzak, O.; Murlewska, J.; Biały, L.; Krekora, M.; Tadros-Zins, M.; Gulczyńska, E.; Strzelecka, I. Fetal Third-Trimester Functional Cardiovascular Abnormalities and Neonatal Elevated Bilirubin Level. J. Clin. Med. 2023, 12, 6021. [Google Scholar] [CrossRef]

| Maternal Information | NHA-LGA (n = 167) | NHA-AGA (n = 835) | p Value |
|---|---|---|---|
| Maternal age (median, Q1–Q3) | 32.0 (28–35) | 31.0 (28–36) | >0.05 |
| Gestational age at exam (median, Q1–Q3) | 33.0 (30.0–35.2) | 33.5 (30.6–36.0) | 0.01 |
| Pregestational diabetes mellitus (n, %) | 23 (13.8%) | 36 (4.3%) | <0.001 |
| Gestational diabetes mellitus (n, %) | 35 (21.0%) | 159 (18.8%) | >0.05 |
| BMI > 30 (n, %) | 30 (18.0%) | 71 (8.5%) | <0.001 |
| Functional Abnormality (n, %) | NHA-LGA (n = 167) | NHA-AGA (n = 835) | p Value |
|---|---|---|---|
| Ductal constriction (DC) | 24 (14.4%) | 11 (1.3%) | <0.001 |
| Hypertrophy | 30 (18.0%) | 72 (8.6%) | <0.001 |
| Cardiomegaly | 19 (11.4%) | 37 (4.4%) | 0.001 |
| TR | 25 (15.0%) | 121 (14.5%) | >0.05 |
| Pericardial effusion | 11 (6.6%) | 51 (6.1%) | >0.05 |
| Disproportion | 11 (6.6%) | 51 (6.1%) | >0.05 |
| Bright spot | 5 (3.0%) | 40 (4.8%) | >0.05 |
| Arrhythmia | 3 (1.8%) | 13 (1.6%) | >0.05 |
| Tricuspid monophasic flow | 4 (2.4%) | 10 (1.2%) | >0.05 |
| FO flow abnormal | 4 (2.4%) | 12 (1.4%) | >0.05 |
| Pulmonary valve insufficiency | 2 (1.2%) | 4 (0.5%) | >0.05 |
| FO spinnaker | 1 (0.6%) | 10 (1.2%) | >0.05 |
| MV insufficiency | 1 (0.6%) | 1 (0.1%) | >0.05 |
| Aortic valve insufficiency | 1 (0.6%) | 1 (0.1%) | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biały, Ł.H.; Sylwestrzak, O.; Murlewska, J.; Sokołowski, Ł.; Strzelecka, I.; Respondek-Liberska, M. Prevalence of Cardiovascular Functional Anomalies in Large-for-Gestational-Age (LGA) Fetuses by Fetal Echocardiography. J. Clin. Med. 2025, 14, 4500. https://doi.org/10.3390/jcm14134500
Biały ŁH, Sylwestrzak O, Murlewska J, Sokołowski Ł, Strzelecka I, Respondek-Liberska M. Prevalence of Cardiovascular Functional Anomalies in Large-for-Gestational-Age (LGA) Fetuses by Fetal Echocardiography. Journal of Clinical Medicine. 2025; 14(13):4500. https://doi.org/10.3390/jcm14134500
Chicago/Turabian StyleBiały, Łucja Hanna, Oskar Sylwestrzak, Julia Murlewska, Łukasz Sokołowski, Iwona Strzelecka, and Maria Respondek-Liberska. 2025. "Prevalence of Cardiovascular Functional Anomalies in Large-for-Gestational-Age (LGA) Fetuses by Fetal Echocardiography" Journal of Clinical Medicine 14, no. 13: 4500. https://doi.org/10.3390/jcm14134500
APA StyleBiały, Ł. H., Sylwestrzak, O., Murlewska, J., Sokołowski, Ł., Strzelecka, I., & Respondek-Liberska, M. (2025). Prevalence of Cardiovascular Functional Anomalies in Large-for-Gestational-Age (LGA) Fetuses by Fetal Echocardiography. Journal of Clinical Medicine, 14(13), 4500. https://doi.org/10.3390/jcm14134500





