Effects of Endometriosis on Anti-Müllerian Hormone
Abstract
1. Introduction
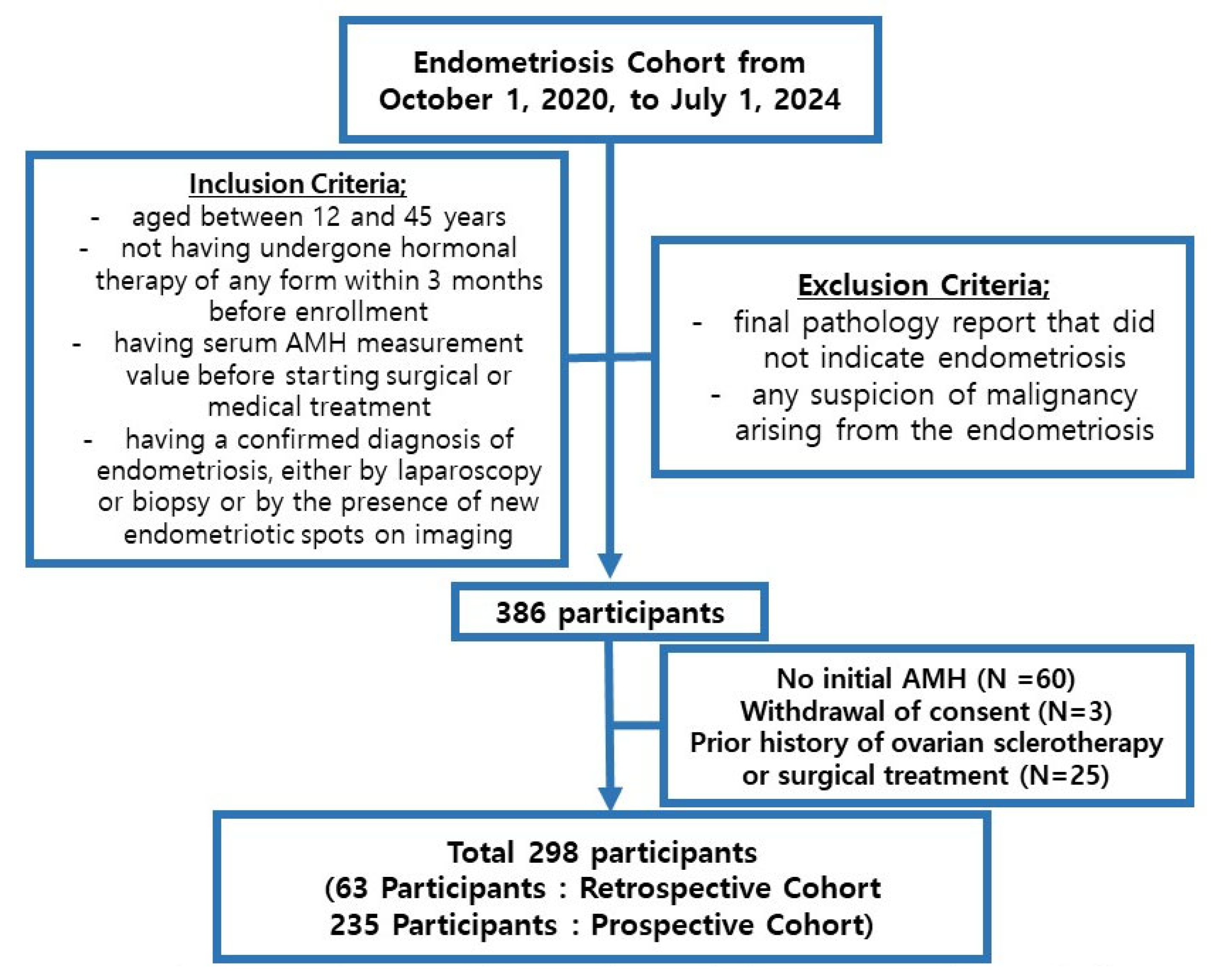
2. Materials and Methods
2.1. Study Participants and Data Collection
2.2. AMH Assay
2.3. Reference AMH
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
3.1. Mean Age and Basal Characteristics
3.2. Mean AMH of Each Age Group
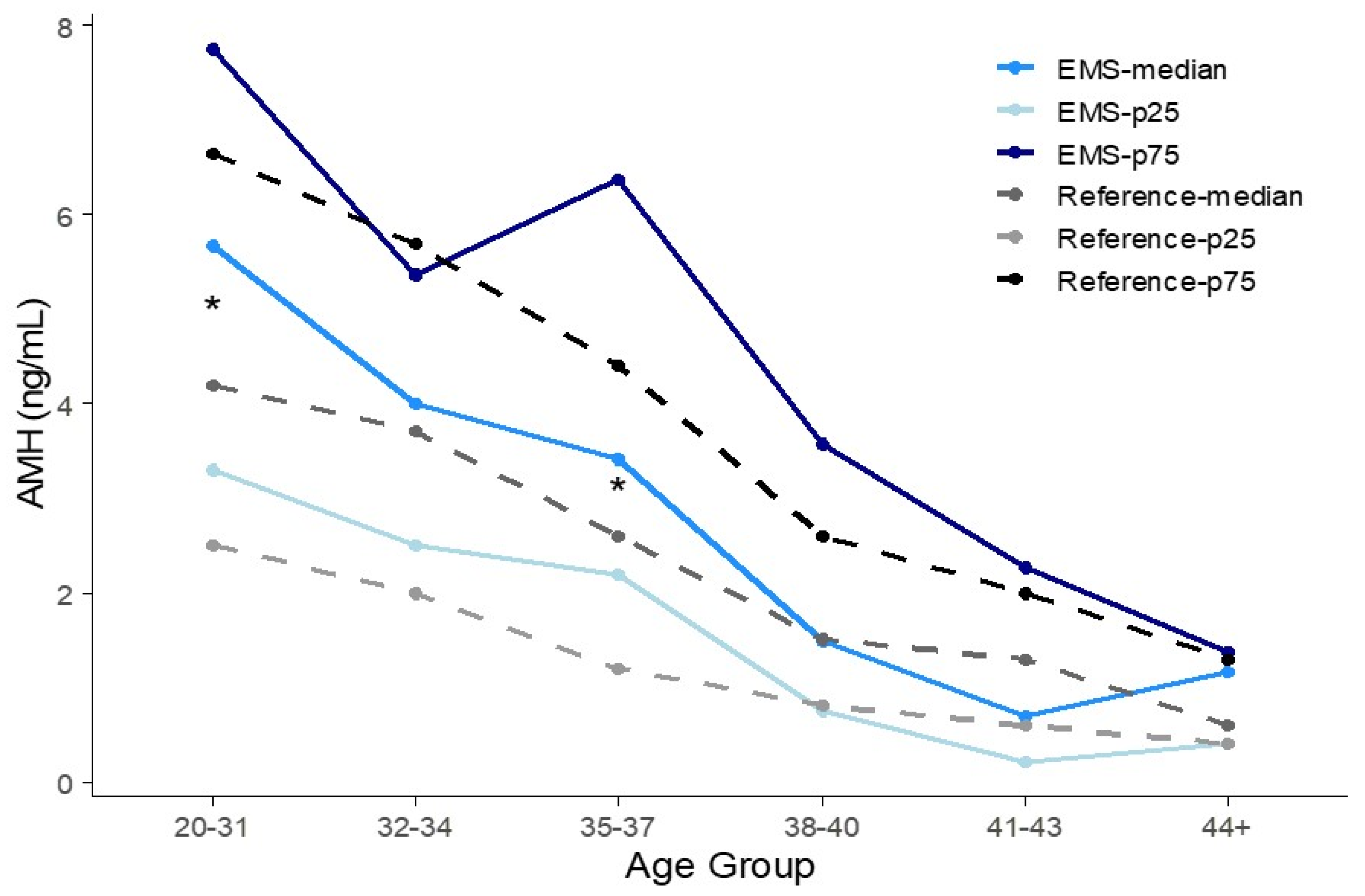
3.3. Percentile AMH Concentration of Each Age Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.; Lee, M.; Hwang, H.; Chung, Y.-J.; Cho, H.-H.; Yoon, H.; Kim, M.; Chae, K.-H.; Jung, C.Y.; Kim, S. The estimated prevalence and incidence of endometriosis with the korean national health insurance service-national sample cohort (nhis-nsc): A national population-based study. J. Epidemiol. 2021, 31, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Kim, E.-S.; Park, K.S.; Lee, Y.J.; Ha, I.-H. Current treatments for endometriosis in south korea: An analysis of nationwide data from 2010 to 2019. Sci. Rep. 2023, 13, 9573. [Google Scholar] [CrossRef] [PubMed]
- Medina-Perucha, L.; Pistillo, A.; Raventós, B.; Jacques-Aviñó, C.; Munrós-Feliu, J.; Martínez-Bueno, C.; Valls-Llobet, C.; Carmona, F.; López-Jiménez, T.; Pujolar-Díaz, G. Endometriosis prevalence and incidence trends in a large population-based study in catalonia (spain) from 2009 to 2018. Women’s Health 2022, 18, 17455057221130566. [Google Scholar] [CrossRef] [PubMed]
- Moradi, Y.; Shams-Beyranvand, M.; Khateri, S.; Gharahjeh, S.; Tehrani, S.; Varse, F.; Tiyuri, A.; Najmi, Z. A systematic review on the prevalence of endometriosis in women. Indian J. Med. Res. 2021, 154, 446–454. [Google Scholar] [CrossRef]
- Lessans, N.; Gilan, A.; Dick, A.; Bibar, N.; Saar, T.D.; Porat, S.; Dior, U.P. Ovarian reserve markers of women with superficial endometriosis. Int. J. Gynecol. Obstet. 2024, 165, 696–702. [Google Scholar] [CrossRef]
- Shin, J.S.; Kim, S.; Choi, J.Y.; Hong, K.; Shim, S.; Jung, Y.W.; Seong, S.J.; Jun, H.S.; Kim, M.-L. Pregnancy outcomes and obstetrical complications of twin pregnancies with endometriosis: A single-center cohort study. Yonsei Med. J. 2024, 65, 356–362. [Google Scholar] [CrossRef]
- Bai, S.W.; Cho, H.J.; Kim, J.Y.; Jeong, K.A.; Kim, S.K.; Cho, D.J.; Song, C.H.; Park, K.H. Endometriosis in an adolescent population: The severance hospital in korean experience. Yonsei Med. J. 2002, 43, 48–52. [Google Scholar] [CrossRef]
- Pacchiarotti, A.; Iaconianni, P.; Caporali, S.; Vitillo, M.; Meledandri, M.; Monaco, G.; Sergio, C.; Boza, M.; Saccucci, P. Severe endometriosis: Low value of amh did not affect oocyte quality and pregnancy outcome in ivf patients. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11488–11495. [Google Scholar]
- Muzii, L.; Di Tucci, C.; Di Feliciantonio, M.; Galati, G.; Di Donato, V.; Musella, A.; Palaia, I.; Panici, P.B. Antimüllerian hormone is reduced in the presence of ovarian endometriomas: A systematic review and meta-analysis. Fertil. Steril. 2018, 110, 932–940.e931. [Google Scholar] [CrossRef]
- Romanski, P.A.; Brady, P.C.; Farland, L.V.; Thomas, A.M.; Hornstein, M.D. The effect of endometriosis on the antimüllerian hormone level in the infertile population. J. Assist. Reprod. Genet. 2019, 36, 1179–1184. [Google Scholar] [CrossRef]
- Zakhari, A.; Delpero, E.; McKeown, S.; Tomlinson, G.; Bougie, O.; Murji, A. Endometriosis recurrence following post-operative hormonal suppression: A systematic review and meta-analysis. Hum. Reprod. Update 2021, 27, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.M.; Malinak, L.R. Recurrent endometriosis: Incidence, management, and prognosis. Am. J. Obstet. Gynecol. 1983, 146, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-W. Recurrence of endometriosis and its control. Hum. Reprod. Update 2009, 15, 441–461. [Google Scholar] [CrossRef] [PubMed]
- Jirge, P.R. Ovarian reserve tests. J. Hum. Reprod. Sci. 2011, 4, 108–113. [Google Scholar] [CrossRef]
- La Marca, A.; Volpe, A. Anti-müllerian hormone (amh) in female reproduction: Is measurement of circulating amh a useful tool? Clin. Endocrinol. 2006, 64, 603–610. [Google Scholar] [CrossRef]
- Yoo, J.H.; Kim, H.O.; Cha, S.W.; Park, C.W.; Yang, K.M.; Song, I.O.; Koong, M.K.; Kang, I.S. Age specific serum anti-müllerian hormone levels in 1,298 korean women with regular menstruation. Clin. Exp. Reprod. Med. 2011, 38, 93–97. [Google Scholar] [CrossRef]
- Moolhuijsen, L.M.; Visser, J.A. Anti-müllerian hormone and ovarian reserve: Update on assessing ovarian function. J. Clin. Endocrinol. Metab. 2020, 105, 3361–3373. [Google Scholar] [CrossRef]
- Hehenkamp, W.J.; Looman, C.W.; Themmen, A.P.; de Jong, F.H.; Te Velde, E.; Broekmans, F.J. Anti-mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J. Clin. Endocrinol. Metab. 2006, 91, 4057–4063. [Google Scholar] [CrossRef]
- Nelson, S.; La Marca, A. The journey from the old to the new amh assay: How to avoid getting lost in the values. Reprod. Biomed. Online 2011, 23, 411–420. [Google Scholar] [CrossRef][Green Version]
- Kasapoglu, I.; Ata, B.; Uyaniklar, O.; Seyhan, A.; Orhan, A.; Oguz, S.Y.; Uncu, G. Endometrioma-related reduction in ovarian reserve (error): A prospective longitudinal study. Fertil. Steril. 2018, 110, 122–127. [Google Scholar] [CrossRef]
- Pedachenko, N.; Anagnostis, P.; Shemelko, T.; Tukhtarian, R.; Alabbas, L. Serum anti-mullerian hormone, prolactin and estradiol concentrations in infertile women with endometriosis. Gynecol. Endocrinol. 2021, 37, 162–165. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, Y.; Zhang, C.; Wang, Y.; Zhu, H.-L. Antral follicle count is reduced in the presence of endometriosis: A systematic review and meta-analysis. Reprod. Biomed. Online 2021, 42, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Gong, X.; Wang, C.C.; Zhang, T.; Huang, J. Diminished ovarian reserve in endometriosis: Insights from in vitro, in vivo, and human studies—A systematic review. Int. J. Mol. Sci. 2023, 24, 15967. [Google Scholar] [CrossRef] [PubMed]
- Streuli, I.; de Ziegler, D.; Gayet, V.; Santulli, P.; Bijaoui, G.; de Mouzon, J.; Chapron, C. In women with endometriosis anti-müllerian hormone levels are decreased only in those with previous endometrioma surgery. Hum. Reprod. 2012, 27, 3294–3303. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, D.; Laven, J. Amh as the primary marker for fertility. Eur. J. Endocrinol. 2019, 181, D45–D51. [Google Scholar] [CrossRef]
- Choi, R.; Lee, S.G.; Lee, E.H. Reference intervals of anti-müllerian hormone in korean women. J. Clin. Lab. Anal. 2022, 36, e24525. [Google Scholar] [CrossRef]
- Kanakatti Shankar, R.; Dowlut-McElroy, T.; Dauber, A.; Gomez-Lobo, V. Clinical utility of anti-mullerian hormone in pediatrics. J. Clin. Endocrinol. Metab. 2022, 107, 309–323. [Google Scholar] [CrossRef]
- Shrikhande, L.; Shrikhande, B.; Shrikhande, A. Amh and its clinical implications. J. Obstet. Gynecol. India 2020, 70, 337–341. [Google Scholar] [CrossRef]
- Ji, M.; Kim, K.-R.; Kim, H.-K.; Lee, W.; Yun, Y.-M.; Chun, S.; Min, W.-K. Age group-specific reference intervals for the elecsys anti-müllerian hormone assay in healthy korean women: A nationwide population-based study. Ann. Lab. Med. 2022, 42, 621–629. [Google Scholar] [CrossRef]
- Jeon, J.H.; Park, S.Y.; Lee, S.R.; Jeong, K.; Chung, H.W. Serum anti-müllerian hormone levels before surgery in patients with ovarian endometriomas compared to other benign ovarian cysts. J. Menopausal Med. 2015, 21, 142–148. [Google Scholar] [CrossRef]
- Chen, Y.; Pei, H.; Chang, Y.; Chen, M.; Wang, H.; Xie, H.; Yao, S. The impact of endometrioma and laparoscopic cystectomy on ovarian reserve and the exploration of related factors assessed by serum anti-mullerian hormone: A prospective cohort study. J. Ovarian Res. 2014, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Celik, H.G.; Dogan, E.; Okyay, E.; Ulukus, C.; Saatli, B.; Uysal, S.; Koyuncuoglu, M. Effect of laparoscopic excision of endometriomas on ovarian reserve: Serial changes in the serum antimüllerian hormone levels. Fertil. Steril. 2012, 97, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Somigliana, E.; Vercellini, P.; Pagliardini, L.; Candiani, M.; Vigano, P. Endometriosis as a detrimental condition for granulosa cell steroidogenesis and development: From molecular alterations to clinical impact. J. Steroid Biochem. Mol. Biol. 2016, 155, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Casalechi, M.; Di Stefano, G.; Fornelli, G.; Somigliana, E.; Viganò, P. Impact of endometriosis on the ovarian follicles. Baillieres Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 92, 102430. [Google Scholar] [CrossRef]
- Fan, W.; Yuan, Z.; Li, M.; Zhang, Y.; Nan, F. Decreased oocyte quality in patients with endometriosis is closely related to abnormal granulosa cells. Front. Endocrinol. 2023, 14, 1226687. [Google Scholar] [CrossRef]
- Zubrzycka, A.; Migdalska-Sęk, M.; Jędrzejczyk, S.; Brzeziańska-Lasota, E. The expression of tgf-β1, smad3, ilk and mirna-21 in the ectopic and eutopic endometrium of women with endometriosis. Int. J. Mol. Sci. 2023, 24, 2453. [Google Scholar] [CrossRef]
- Juhasz-Böss, I.; Fischer, C.; Lattrich, C.; Skrzypczak, M.; Malik, E.; Ortmann, O.; Treeck, O. Endometrial expression of estrogen receptor β and its splice variants in patients with and without endometriosis. Arch. Gynecol. Obstet. 2011, 284, 885–891. [Google Scholar] [CrossRef]
- Han, S.J.; Jung, S.Y.; Wu, S.-P.; Hawkins, S.M.; Park, M.J.; Kyo, S.; Qin, J.; Lydon, J.P.; Tsai, S.Y.; Tsai, M.-J. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell 2015, 163, 960–974. [Google Scholar] [CrossRef]
- Demirel, L.C.; Cengiz, B.; Ünlü, C. Severe endometriosis and apoptotic granulosa cells. Fertil. Steril. 2001, 75, 642. [Google Scholar] [CrossRef]
- Toya, M.; Saito, H.; Ohta, N.; Saito, T.; Kaneko, T.; Hiroi, M. Moderate and severe endometriosis is associated with alterations in the cell cycle of granulosa cells in patients undergoing in vitro fertilization and embryo transfer. Fertil. Steril. 2000, 73, 344–350. [Google Scholar] [CrossRef]
- Inal, Z.O.; Engin Ustun, Y.; Yilmaz, N.; Aktulay, A.; Bardakci, Y.; Gulerman, C. Does the anti-müllerian hormone truly reflect ovarian response in women with endometrioma? J. Obstet. Gynaecol. 2019, 39, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Kacem-Berjeb, K.; Braham, M.; Massoud, C.B.; Hannachi, H.; Hamdoun, M.; Chtourou, S.; Debbabi, L.; Bouyahia, M.; Fadhlaoui, A.; Zhioua, F. Does endometriosis impact the composition of follicular fluid in il6 and amh? A case-control study. J. Clin. Med. 2023, 12, 1829. [Google Scholar] [CrossRef] [PubMed]
- Velarde, M.C.; Bucu, M.E.M.; Habana, M.A.E. Endometriosis as a highly relevant yet neglected gynecologic condition in asian women. Endocr. Connect. 2023, 12, e230169. [Google Scholar] [CrossRef] [PubMed]

| Age (Yr) | 20–31 | 32–34 | 35–37 | 38–40 | 41–43 | ≥44 | |
|---|---|---|---|---|---|---|---|
| N | 168 | 30 | 31 | 34 | 26 | 9 | |
| Average Age (yr) | 26.85 | 32.80 | 36.13 | 38.92 | 41.81 | 44.67 | |
| Initial treatment after the diagnosis | Sclerotherapy (%) | 80 (47.6) | 15 (50) | 13 (41.9) | 17 (50) | 11 (42.3) | 1 (11.1) |
| Operation d/t endometrioma (%) | 50 (29.8) | 5 (16.7) | 10 (32.3) | 15 (44.1) | 15 (57.7) | 8 (88.9) | |
| Medication (%) | 36 (21.4) | 10 (33.3) | 7 (22.6) | 2 (5.9) | |||
| Operation d/t EMS other than ovary (%) | 2 (1.2) | 0 | 1 (3.2) | ||||
| Tracking loss after initial visit (%) | 1 (3.2) | ||||||
| Endometriosis | Peritoneum (%) | 4 (2.4) | 2 (6.7) | 1 (2.8) | 0 (0) | 0 (0) | 1 (8.3) |
| Unilateral ovary (%) | 114 (67.9) | 18 (60) | 26 (80.6) | 21 (64.1) | 19 (68.8) | 5 (58.3) | |
| Bilateral ovary (%) | 50 (29.8) | 10 (33.3) | 4 (16.7) | 13 (35.9) | 7 (31.3) | 3 (33.3) | |
| Recurrence of endometriosis (%) | 0(0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Previous treatment of endometriosis | Surgery (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Medication (%) | 11 (6.5) | 4 (13.3) | 2 (6.5) | 2 (5.9) | 2 (7.7) | 0 (0) | |
| None (%) | 157 (93.5) | 26 (86.7) | 29 (93.5) | 32 (94.1) | 24 (92.3) | 9 (100) | |
| Sclerotherapy (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Age (yr) | N | Age (yr) Mean ± SD | AMH (ng/mL) Mean ± SD (95% CI) | 25th Percentile | 50th Percentile (Median) | 75th Percentile | Bootstrap 95% CI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. | EMS | Ref. | EMS | p-Value | Ref. | EMS * | p-Value | Ref. | EMS * (N) | Ref. | EMS * (N) | Ref. | EMS * (N) | EMS * | |
| 20–31 | 377 | 168 | 29.1 ± 0.1 | 26.9 ± 3.1 | <0.01 | 4.94 ± 0.17 (4.61–5.25) | 5.96 ± 3.22 (5.47–6.44) | <0.01 | 2.5 | 3.29(24) | 4.2 | 5.68 (57) | 6.65 | 7.75 (108) | 5.14–6.05 |
| 32–34 | 331 | 30 | 33.1 ± 0.4 | 32.8 ± 0.8 | 0.06 | 4.25 ± 0.17 (3.92–4.57) | 4.49 ± 3.33 (3.30–5.68) | 0.70 | 2.00 | 2.50 (5) | 3.70 | 4.00 (13) | 5.70 | 5.36 (23) | 2.85–4.56 |
| 35–37 | 283 | 31 | 35.9 ± 0.5 | 36.1 ± 0.8 | 0.85 | 3.22 ± 0.15 (2.92–3.51) | 4.33 ± 3.06 (3.25–5.40) | 0.05 | 1.20 | 2.19 (2) | 2.60 | 3.42 (9) | 4.40 | 6.37 (17) | 2.70–5.08 |
| 38–40 | 173 | 34 | 39.0 ± 0.6 | 38.9 ± 0.8 | 0.94 | 2.13 ± 0.15 (1.83–2.44) | 2.51 ± 2.58 (1.65–3.38) | 0.39 | 0.80 | 0.76 (9) | 1.50 | 1.49 (17) | 2.60 | 3.58 (24) | 1.13–2.28 |
| 41–43 | 87 | 26 | 41.7 ± 0.1 | 41.8 ± 0.8 | 0.52 | 1.47 ± 0.13 (1.21–1.71) | 1.33 ± 1.43 (0.78–1.88) | 0.62 | 0.60 | 0.21 (12) | 1.30 | 0.70 (15) | 2.00 | 2.26 (17) | 0.34–2.06 |
| ≥44 | 47 | 9 | 45.6 ± 0.3 | 44.7 ± 0.5 | <0.01 | 0.95 ± 0.14 (0.68–1.23) | 0.93 ± 0.68 (0.49–1.37) | 0.94 | 0.40 | 0.40 (2) | 0.60 | 1.15 (3) | 1.30 | 1.37 (6) | 0.13–1.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, Y.S.; Choi, E.; Baek, J.K.; Kim, H.; Yun, B.H. Effects of Endometriosis on Anti-Müllerian Hormone. J. Clin. Med. 2025, 14, 4495. https://doi.org/10.3390/jcm14134495
Chung YS, Choi E, Baek JK, Kim H, Yun BH. Effects of Endometriosis on Anti-Müllerian Hormone. Journal of Clinical Medicine. 2025; 14(13):4495. https://doi.org/10.3390/jcm14134495
Chicago/Turabian StyleChung, Yun Soo, Euna Choi, Jin Kyung Baek, Heeyon Kim, and Bo Hyon Yun. 2025. "Effects of Endometriosis on Anti-Müllerian Hormone" Journal of Clinical Medicine 14, no. 13: 4495. https://doi.org/10.3390/jcm14134495
APA StyleChung, Y. S., Choi, E., Baek, J. K., Kim, H., & Yun, B. H. (2025). Effects of Endometriosis on Anti-Müllerian Hormone. Journal of Clinical Medicine, 14(13), 4495. https://doi.org/10.3390/jcm14134495





