The Role of the Inflammatory Prognostic Index in Patients with Non-ST Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention
Abstract
1. Introduction
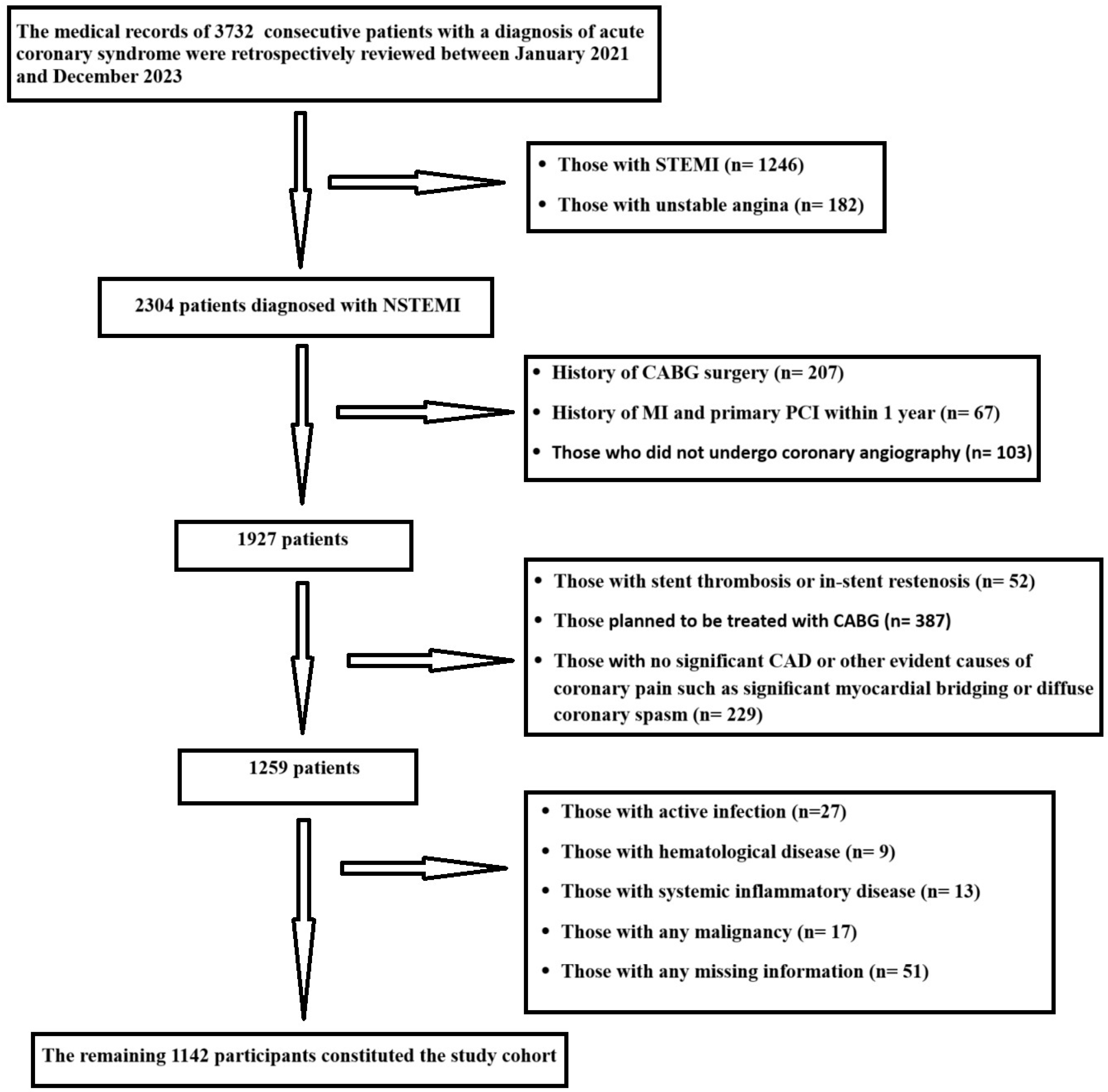
2. Materials and Methods
2.1. Study Population
2.2. Demographic and Clinical Characteristics
2.3. Laboratory Parameters
2.4. Coronary Angiography Findings
2.5. Study Endpoint
2.6. Statistical Analysis
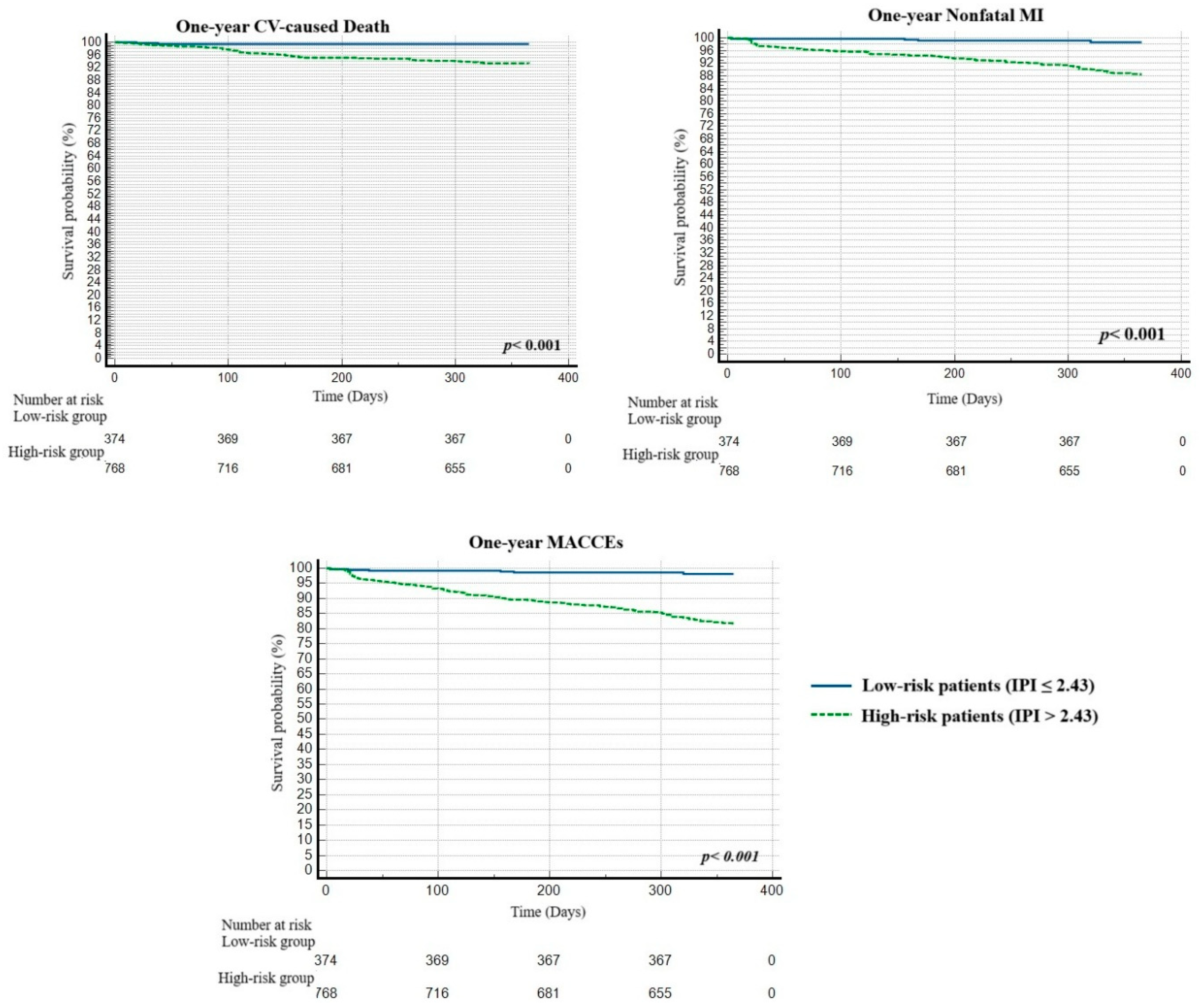
3. Results
3.1. Baseline Characteristics
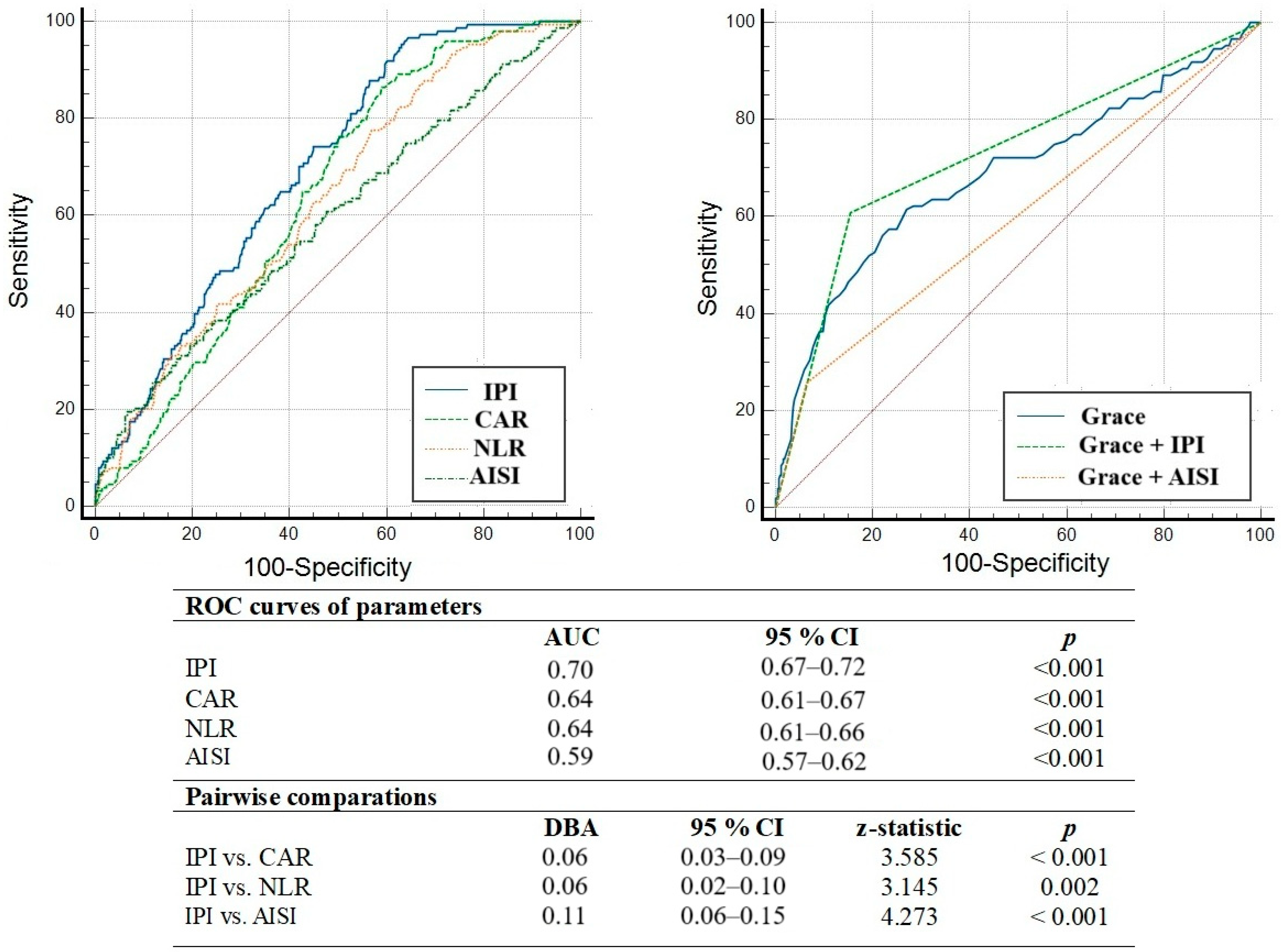
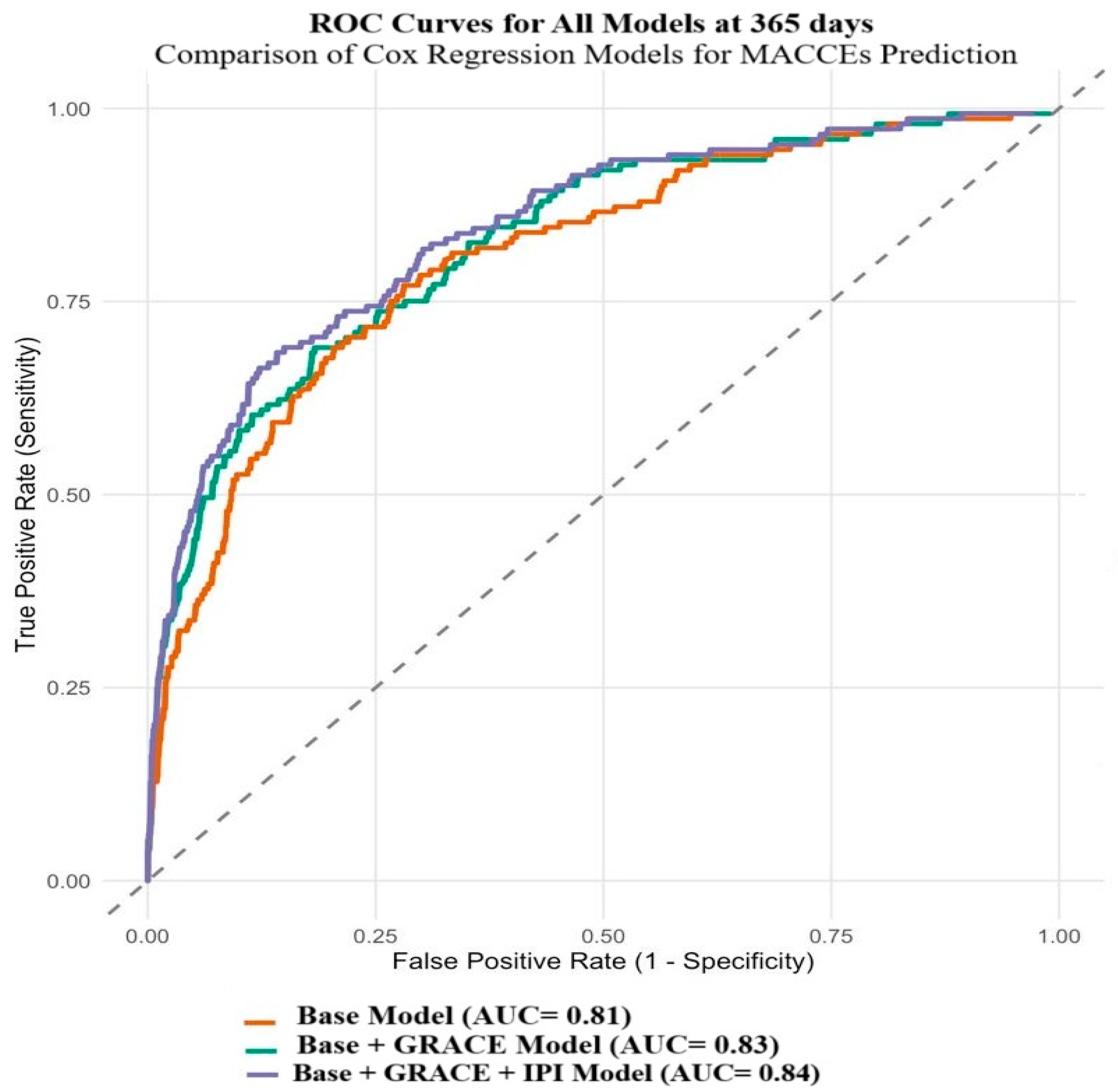
3.2. Independent Predictors of MACCE Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banahene, N.O.; Sinha, T.; Shaikh, S.; Zin, A.K.; Khreis, K.; Chaudhari, S.S.; Wei, C.R.; Palleti, S.K. Effect of Elevated Neutrophil-to-Lymphocyte Ratio on Adverse Outcomes in Patients with Myocardial Infarction: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e61647. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Yoon, C.H.; Kang, S.H.; Choi, D.J.; Kim, H.S.; Cho, M.C.; Kim, Y.J.; Chae, S.C.; Yoon, J.H.; Gwon, H.C.; et al. Early- and late-term clinical outcome and their predictors in patients with ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction. Int. J. Cardiol. 2013, 169, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Mc Manus, D.D.; Gore, J.; Yarzebski, J.; Spencer, F.; Lessard, D.; Goldberg, R.J. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am. J. Med. 2011, 124, 40–47. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Melichova, D.; Aabel, E.W.; Lie, Ø.H.; Klæboe, L.G.; Grenne, B.; Sjøli, B.; Brunvand, H.; Haugaa, K.; Edvardsen, T. Mortality in Patients with Acute Coronary Syndrome-A Prospective 5-Year Follow-Up Study. J. Clin. Med. 2023, 12, 6598. [Google Scholar] [CrossRef]
- Garcia-Osuna, A.; Sans-Rosello, J.; Ferrero-Gregori, A.; Alquezar-Arbe, A.; Sionis, A.; Ordóñez-Llanos, J. Risk Assessment after ST-Segment Elevation Myocardial Infarction: Can Biomarkers Improve the Performance of Clinical Variables? J. Clin. Med. 2022, 11, 1266. [Google Scholar] [CrossRef]
- Eyiol, A.; Ertekin, B.; Acar, T. The association between systemic inflammatory indices and prognosis in acute coronary syndrome. Ann. Clin. Anal. Med. 2024, 15, 514–518. [Google Scholar] [CrossRef]
- Yukselen, Z.; Majmundar, V.; Dasari, M.; Arun Kumar, P.; Singh, Y. Chest Pain Risk Stratification in the Emergency Department: Current Perspectives. Open Access Emerg. Med. 2024, 16, 29–43. [Google Scholar] [CrossRef]
- Guclu, K.; Celik, M. Prognostic Value of Inflammation Parameters in Patients With Non-ST Elevation Acute Coronary Syndromes. Angiology 2020, 71, 825–830. [Google Scholar] [CrossRef]
- Kaski, J.C.; Cruz-Fernández, J.M.; Fernández-Bergés, D.; García-Moll, X.; Martín Jadraque, L.; Mostaza, J.; López García-Aranda, V.; González Juanatey, J.R.; Castro Beiras, A.; Martín Luengo, C.; et al. Investigadores del estudio SIESTA Inflammation markers and risk stratification in patients with acute coronary syndromes: Design of the SIESTA Study (Systemic Inflammation Evaluation in Patients with non-ST segment elevation Acute coronary syndromes). Rev. Esp. Cardiol. 2003, 56, 389–395. (In Spanish) [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Nanchen, D.; Klingenberg, R.; Gencer, B.; Räber, L.; Carballo, D.; von Eckardstein, A.; Windecker, S.; Rodondi, N.; Lüscher, T.F.; Mach, F.; et al. Inflammation during acute coronary syndromes—Risk of cardiovascular events and bleeding. Int. J. Cardiol. 2019, 287, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Menekşe, T.S.; Kaçer, İ.; Hacımustafaoğlu, M.; Gül, M.; Ateş, C. C-reactive protein to albumin ratio may predict in-hospital mortality in non-ST elevation myocardial infarction. Biomark. Med. 2024, 18, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Şaylık, F.; Çınar, T.; Tanboğa, İ.H. The Predictive Value of the Inflammatory Prognostic Index for Detecting No-Reflow in ST-Elevation Myocardial Infarction Patients. Arq. Bras. Cardiol. 2024, 121, e20230644. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, B.; Lu, W.; Chen, Y.; Peng, Y.; Chen, L.; Lin, Y. Association Between the Aggregate Index of Systemic Inflammation and Clinical Outcomes in Patients with Acute Myocardial Infarction: A Retrospective Study. J. Inflamm. Res. 2024, 17, 7057–7067. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, B.; Chen, Y.; Peng, Y.; Lu, W.; Chen, L.; Lin, Y. Predictive value of inflammatory prognostic index for contrast-induced nephropathy in patients undergoing coronary angiography and/or percutaneous coronary intervention. Sci. Rep. 2024, 14, 15861. [Google Scholar] [CrossRef]
- Badem, S.; Pekcolaklar, A. Inflammatory prognostic index predicts new-onset atrial fibrillation and mortality after on-pump coronary artery bypass grafting. Rev. Assoc. Med. Bras. 2023, 69, e20230226. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41 (Suppl. 1), S13–S27. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar]
- European Stroke Organisation; Tendera, M.; Aboyans, V.; Bartelink, M.L.; Baumgartner, I.; Clément, D.; Collet, J.P.; Cremonesi, A.D.; De Carlo, M.; Erbel, R.; et al. ESC Committee for Practice Guidelines. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: The Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2011, 32, 2851–2906. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Rev. Esp. Cardiol. 2021, 74, 544. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M.; et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients with Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2025, 151, e771–e862. [Google Scholar] [CrossRef]
- Granger, C.B.; Goldberg, R.J.; Dabbous, O.; Pieper, K.S.; Eagle, K.A.; Cannon, C.P.; Van De Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch. Intern. Med. 2003, 163, 2345–2353. [Google Scholar] [CrossRef]
- Benson, B.; Belle, A.; Lee, S.; Bassin, B.S.; Medlin, R.P.; Sjoding, M.W.; Ward, K.R. Prediction of episode of hemodynamic instability using an electrocardiogram based analytic: A retrospective cohort study. BMC Anesthesiol. 2023, 23, 324. [Google Scholar] [CrossRef]
- Garcia-Garcia, H.M.; McFadden, E.P.; Farb, A.; Mehran, R.; Stone, G.W.; Spertus, J.; Onuma, Y.; Morel, M.A.; van Es, G.A.; Zuckerman, B.; et al. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Circulation 2018, 137, 2635–2650. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Novotny, N.L. Clinical Prediction Model of Medical Inpatients at Risk of Early Readmission: Development and Validation; ProQuest: Ann Arbor, MI, USA, 2008; pp. 82–83. [Google Scholar]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Perkins, N.J.; Schisterman, E.F. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef]
- Karaca, M.; Kalyoncuoğlu, M.; Zengin, A.; Eren, S.; Keskin, K.; Oflar, E.; Karataş, M.B.; Çalık, A.N. The Prognostic Value of the Advanced Lung Cancer Inflammation Index for Major Cardiovascular and Cerebrovascular Events in Patients with Non-ST Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention. J. Clin. Med. 2025, 14, 1403. [Google Scholar] [CrossRef] [PubMed]
- Ozveren, A.; Erdogan, A.P.; Ekinci, F. The Inflammatory Prognostic Index as a Potential Predictor of Prognosis in Metastatic Gastric Cancer. Sci. Rep. 2023, 13, 7755. [Google Scholar] [CrossRef] [PubMed]
- Dirican, N.; Dirican, A.; Anar, C.; Atalay, S.; Ozturk, O.; Bircan, A.; Akkaya, A.; Cakir, M. A New Inflammatory Prognostic Index, Based on C-reactive Protein, the Neutrophil to Lymphocyte Ratio and Serum Albumin is Useful for Predicting Prognosis in Non-Small Cell Lung Cancer Cases. Asian Pac. J. Cancer Prev. 2016, 17, 5101–5106. [Google Scholar] [CrossRef]
- Yang, X.; Tao, N.; Wang, T.; Zhang, Z.; Wu, Q. The relationship between composite inflammatory indicators and short-term outcomes in patients with heart failure. Int. J. Cardiol. 2025, 420, 132755. [Google Scholar] [CrossRef]
- Sueta, D.; Hokimoto, S.; Sakamoto, K.; Akasaka, T.; Tabata, N.; Kaikita, K.; Honda, O.; Naruse, M.; Ogawa, H.; Multi-center Study of Hemodialysis Patients Undergoing Invasive Cardiovascular Procedures Study Investigators. Validation of the high mortality rate of Malnutrition-Inflammation-Atherosclerosis syndrome: -Community-based observational study. Int. J. Cardiol. 2017, 230, 97–102. [Google Scholar] [CrossRef]
- Kalyoncuoğlu, M.; Katkat, F.; Biter, H.I.; Cakal, S.; Tosu, A.R.; Can, M.M. Predicting One-Year Deaths and Major Adverse Vascular Events with the Controlling Nutritional Status Score in Elderly Patients with Non-ST-Elevated Myocardial Infarction Undergoing Percutaneous Coronary Intervention. J. Clin. Med. 2021, 10, 2247. [Google Scholar] [CrossRef]
- Kalyoncuoglu, M.; Durmus, G. Relationship between C-reactive protein-to-albumin ratio and the extent of coronary artery disease in patients with non-ST-elevated myocardial infarction. Coron. Artery Dis. 2020, 31, 130–136. [Google Scholar] [CrossRef]
- Puhl, S.L.; Steffens, S. Neutrophils in Post-myocardial Infarction Inflammation: Damage vs. Resolution? Front. Cardiovasc. Med. 2019, 6, 25. [Google Scholar] [CrossRef]
- Ohmure, K.; Kanda, D.; Ikeda, Y.; Tokushige, A.; Sonoda, T.; Arikawa, R.; Anzaki, K.; Ohishi, M. Impact of co-presence of malnutrition-inflammation-atherosclerosis factors on prognosis in lower extremity artery disease patients after endovascular therapy. Cardiovasc. Interv. Ther. 2025, 40, 102–111. [Google Scholar] [CrossRef]
- Don, B.R.; Kaysen, G. Serum albumin: Relationship to inflammation and nutrition. Semin. Dial 2004, 17, 432–437. [Google Scholar] [CrossRef]
- Eckart, A.; Struja, T.; Kutz, A.; Baumgartner, A.; Baumgartner, T.; Zurfluh, S.; Neeser, O.; Huber, A.; Stanga, Z.; Mueller, B.; et al. Relationship of Nutritional Status, Inflammation, and Serum Albumin Levels During Acute Illness: A Prospective Study. Am. J. Med. 2020, 133, 713–722.e7. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.; Huang, Z.; Lu, J.; Yang, Y.; Zhao, X.; Tu, J.; Pan, Y.; Bao, K.; Chen, W.; et al. A Synergistic Association Between Inflammation, Malnutrition, and Mortality in Patients With Diabetics. Front. Nutr. 2022, 9, 872512. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.L.; Kennedy, K.F.; Cohen, D.J.; Diercks, D.B.; Moscucci, M.; Ramee, S.; Wang, T.Y.; Connolly, T.; Spertus, J.A. Predicting In-Hospital Mortality in Patients With Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 68, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Jäckel, M.; Kaier, K.; Rilinger, J.; Wolf, D.; Peikert, A.; Roth, K.; Oettinger, V.; Dawid Leander, S.; Zehender, M.; Bode, C.; et al. Outcomes of female and male patients suffering from coronary artery disease: A nation-wide registry of patients admitted as emergency. Medicine 2021, 100, e27298. [Google Scholar] [CrossRef]
| Variables | All Population (n = 1142) | Low IPI (<3.40) (n = 957; 83.8%) | High IPI (≥3.40) (n = 185; 16.2%) | p |
|---|---|---|---|---|
| Male gender, n% | 848 (74.3) | 738 (77.1) | 110 (59.5) | <0.001 |
| Age, years | 61.9 ± 12.5 | 60.3 ± 12.4 | 70.3 ± 9.8 | <0.001 |
| BMI (kg/m2) | 27.7 ± 3.3 | 27.7 ± 3.3 | 27.5 ± 3.1 | 0.482 |
| Hypertension, n (%) | 659 (57.7) | 531 (55.5) | 128 (69.2) | 0.001 |
| Diabetes, n (%) | 402 (35.2) | 312 (32.6) | 90 (48.6) | <0.001 |
| Hyperlipidemia, n (%) | 532 (46.6) | 454 (47.4) | 78 (42.2) | 0.188 |
| Smoking, n (%) | 504 (44.1) | 431 (45.0) | 73 (39.5) | 0.162 |
| Family history, n (%) | 407 (35.6) | 338 (35.3) | 69 (37.3) | 0.607 |
| CAD history, n (%) | 483 (42.3) | 394 (41.2) | 89 (48.1) | 0.080 |
| Previous MI, n (%) | 341 (29.9) | 277 (28.9) | 64 (34.6) | 0.124 |
| Previous PCI, n (%) | 360 (31.5) | 302 (31.6) | 58 (31.4) | 0.956 |
| PAD history, n (%) | 32 (2.8) | 24 (2.5) | 8 (4.3) | 0.171 |
| Heart Failure, n (%) | 208 (18.2) | 152 (15.9) | 56 (30.3) | <0.001 |
| CRF, n (%) | 144 (12.6) | 94 (9.8) | 50 (27.0) | <0.001 |
| Dialysis, n (%) | 13 (1.1) | 8 (0.8) | 5 (2.7) | 0.028 |
| Killip III-IV, n (%) | 97 (8.5) | 54 (5.6) | 43 (23.2) | <0.001 |
| Hemodynamic instability, n (%) | 31 (2.7) | 18 (1.9) | 13 (7.0) | <0.001 |
| GRACE risk score | 98.1 ± 22.9 | 92.8 ± 20.3 | 125.6 ± 14.5 | <0.001 |
| LVEF, % | 51.3 ± 9.8 | 51.5 ± 9.7 | 50.3 ± 10.6 | 0.134 |
| Medications, n (%) | ||||
| Acetylsalicylic acid | 413 (36.2) | 340 (35.5) | 73 (39.5) | 0.308 |
| ADP blockers | 132 (11.6) | 110 (11.5) | 22 (11.9) | 0.877 |
| OACs, n (%) | 56 (4.9) | 36 (3.8) | 20 (10.8) | <0.001 |
| Beta-blockers | 335 (29.3) | 274 (29.3) | 61 (33.0) | 0.235 |
| RAS blockers | 490 (42.9) | 400 (41.8) | 90 (48.6) | 0.085 |
| CCBs, n (%) | 421 (36.9) | 341 (35.6) | 80 (43.2) | 0.049 |
| Statin | 250 (21.9) | 204 (21.3) | 46 (24.9) | 0.285 |
| Antianginals, n (%) | 110 (9.6) | 96 (10.0) | 14 (7.6) | 0.298 |
| OADs, n (%) | 382 (33.5) | 296 (30.9) | 86 (46.5) | <0.001 |
| Insulin, (%) | 67 (5.9) | 47 (4.9) | 20 (10.8) | 0.002 |
| Syntax score I | 20.1 ± 6.0 | 19.9 ± 5.8 | 20.8 ± 7.0 | 0.070 |
| TIMI < 3 flow, n (%) | 100 (8.8) | 82 (8.6) | 18 (9.7) | 0.609 |
| TVR, year, n (%) | 37 (3.2) | 26 (2.7) | 11 (5.9) | 0.023 |
| Nonfatal MI, 30 days, n (%) | 23 (2.0) | 12 (1.3) | 11 (5.9) | <0.001 |
| Nonfatal stroke, 30 days, n (%) | 2 (0.2) | 1 (0.1) | 1 (0.5) | 0.194 |
| CV-caused mortality, 30 days, n (%) | 5 (0.4) | 2 (0.2) | 3 (1.6) | 0.008 |
| MACCEs, 30 days, n (%) | 30 (2.6) | 15 (1.6) | 15 (8.1) | <0.001 |
| Nonfatal MI, year, n (%) | 90 (7.9) | 45 (4.7) | 45 (24.3) | <0.001 |
| Nonfatal stroke, year, n (%) | 7 (0.6) | 5.0 (0.5) | 2 (1.1) | 0.373 |
| CV-caused mortality, year, n (%) | 51 (4.5) | 23 (2.4) | 28 (15.1) | <0.001 |
| MACCEs, 1 year, n (%) | 148 (13.0) | 73 (7.6) | 75 (40.5) | <0.001 |
| Variables | All Population (n = 1142) | Low IPI (<3.40) (n = 957; 83.8%) | High IPI (≥3.40) (n = 185; 16.2%) | p |
|---|---|---|---|---|
| Glucose, mg/dL | 112.9 ± 27.2 | 112.4 ± 26.6 | 115.5 ± 30.2 | 0.151 |
| eGFR, mL/min/1.73 m2 | 81.0 ± 23.7 | 83.8 ± 22.4 | 66.5 ± 24.6 | <0.001 |
| Uric acid, mg/dL | 5.49 ± 1.7 | 5.52 ± 1.7 | 5.38 ± 1.6 | 0.329 |
| Albumin, g/dL | 3.88 ± 0.48 | 3.91 ± 0.47 | 3.73 ± 0.50 | <0.001 |
| CRP, mg/dL, IQR | 5.36 [3.45–8.20] | 4.95 [3.20–7.68] | 7.76 [5.55–9.80] | <0.001 |
| Troponin I, ng/mL, IQR | 0.06 [0.02–0.21] | 0.06 [0.02–0.19] | 0.07 [0.03–0.30] | 0.047 |
| CAR, IQR | 1.42 [0.88–2.15] | 1.28 [0.82–1.99] | 2.0 [1.49–2.74] | <0.001 |
| TC, mg/dL | 193.5 ± 43.7 | 194.2 ± 44.2 | 189.5 ± 40.4 | 0.174 |
| LDL-C, mg/dL | 117.4 ± 37.7 | 118.2 ± 37.8 | 113.2 ± 37.4 | 0.10 |
| HDL-C, mg/dL | 39.2 ± 10.3 | 39.2 ± 10.2 | 39.53 ± 10.5 | 0.907 |
| Triglycerides, mg/dL, IQR | 122 [99.0–167.0] | 122 [99.0–164] | 122 [99.0–179.5] | 0.112 |
| Hemoglobin, g/dL | 13.5 ± 1.9 | 13.7 ± 1.8 | 12.6 ± 1.9 | <0.001 |
| WBC, 109/L | 8.51 ± 2.2 | 8.47 ± 2.2 | 8.77 ± 2.6 | <0.001 |
| Neutrophils, 109/L | 5.22 ± 1.8 | 5.13 ± 1.8 | 5.76 ± 2.0 | <0.001 |
| Lymphocytes, 109/L, IQR | 2.06 [1.60–2.59] | 2.13 [1.68–2.65] | 1.74 [1.28–2.36] | <0.001 |
| Monocytes, 109/L, IQR | 0.53 [0.43–0.66] | 0.53 [0.43–0.65] | 0.57 [0.45–0.71] | 0.037 |
| Platelets, 109/L | 254.1 ± 74.3 | 253.0 ± 72.1 | 259.9 ± 84.8 | 0.243 |
| NLR, IQR | 2.36 [1.84–3.24] | 2.29 [1.78–3.06] | 3.05 [2.33–4.22] | <0.001 |
| AISI, IQR | 307.8 [206.6–506.8] | 289.9 [194.0–470.5] | 429.9 [267.8–733.7] | <0.001 |
| IPI, IQR | 3.40 [1.94–5.85] | 2.99 [1.71–5.14] | 6.08 [4.50–8.28] | <0.001 |
| Variables | HR (95% CI) | p | Variables | HR (95% CI) | p |
|---|---|---|---|---|---|
| Male gender | 1.83 (1.31–2.55) | <0.001 | TIMI < 3 flow | 1.83 (1.15–2.90) | 0.010 |
| Age (years) | 1.03 (1.02–1.05) | <0.001 | Troponin I (ng/mL) | 1.44 (1.35–1.54) | <0.001 |
| Diabetes | 2.24 (1.62–3.09) | <0.001 | CRP (mg/dL) | 1.08 (1.04–1.13) | <0.001 |
| Previous CAD | 1.49 (1.08–2.05) | 0.016 | Albumin (g/dL) | 0.47 (0.35–0.63) | <0.001 |
| Previous HF | 2.95 (2.11–4.12) | <0.001 | Hemoglobin (g/dL) | 0.81 (0.75–0.88) | <0.001 |
| Previous CRF | 3.07 (2.15–4.39) | <0.001 | Lymphopenia | 0.52 (0.41–0.65) | <0.001 |
| Killip class II-IV | 1.66 (1.02–2.68) | 0.040 | CAR | 1.41 (1.22–1.62) | <0.001 |
| LVEF (%) | 0.94 (0.93–0.96) | <0.001 | NLR | 1.22 (1.14–1.30) | <0.001 |
| GRACE risk score | 1.03 (1.02–1.04) | <0.001 | AISI | 1.00 (1.00–1.00) | <0.001 |
| Syntax score I | 1.06 (1.03–1.08) | <0.001 | IPI | 1.09 (1.07–1.11) | <0.001 |
| Variables | Base Model | p | Base + GRACE Model | p | Base + GRACE + IPI Model | p |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| Male gender | 1.49 (1.05–2.12) | 0.027 | 1.47 (1.02–2.09) | 0.036 | 1.53 (1.07–2.21) | 0.021 |
| Diabetes | 1.82 (1.29–2.55) | <0.001 | 1.43 (1.00–2.03) | 0.048 | 1.62 (1.13–2.31) | 0.008 |
| Previous CAD | 1.08 (0.78–1.50) | 0.641 | 1.10 (0.79–1.53) | 0.577 | 1.09 (0.78–1.51) | 0.620 |
| Previous HF | 2.11 (1.50–2.97) | <0.001 | 1.66 (1.16–2.37) | 0.005 | 1.70 (1.19–2.42) | 0.003 |
| LVEF (%) | 0.95 (0.93–0.96) | <0.001 | 0.94 (0.93–0.95) | <0.001 | 0.94 (0.93–0.96) | <0.001 |
| Syntax score I | 1.04 (1.02–1.07) | <0.001 | 1.04 (1.01–1.06) | 0.004 | 1.03 (1.00–1.05) | 0.019 |
| TIMI < 3 flow | 1.17 (0.72–1.91) | 0.519 | 1.11 (0.68–1.82) | 0.668 | 1.24 (0.76–2.03) | 0.383 |
| Troponin I (ng/mL) | 1.39 (1.29–1.50) | <0.001 | 1.41 (1.30–1.53) | <0.001 | 1.43 (1.32–1.55) | <0.001 |
| Hemoglobin (g/dL) | 0.90 (0.82–0.98) | 0.021 | 0.97 (0.88–1.08) | 0.618 | 0.98 (0.89–1.09) | 0.734 |
| GRACE risk score | - | - | 1.02 (1.01–1.03) | <0.001 | 1.02 (1.01–1.03) | <0.001 |
| IPI | - | - | - | - | 1.07 (1.04–1.09) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oflar, E.; Kalyoncuoğlu, M.; Koyuncu, A.; Yıldız Erbaş, C.; Sinoplu, H.A.; Katkat, F.; Durmuş, G. The Role of the Inflammatory Prognostic Index in Patients with Non-ST Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention. J. Clin. Med. 2025, 14, 4491. https://doi.org/10.3390/jcm14134491
Oflar E, Kalyoncuoğlu M, Koyuncu A, Yıldız Erbaş C, Sinoplu HA, Katkat F, Durmuş G. The Role of the Inflammatory Prognostic Index in Patients with Non-ST Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Journal of Clinical Medicine. 2025; 14(13):4491. https://doi.org/10.3390/jcm14134491
Chicago/Turabian StyleOflar, Ersan, Muhsin Kalyoncuoğlu, Atilla Koyuncu, Cennet Yıldız Erbaş, Hasan Ali Sinoplu, Fahrettin Katkat, and Gündüz Durmuş. 2025. "The Role of the Inflammatory Prognostic Index in Patients with Non-ST Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention" Journal of Clinical Medicine 14, no. 13: 4491. https://doi.org/10.3390/jcm14134491
APA StyleOflar, E., Kalyoncuoğlu, M., Koyuncu, A., Yıldız Erbaş, C., Sinoplu, H. A., Katkat, F., & Durmuş, G. (2025). The Role of the Inflammatory Prognostic Index in Patients with Non-ST Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Journal of Clinical Medicine, 14(13), 4491. https://doi.org/10.3390/jcm14134491






