Pain Control in Oral Mucositis According to the Severity Scale: A Narrative Literature Review
Abstract
1. Introduction
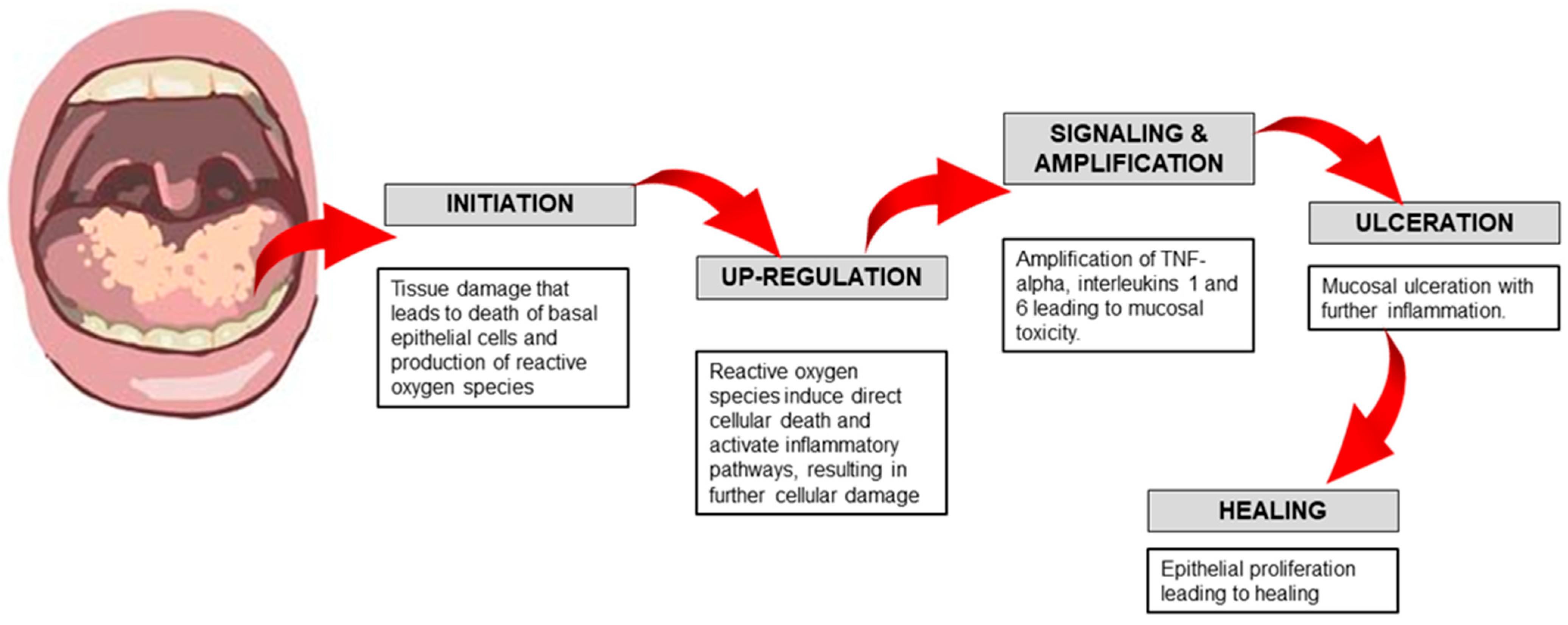
1.1. Phases of Pathogenesis of Oral Mucositis
1.2. Severity of OM
2. Methods
3. Results
3.1. Pharmacological Interventions
3.1.1. Topical Agents
Topical Anesthetic Agents
- Lidocaine
- b.
- Ketamine
- c.
- Benzydamine mouthwash
Topical Corticosteroids
Topical Antiseptics and Antimicrobials
Topical Coatings and Protective Agents
- Hyaluronate gel
- b.
- Sucralfate
Antidepressant Topical Rinse
3.1.2. Systemic Agents
Opioids
Corticosteroids
Cytoprotective Agent
Growth Factor
3.2. Non-Pharmacological Intervention
3.2.1. Oral Cryotherapy
3.2.2. Low-Level Laser Therapy/Photobiomodulation
3.2.3. Oral Capsaicin
3.2.4. Herbal and Natural Remedies
- Aloe vera
- b.
- Honey
3.2.5. Comprehensive Oral Care
| No. | Authors and Year | Type of Study | Number of Subjects | Brief Description of Study | Severity of OM | Grading Used | Interventions |
|---|---|---|---|---|---|---|---|
| 1 | Alfieri et al., 2016 [21] | Retrospective record study | 75 | Opioid therapy for patients with oropharyngeal cancer treated with chemoradiotherapy | 2 and above | WHO | Weak and strong opioids |
| 2 | Shillingburg et al., 2017 [25] | Open-label, prospective, phase II interventional study | 30 | Patient to gargle with ketamine mouthwash | 3–4 | WHO | Oral ketamine mouthwash 20 mg/5 mL four times daily and every 4 h |
| 3 | Cheng, 2006 [27] | RCT | 14 | Chlorhexidine vs. Benzydamine mouthwash from 1–14 days completion of RT | 2 and above | WHO | Chlorhexidine 0.2% vs. Benzydamine 0.15% mouthwash |
| 4 | Ahmed et al., 2019 [29] | RCT | 30 | Rheumatoid arthritis pts on methotrexate treatment randomly assigned to receive human platelet lysate vs. Clobetasol Propionate | 3 | WHO | Intervention: human platelet lysate Control: Clobetasol Propionate |
| 5 | Sio et al., 2020 [34] | RCT | 275 | 92 patients: doxepin mouthwash (25 mg/5 mL water); 91 patients: diphenhydramine-lidocaine-antacid; 92 patients: placebo. | 4 and greater | Pain score (scale, 0–10) | doxepin mouthwash or diphenhydramine-lidocaine-antacid mouthwash |
| 6 | Saito et al.; 2022 [37] | Retrospective analysis of medical record | 131 | Case: High dose of dexamethasone (9.9 mg infusion on day 1 and 8 mg orally on days 2–4) Control: Low dose of dexamethasone (6.6 mg infusion on day 1 and 4 mg orally on days 2–4 | 1–2 | WHO | Dexamethasone infusion |
| 7 | Dodd et al., 2022 [40] | RCT | 91 | 1. GG (GM CSF/GM CSF): GM CSF mouthwash during both prevention and treatment phases. 2. SS (Salt and Soda/Salt and Soda): Salt and soda mouthwash throughout. 3. SG (Salt and Soda then GM CSF): Salt and soda during prevention, switching to GM CSF at the onset of mucositis | unspecified | Radiation Therapy Oncology Group’s Acute Radiation Morbidity Scoring Criteria | Granulocyte-macrophage colony-stimulating factor (GM-CSF) mouthwash |
| 8 | Vadhan-Raj et al., 2010 [41] | RCT | 48 | Case: Palifermin as a single dose before each cycle of chemotherapy Control: Placebo | 2–4 | WHO | Palifermin (180 µg per kg of body weight) administered intravenously as a single dose 3 days before each chemotherapy cycle (maximum, 6 cycles) |
| 9 | Silva et al., 2011 [43] | RCT | 42 | Case: Low light laser therapy and oral hygiene protocol Control: Oral hygiene protocol | 0–4 | WHO | Low-level laser therapy 600 nm irradiation |
| 10 | Berger et al., 1995 [44] | Prospective case series | 11 | Patients were instructed to allow the candy to dissolve in the mouth | 1–4 | Easter Cooperative Oncology Group | Capsaicin taffy |
| 11 | Puataweeponga et al., 2009 [45] | Phase II trial | 61 | Case: Aloe vera juice Control: Placebo | 0–4 | Radiation Therapy Oncology Group’s Acute Radiation Morbidity Scoring Criteria | Aloe vera juice |
| 12 | Amanat et al., 2017 [48] | RCT | 82 | Case: Honey Control: Saline | 0–4 | Radiation Therapy Oncology Group’s Acute Radiation Morbidity Scoring Criteria | 20 mL of honey daily during radiotherapy |
| 13 | AkhavanKarbassi et al., 2016 [49] | RCT | 40 | Case: Propolis mouthwash Control: Placebo | 0–4 | WHO | 30% Propolis extract mouthwash (5 mL swished for 60 s and expectorated three times daily for 7 days) |
| 14 | Erdem & Güngörmüş, 2014 [50] | RCT | 103 | Case: Royal jelly with mouthwash therapy (Benzydamine hydrochloride and nystatin rinse) Control: Mouthwash therapy | 1–3 | WHO | Royal jelly, swished for 30 s and swallowed, daily dose of 1 g, twice a day |
4. Discussion
Limitations of This Review
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Harris, D.J. Cancer treatment-induced mucositis pain: Strategies for assessment and management. Ther. Clin. Risk Manag. 2006, 2, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Sroussi, H.Y.; Epstein, J.B.; Bensadoun, R.J.; Saunders, D.P.; Lalla, R.V.; Migliorati, C.A.; Heaivilin, N.; Zumsteg, Z.S. Common oral complications of head and neck cancer radiation therapy: Mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017, 6, 2918–2931. [Google Scholar] [CrossRef]
- Rivers, C.I.; Iovoli, A.J.; Chatterjee, U.; Hermann, G.M.; Singh, A.K. Intravenous fluids for pain management in head and neck cancer patients undergoing chemoradiation. Ann. Transl. Med. 2021, 9, 912. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Yang, S.; Wu, X.; Wang, S.; Li, X.; Zhang, F.; Wang, P.; Zhao, J. The quality of life in nasopharyngeal carcinoma radiotherapy: A longitudinal study. Asia-Pac. J. Oncol. Nurs. 2023, 10, 100251. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, J.; Lam, S.; Zhang, X.; Liu, A.; Teng, X.; Han, X.; Cao, J.; Li, H.; Lee, F.K.H.; et al. Multimodal data integration to predict severe acute oral mucositis of nasopharyngeal carcinoma patients following radiation therapy. Cancers 2023, 15, 2032. [Google Scholar] [CrossRef]
- Zrubáková, K.; Lehotská, M.; Herinková, A.; Podoba, R. The nurse as a member of an interprofessional team in the care of the oral cavity of cancer patients. Med. Stud. 2020, 36, 230. [Google Scholar] [CrossRef]
- Sonis, S.; Antin, J.; Tedaldi, M.; Alterovitz, G. SNP-based Bayesian networks can predict OM risk in autologous stem cell transplant recipients. Oral Dis. 2013, 19, 721–727. [Google Scholar] [CrossRef]
- Sonis, S.T.; Peterson, R.L.; Edwards, L.J.; Lucey, C.A.; Wang, L.; Mason, L.; Login, G.; Ymamkawa, M.; Moses, G.; Bouchard, P.; et al. Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral Oncol. 2000, 36, 373–381. [Google Scholar] [CrossRef]
- Kinhult, S.; Albertsson, M.; Eskilsson, J.; Cwikiel, M. Effects of probucol on endothelial damage by 5-fluorouracil. Acta Oncol. 2003, 42, 304–308. [Google Scholar] [CrossRef]
- Kuenen, B.C.; Levi, M.; Meijers, J.C.; van Hinsbergh, V.W.; Berkhof, J.; Kakkar, A.K.; Hoekman, K.; Pinedo, H.M. Potential role of platelets in endothelial damage during treatment with cisplatin, gemcitabine, and the angiogenesis inhibitor SU5416. J. Clin. Oncol. 2003, 21, 2192–2198. [Google Scholar] [CrossRef]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Chaveli-López, B.; Bagán-Sebastián, J.V. Treatment of oral mucositis due to chemotherapy. J. Clin. Exp. Dent. 2016, 8, e201–e209. [Google Scholar] [CrossRef] [PubMed]
- Wardley, A.M.; Jayson, G.C.; Swindell, R.; Morgenstern, G.R.; Chang, J.; Bloor, R.; Fraser, C.J.; Scarffe, J.H. Prospective evaluation of oral mucositis in patients receiving myeloablative conditioning regimens and haemopoietic progenitor rescue. Br. J. Haematol. 2000, 110, 292–299. [Google Scholar] [CrossRef]
- Wong, H.M. Oral complications and management strategies for patients undergoing cancer therapy. Sci. World. J. 2014, 2014, 581795. [Google Scholar] [CrossRef]
- Robien, K.; Schubert, M.M.; Bruemmer, B.; Lloid, M.E.; Potter, J.D.; Ulrich, C.M. Predictors of oral mucositis in patients receiving hematopoietic cell transplants for chronic myelogenous leukemia. J. Clin. Oncol. 2004, 22, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Payeras, M.R.; Cherubini, K.; de Figueiredo, M.A.Z.; Salum, F.G. Oral lichen planus: Focus on etiopathogenesis. Arch. Oral Biol. 2013, 58, 1057. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment; World Health Organization: Geneva, Switzerland, 1979; pp. 15–22.
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef]
- Chaudhry, S.; Ehtesham, Z. Treatment options for cancer patients suffering from oral mucositis. Asian Pac. J. Cancer Care 2023, 8, 181–184. [Google Scholar]
- Cheng, K.K. Oral mucositis: A phenomenological study of pediatric patients’ and their parents’ perspectives and experiences. Support Care Cancer 2009, 17, 829–837. [Google Scholar] [CrossRef]
- Alfieri, S.; Ripamonti, C.I.; Marceglia, S.; Orlandi, E.; Iacovelli, N.A.; Granata, R.; Cavallo, A.; Pozzi, P.; Boffi, R.; Bergamini, C.; et al. Temporal course and predictive factors of analgesic opioid requirement for chemoradiation-induced oral mucositis in oropharyngeal cancer. Head Neck. 2016, 38 (Suppl. S1), E1521–E1527. [Google Scholar] [CrossRef]
- Buchsel, P.C. Polyvinylpyrrolidone-sodium hyaluronate gel (Gelclair): A bioadherent oral gel for the treatment of oral mucositis and other painful oral lesions. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Ignoffo, R.J. Survey of topical oral solutions for the treatment of chemo-induced oral mucositis. J. Oncol. Pharm. Pract. 2005, 11, 139–143. [Google Scholar] [CrossRef]
- Abo Enin, H.A.; El Nabarawy, N.A.; Elmonem, R.A. Treatment of Radiation-Induced Oral Mucositis Using a Novel Accepted Taste of Prolonged Release Mucoadhesive Bi-medicated Double-Layer Buccal Films. AAPS PharmSciTech. 2017, 18, 563–575. [Google Scholar] [CrossRef]
- Shillingburg, A.; Kanate, A.S.; Hamadani, M.; Wen, S.; Craig, M.; Cumpston, A. Treatment of severe mucositis pain with oral ketamine mouthwash. Support Care Cancer 2017, 25, 2215–2219. [Google Scholar] [CrossRef] [PubMed]
- Nicolatou-Galitis, O.; Bossi, P.; Orlandi, E.; Bensadoun, R.J. The role of benzydamine in prevention and treatment of chemoradiotherapy-induced mucositis. Support Care Cancer 2021, 29, 5701–5709. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.F.; Tsui, Y.J. A pilot study of chlorhexidine and benzydamine oral rinses for the prevention and treatment of irradiation mucositis in patients with head and neck cancer. Cancer Nurs. 2006, 29, 423–430. [Google Scholar] [CrossRef]
- BC Cancer Agency. Symptom Management Guidelines: Oral Mucositis. Available online: http://www.bccancer.bc.ca/nursing-site/documents/12.%20oral%20mucositis.pdf (accessed on 20 January 2025).
- Ahmed, E.M.; Ali, S.; Gaafar, S.M.; Rashed, L.M.; Fayed, H.L. Evaluation of topical human platelet lysate versus topical clobetasol in management of methotrexate-induced oral ulceration in rheumatoid arthritis patients: Randomized-controlled clinical trial. Int. Immunopharmacol. 2019, 73, 389–394. [Google Scholar] [CrossRef]
- Barasch, A.; Elad, S.; Altman, A.; Damato, K.; Epstein, J. Antimicrobials, mucosal coating agents, anesthetics, analgesics, and nutritional supplements for alimentary tract mucositis. Support Care Cancer 2006, 14, 528–532. [Google Scholar] [CrossRef]
- Mohammed, A.I.; Celentano, A.; Paolini, R.; Low, J.T.; Silke, J.; O’Reilly, L.A.; McCullough, M.; Cirillo, N. High molecular weight hyaluronic acid drastically reduces chemotherapy-induced mucositis and apoptotic cell death. Cell Death Dis. 2023, 14, 453. [Google Scholar] [CrossRef]
- Coppini, M.; Caponio, V.C.A.; Mauceri, R.; Bizzoca, M.E.; Laino, L.; Lorenzo-Pouso, A.I.; Russo, D.; Troiano, G.; Silva, F.F.V.E.; Lo Muzio, L.; et al. Efficacy of topical agents in oral mucositis prevention: Systematic review and network meta-analysis. Oral Dis. 2024, 30, 4126–4144. [Google Scholar] [CrossRef]
- Keefe, D.M.; Schubert, M.M.; Elting, L.S.; Sonis, S.T.; Epstein, J.B.; Raber-Durlacher, J.E.; Migliorati, C.A.; McGuire, D.B.; Hutchins, R.D.; Peterson, D.E. Mucositis study section of the multinational association of supportive care in cancer and the International Society for Oral Oncology. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 2007, 109, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Sio, T.T.; Le-Rademacher, J.G.; Leenstra, J.L.; Loprinzi, C.L.; Rine, G.; Curtis, A.; Singh, A.K.; Martenson, J.A., Jr.; Novotny, P.J.; Tan, A.D.; et al. Effect of doxepin mouthwash or diphenhydramine-lidocaine-administered mouthwash on mucositis pain in patients with head and neck cancer. J Clin Oncol. 2020, 38, e48–e50. [Google Scholar]
- Fielding, F.; Sanford, T.M.; Davis, M.P. Achieving effective control in cancer pain: A review of current guidelines. Int. J. Palliat. Nurs. 2013, 19, 584. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.; Lambert, D.G. Opioid mechanisms and opioid drugs. Anaesth. Intensive Care Med. 2008, 9, 33–37. [Google Scholar] [CrossRef]
- Saito, Y.; Takekuma, Y.; Takeshita, T.; Oshino, T.; Sugawara, M. Impact of systemic dexamethasone administration on oral mucositis induced by anthracycline-containing regimens in breast cancer treatment. Sci Rep. 2022, 12, 12587. [Google Scholar] [CrossRef]
- Redding, S.W. Cancer therapy-related oral mucositis. J. Dent. Educ. 2005, 69, 919–929. [Google Scholar] [CrossRef]
- Raber-Durlacher, J.E.; von Bültzingslöwen, I.; Logan, R.M.; Bowen, J.; Al-Azri, A.R.; Everaus, H.; Gerber, E.; Gomez, J.G.; Pettersson, B.G.; Soga, Y.; et al. Systematic review of cytokines and growth factors for the management of oral mucositis in cancer patients. Support Care Cancer 2013, 21, 343–355. [Google Scholar] [CrossRef]
- Dodd, M.J.; Cho, M.H.; Cooper, B.A.; MacPhail, L.; Miaskowski, C. A randomized clinical trial of granulocyte macrophage colony stimulating factor mouthwash for oral mucositis in head and neck cancer. Eur. J. Oncol. Nurs. 2022, 56, 102093. [Google Scholar] [CrossRef]
- Vadhan-Raj, S.; Trent, J.; Patel, S.; Zhou, X.; Johnson, M.M.; Araujo, D.; Ludwig, J.A.; O’Roark, S.; Gillenwater, A.M.; Bueso-Ramos, C.; et al. Single-dose palifermin prevents severe oral mucositis during multicycle chemotherapy in patients with cancer: A randomized trial. Ann. Intern. Med. 2010, 153, 358–367. [Google Scholar] [CrossRef]
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of oral mucositis in patients who have cancer. Dent. Clin. N. Am. 2008, 52, 61–77. [Google Scholar] [CrossRef]
- Silva, G.B.; Mendonça, E.F.; Bariani, C.; Antunes, H.S.; Silva, M.A.G. The prevention of induced oral mucositis with low-level laser therapy in bone marrow transplantation patients: A randomized clinical trial. Photomed. Laser Surg. 2011, 29, 27–31. [Google Scholar] [CrossRef]
- Berger, A.; Henderson, M.; Nadoolman, W.; Duffy, V.; Cooper, D.; Saberski, L.; Bartoshuk, L. Oral capsaicin provides temporary relief for oral mucositis pain secondary to chemotherapy/radiation therapy. J. Pain Symptom Manag. 1995, 10, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Puataweeponga, P.; Dangpraserta, S.; Chomporn, S.; Sawangsilpa, T.; Narkwonga, L.; Puttikarana, P.; Intragumtornchaib, T. The efficacy of oral Aloe vera juice for radiation induced mucositis in head and neck cancer patients: A double-blind placebo-controlled study. Asian Biomed. 2009, 3, 375–382. [Google Scholar]
- Maria, O.M.; Eliopoulos, N.; Muanza, T. Radiation-induced OM. Front Oncol. 2017, 7, 89. [Google Scholar] [CrossRef]
- Zakaria, S. Natural remedies target different therapeutic pathways in oral mucositis induced by cancer chemo or radiotherapy. Am. J. Phytomed. Clin. Ther. 2017, 5, 250–256. [Google Scholar]
- Amanat, A.; Ahmed, A.; Kazmi, A.; Aziz, B. The effect of honey on radiation-induced oral mucositis in head and neck cancer patients. Indian J. Palliat. Care. 2017, 23, 317–320. [Google Scholar] [CrossRef] [PubMed]
- AkhavanKarbassi, M.H.; Yazdi, M.F.; Ahadian, H.; Sadr-Abad, M.J. Randomized double-blind placebo-controlled trial of propolis for oral mucositis in patients receiving chemotherapy for head and neck cancer. Asian Pac. J. Cancer Prev. 2016, 17, 3611–3614. [Google Scholar]
- Erdem, O.; Güngörmüş, Z. The effect of royal jelly on oral mucositis in patients undergoing radiotherapy and chemotherapy. Holist. Nurs. Pract. 2014, 28, 242–246. [Google Scholar] [CrossRef]
- Schneider, S.; Hess, K.; Gosselin, T. Interventions to promote adherence with oral agents. Semin. Oncol. Nurs. 2011, 27, 133. [Google Scholar] [CrossRef]
- Al-Ansari, S.; Zecha, J.A.; Barasch, A.; de Lange, J.; Rozema, F.R.; Raber-Durlacher, J.E. Oral mucositis induced by anticancer therapies. Curr. Oral Health Rep. 2015, 2, 202–211. [Google Scholar] [CrossRef]
- Ariyawardana, A.; Cheng, K.K.F.; Kandwal, A.; Tilly, V.; Al-Azri, A.R.; Galiti, D.; Chiang, K.; Vaddi, A.; Ranna, V.; Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society for Oral Oncology (MASCC/ISOO); et al. Systematic review of anti-inflammatory agents for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2019, 27, 3985–3995. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.E.; Kim, H.S. Sodium bicarbonate solution versus chlorhexidine mouthwash in oral care of acute leukemia patients undergoing induction chemotherapy: A randomized controlled trial. Asian Nurs. Res. (Korean Soc. Nurs. Sci.) 2012, 6, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.E.P.; Cheng, K.K.F.; Chiang, K.; Kandwal, A.; Loprinzi, C.L.; Mori, T.; Potting, C.; Rouleau, T.; Toro, J.J.; Ranna, V.; et al. Systematic review of oral cryotherapy for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2020, 28, 2449–2456. [Google Scholar] [CrossRef]
- Yarom, N.; Hovan, A.; Bossi, P.; Ariyawardana, A.; Jensen, S.B.; Gobbo, M.; Saca-Hazboun, H.; Kandwal, A.; Majorana, A.; Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO); et al. Systematic review of natural and miscellaneous agents, for the management of oral mucositis in cancer patients and clinical practice guidelines—Part 2: Honey, herbal compounds, saliva stimulants, probiotics, and miscellaneous agents. Support Care Cancer 2020, 28, 2457–2472. [Google Scholar] [CrossRef]
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO); et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461. [Google Scholar] [CrossRef]
- Saunders, D.P.; Rouleau, T.; Cheng, K.; Yarom, N.; Kandwal, A.; Joy, J.; Bektas Kayhan, K.; van de Wetering, M.; Brito-Dellan, N.; Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO); et al. Systematic review of antimicrobials, mucosal coating agents, anesthetics, and analgesics for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2020, 28, 2473–2484. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.; Glenny, A.M.; Worthington, H.V.; Littlewood, A.; Fernandez Mauleffinch, L.M.; Clarkson, J.E.; McCabe, M.G. Interventions for preventing oral mucositis in patients with cancer receiving treatment: Cytokines and growth factors. Cochrane Database Syst Rev. 2017, 11, CD011990. [Google Scholar] [CrossRef]
- Kulkarni, R.; Fanse, S.; Burgess, D.J. Mucoadhesive drug delivery systems: A promising noninvasive approach to bioavailability enhancement. Part II: Formulation considerations. Expert Opin. Drug Deliv. 2023, 20, 413–434. [Google Scholar] [CrossRef]
- Priya, K.L.; Maheswary, D.; Ravi, S.S.S.; Leela, K.V.; Lathakumari, R.H.; Malavika, G. The impact of probiotics on oral cancer: Mechanistic insights and therapeutic strategies. Oral Oncol. Rep. 2025, 13, 100715. [Google Scholar] [CrossRef]
- Cheng, K.; Martin, L.; Slepian, M.; Patwardhan, A.; Ibrahim, M. Mechanisms and pathways of pain photobiomodulation: A narrative review. J. Pain. 2021, 22, 763–777. [Google Scholar] [CrossRef]
| World Health Organization OM Scale [17] | |
| Grade | Description |
| 0 | No mucositis |
| 1 | Soreness/erythema |
| 2 | Erythema and ulcers but able to tolerate solid diet. |
| 3 | Unable to tolerate solids but able to tolerate liquids |
| 4 | Unable to tolerate solids or liquids. Oral alimentation is not possible. |
| Radiation Therapy Oncology Group’s Acute Radiation Morbidity Scoring Criteria for Mucosa [18] | |
| 0 | No change over baseline |
| 1 | Injection/may experience mild pain not requiring analgesic |
| 2 | Patchy mucositis, which may produce an inflammatory serosanguinitis discharge/may experience moderate pain requiring analgesia |
| 3 | Confluent fibrinous mucositis/may include severe pain requiring narcotic |
| 4 | Ulceration, hemorrhage, or necrosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhaimi, N.; Abdul Razak, N.A.H.; Ramli, R. Pain Control in Oral Mucositis According to the Severity Scale: A Narrative Literature Review. J. Clin. Med. 2025, 14, 4478. https://doi.org/10.3390/jcm14134478
Suhaimi N, Abdul Razak NAH, Ramli R. Pain Control in Oral Mucositis According to the Severity Scale: A Narrative Literature Review. Journal of Clinical Medicine. 2025; 14(13):4478. https://doi.org/10.3390/jcm14134478
Chicago/Turabian StyleSuhaimi, Nawal, Noor Azura Hani Abdul Razak, and Roszalina Ramli. 2025. "Pain Control in Oral Mucositis According to the Severity Scale: A Narrative Literature Review" Journal of Clinical Medicine 14, no. 13: 4478. https://doi.org/10.3390/jcm14134478
APA StyleSuhaimi, N., Abdul Razak, N. A. H., & Ramli, R. (2025). Pain Control in Oral Mucositis According to the Severity Scale: A Narrative Literature Review. Journal of Clinical Medicine, 14(13), 4478. https://doi.org/10.3390/jcm14134478




