Trajectories of Pain in Low-Opioid and Opioid-Based Postoperative Analgesia in Older Patients—Perioperative Clinical Study
Abstract
1. Introduction
2. Materials and Methods
Analgesic Scheme in Study and Control Group
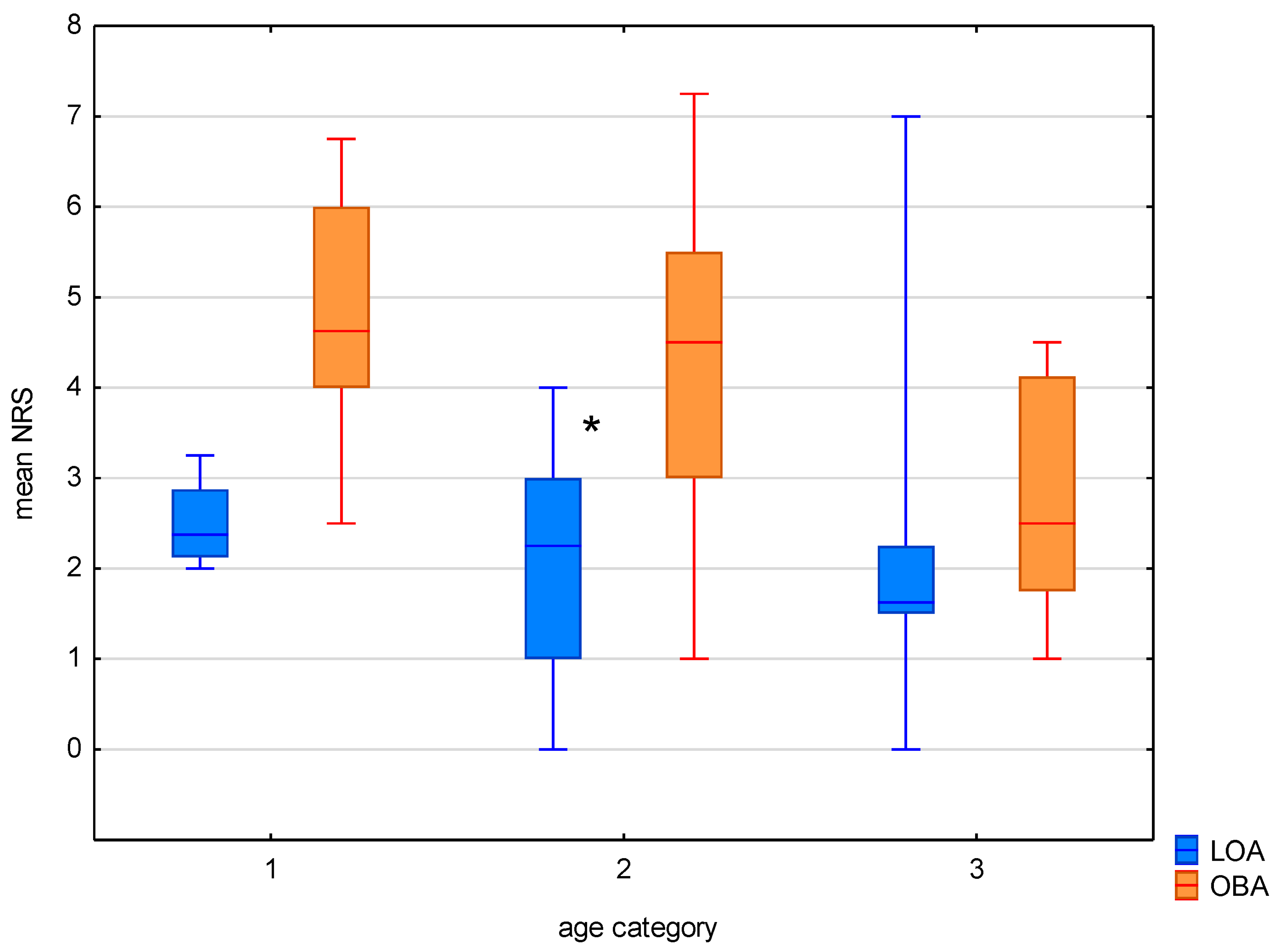
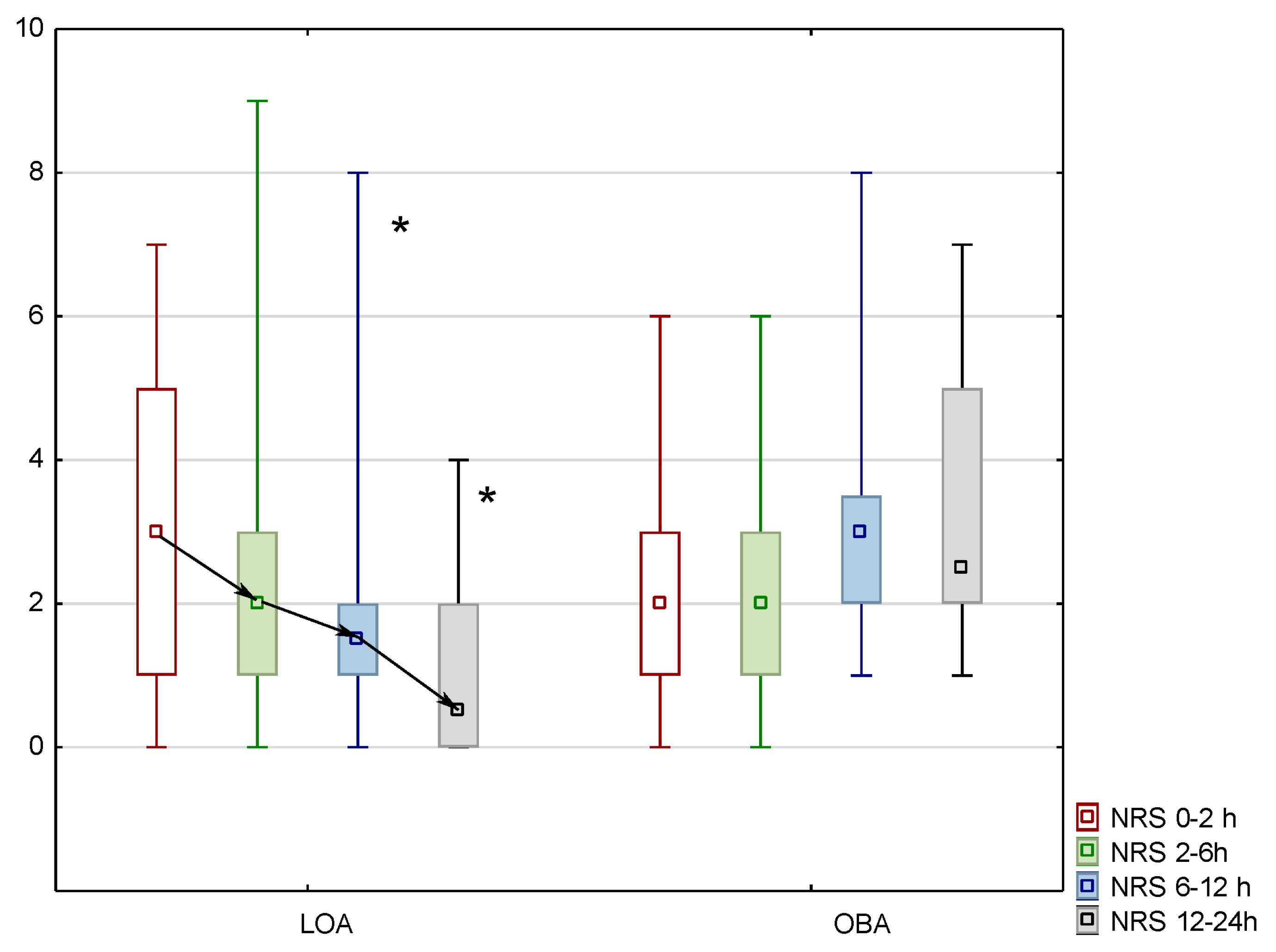
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, J.; Zhang, T.; Xiong, X.; Chen, H.; Jiang, Y.; He, S. A prospective study of chronic postsurgical pain in elderly patients: Incidence, characteristic and risk factors. BMC Geriatr. 2023, 23, 289. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S. Infuence of aging on opioid dosing for perioperative pain management:a focus on pharmacokinetics. J. Anesth. Analg. Crit. Care 2024, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Van Zundert, T.; Gatt, S.; Van Zundert, A. Anesthesia and perioperative pain relief in the frail elderly patient. Saudi J. Anesth. 2023, 17, 566–574. [Google Scholar] [CrossRef] [PubMed]
- McCleane, G. Pharmacological pain management in the elderly patient. Clin. Interv. Aging 2007, 4, 637–643. [Google Scholar] [CrossRef]
- Rakel, B.; Herr, K. Assessment and treatment of postoperative pain in older adults. J. Perianesthesia Nurs. 2004, 3, 194–208. [Google Scholar] [CrossRef]
- Falzone, E.; Hoffmann, C.; Keita, H. Postoperative analgesia in elderly patients. Drugs Aging 2013, 30, 81–90. [Google Scholar] [CrossRef]
- Tighe, P.; Le-Wendling, L.; Patel, A.; Zou, B.; Fillingim, R. Clinically derived early postoperative pain trajectories differ by age, sex, and type of surgery. Pain 2015, 4, 609–617. [Google Scholar] [CrossRef]
- Shellito, A.; Dworsky, J.; Kirkland, P.; Rosenthal, R.; Sarkisian, C.; Ko, C.; Russel, M. Perioperative pain management issues unique to older adults undergoing surgery. Ann. Surg. Open 2021, 3, e072. [Google Scholar] [CrossRef]
- Ramwani, R.; Wernberg, J. The use of opioi analgesia after surgery: Assessing postoperative prescription from patient and surgeon perspective. Clin. Med. Res. 2021, 20, 89–94. [Google Scholar] [CrossRef]
- Cohendy, R.; Brougere, A.; Cuvillon, P. Anaesthesia in the older patient. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 17–21. [Google Scholar] [CrossRef]
- Mędrzycka-Dabrowska, W.; Dabrowski, S.; Basinski, A.; Pilch, D. Perception of barriers to postoperative pain management in elderly patients in Polish hospitals with and without a Hospital Without pain certificate- a multi-center study. Arch. Med. Sci. 2016, 4, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Del Tedesco, F.; Sessa, F.; Xhemalaj, R.; Sollazzi, L.; Russo, C.; Aceto, P. Perioperative analgesia in the elderly. Saudi J. Anesth. 2023, 17, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Pius, A.; Jones, E.; Bonnel, L.; Fujii, M.; MacLean, C. Patients experience with opioid pain medication after discharge from surgery; a mixed- methods study. J. Surg. Res. 2020, 256, 328–337. [Google Scholar] [CrossRef] [PubMed]
- El-Kefraoui Ch Olleik, G.; Chay, M.; Kouyoumdijan, A.; Powanda, P.; Rajabiyazdi, F.; Do, U.; Derksen, A.; Landry, T.; Zifkin, A.; Ramanakumar, A.; et al. Opioid versus opioid-free analgesia after surgical discharge: Protocol for a systemic review and meta-analysis. BMJ Open 2020, 10, e035443. [Google Scholar] [CrossRef]
- Ong, T.; Thiam, C. Special consideration for pain management in the older person. Clin. Med. 2022, 4, 295–297. [Google Scholar] [CrossRef]
- Nowak, H.; Zech, N.; Asmussen, S.; Rahmen, T.; Tryba, M.; Oprea, G.; Grause, L.; Schork, K.; Moeller, M.; Loeser, J.; et al. Effect of therapeutic suggestions during general anaesthesia on postoperative pain and opioid use: Multicentre randomised controlled trial. BMJ 2020, 371, m4284. [Google Scholar] [CrossRef]
- Feinberg, A.; Acuna, S.; Smith, D.; Kashin, B.; Mocon, A.; Yau, B.; Chiu, J.; Srikandarajah, S. Optimizing opioid prescription after laparoscopic appendectomy and cholecystectomy. Can. J. Surg. 2021, 64, E69–E75. [Google Scholar] [CrossRef]
- Apfelbaum, J.; Chen, C.; Mehta, S.; Gan, T. Postoperative pain experience; results from national survey suggest postoperative pain continues to be undermanaged. Anesth. Analg. 2003, 97, 534–540. [Google Scholar] [CrossRef]
- Kosciuczuk, U.; Jakubow, P.; Czyzewska, J.; Knapp, P.; Rynkiewicz-Szczepanska, E. Plasma Brain-Derived Neurotrophic Factor and Opioid Therapy: Results of Pilot Cross-Sectional Study. Clin. Med. Res. 2022, 20, 195–203. [Google Scholar] [CrossRef]
- Do, U.; El-Kefraoui, C.; Pook, M.; Balvardi, S.; Barone, N.; Powanda, P.; Lee, L.; Baldini, G.; Feldman, L.F. Feasibility of prospectively comparing opioid analgesia with opioid-free analgesia after outpatient general surgery. JAMA Netw. Open 2022, 7, e2221430. [Google Scholar] [CrossRef]
- Assaf, G.; Yared, F.; Boutros Ch Maassarani, D.; Seblani, R.; Khalaf, C.; Kaady, J. The efficacy of opioid-free general anesthesia in the management of hip surgeries in elderly patients. Cureus 2020, 12, e11295. [Google Scholar] [CrossRef] [PubMed]
- Guinot, P.; Spitz, A.; Berthoud, V.; Ellouze, O.; Missaoui, A.; Constandache, T.; Grosjean, S.; Raghouani, M.; Anciaux, J.; Parthiot, J.; et al. Effect of opioid-free anaesthesia on postoperative period in cardiac surgery: A retrospective matched case-control study. BMC Anesthesiol. 2019, 19, 136. [Google Scholar] [CrossRef] [PubMed]
- Kosciuczuk, U.; Tarnowska, K.; Rynkiewicz-Szczepanska, E. Are There Any Advantages of the Low Opioid Anaesthesia and Non-Opioid Postoperative Analgesia Protocol: A Clinical Observational Study. J. Pain Res. 2024, 17, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Kosciuczuk, U.; Knapp, P.; Lotowska-Cwiklewska, A.M. Opioid-induced immunosuppression and carcinogenesis promotion theories create the newest trend in acute and chronic pain pharmacotherapy. Clinics 2020, 75, e1554. [Google Scholar] [CrossRef]
- de Boer, H.D.; Detriche, O.; Forget, P. Opioid-related side effects: Postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 499–504. [Google Scholar] [CrossRef]
- Mulier, J.P.; Hunter, J.M.; de Boer, H.D. Seventy-five years since the birth of the Liverpool anaesthetic technique. Br. J. Anaesth. 2021, 126, 343–347. [Google Scholar] [CrossRef]
- Verret, M.; Lalu, M.M.; Assi, A.; Nicholls, S.G.; Turgeon, A.F.; Carrier, F.M.; the Canadian Perioperative Anesthesia Clinical Trials (PACT) Group. Use of opioids and opioids alternatives during general anesthesia: A Canadian survey among anesthesiologists. Can. J. Anesth. 2024, 71, 1694–1704. [Google Scholar] [CrossRef]
- O’Neil, A.; Lirk, P. Multimodal Analgesia. Anesthesiol. Clin. 2022, 40, 455–468. [Google Scholar] [CrossRef]
- Kosciuczuk, U.; Jakubow, P.; Tarnowska, K.; Rynkiewicz-Szczepanska, E. Opioid Therapy and Implications for Oxidative Balance: A Clinical Study of Total Oxidative Capacity (TOC) and Total Antioxidative Capacity (TAC). J. Clin. Med. 2023, 13, 82. [Google Scholar] [CrossRef]
- Carcamo-Cavazos, V.; Cannesson, M. Opioid-Free Anesthesia: The Pros and Cons. Adv. Anesth. 2022, 40, 149–166. [Google Scholar] [CrossRef]
- Salomé, A.; Harkouk, H.; Fletcher, D.; Martinez, V. Opioid-Free Anesthesia Benefit-Risk Balance: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2021, 10, 2069. [Google Scholar] [CrossRef] [PubMed]
- Bégaud, B.; de Germay, S.; Noize, P. Drugs and the elderly: A complex interaction. Therapies 2023, 78, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Giraldo, C.; Rosas-Morales, C.; Vásquez, F.; Isaza-Restrepo, A.; Ibáñez-Pinilla, M.; Vargas-Rubiano, S.; Vargas-Barato, F. Laparoscopic cholecystectomy in super elderly (>90 years of age): Safety and outcomes. Surg. Endosc. 2023, 37, 5989–5998. [Google Scholar] [CrossRef] [PubMed]
- Lord, A.C.; Hicks, G.; Pearce, B.; Tanno, L.; Pucher, P.H. Safety and outcomes of laparoscopic cholecystectomy in the extremely elderly: A systematic review and meta-analysis. Acta Chir. Belg. 2019, 119, 349–356. [Google Scholar] [CrossRef]
- Van Dijk, J.; Zaslansky, R.; van Boeckel, R.; Alam, J.; Baart, S.; Huygen, F.; Rijsdijk, M. Postoperative pain and age: A retrospective cohort association study. Anesthesiology 2021, 135, 1104–1119. [Google Scholar] [CrossRef]
- Lewandowska, A.; Filip, R.; Mucha, M. Postoperative pain combating and evaluation of patient satisfaction from analgesic treatment. Ann. Agric. Environ. Med. 2013, 1, 48–51. [Google Scholar]
- Chia, P.A.; Cannesson, M.; Bui, C.C.M. Opioid free anesthesia: Feasible? Curr. Opin. Anaesthesiol. 2020, 33, 512–517. [Google Scholar] [CrossRef]
- Rogobete, A.F.; Sandesc, D. General Anesthesia as a Multimodal Individualized Clinical Concept. Medicina 2022, 58, 956. [Google Scholar] [CrossRef]
- Oya, R.; Ogawa, S.; Oya, K.; Hirakawa, Y.; Maeda, C.; Amaya, F. Prevalence of preoperative opioid usage and its impact on postoperative outcomes: A retrospective cohort study. J. Anesth. 2023, 37, 532–538. [Google Scholar] [CrossRef]
- Tinnirello, A.; Mazzoleni, S.; Santi, C. Chronic Pain in the Elderly: Mechanisms and Distinctive Features. Biomolecules 2021, 11, 1256. [Google Scholar] [CrossRef]
- Gerbershagen, H.; Aduckathil, S.; van Wijck, A.; Peelen, L.; Kalkman, C.; Meissner, W. Pain intensity on the first day after surgery. Anesthesiology 2013, 118, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Khaled, M.; Sabac, D.; Marcucci, M. Postoperative pain and pain management and neurocognitive outcomes after non-cardiac surgery: A protocol for a series of systematic reviews. Syst. Rev. 2022, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Kneppova, K.; Kralikova, I.; Cambal, M.; Labas, P. Postoperative pain management in geriatric patients after cholecystectomy and studies of glycemia and cortisol levels. Bratisl. Med. 2022, 123, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Van de Velde, M.; Pogatzki-Zahn, E. Opioid-free anaesthesia: Should we all adopt it? An overview of current evidence. Eur. J. Anaesthesiol. 2023, 40, 539–541. [Google Scholar] [CrossRef]
- Feenstra, M.L.; Jansen, S.; Eshuis, W.J.; van Berge Henegouwen, M.I.; Hollmann, M.W.; Hermanides, J. Opioid-free anesthesia: A systematic review and meta-analysis. J. Clin. Anesth. 2023, 90, 111215. [Google Scholar] [CrossRef]
- Park, M.; Kim, B.; Kim, G. Prediction of postoperative pain and analgesic requirements using surgical pleth index: A observational study. J. Clin. Monit. Comp. 2020, 34, 583–587. [Google Scholar] [CrossRef]
- Ibrahim, M.; Elnabtity, A.M.; Hegab, A.; Alnujaidi, O.A.; El Sanea, O. Combined opioid free and loco-regional anaesthesia enhances the quality of recovery in sleeve gastrectomy done under ERAS protocol: A randomized controlled trial. BMC Anesthesiol. 2022, 22, 29. [Google Scholar] [CrossRef]
- Tahota, R.S.; Ramkiran, S.; Garg, R.; Goswami, J.; Baxi, V.; Thomas, M. Opioid free onco-anesthesia: Is it time to convict opioids? A systematic review of literature. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, 441–452. [Google Scholar] [CrossRef]
- Kosciuczuk, U.; Kossakowska, A.; Talalaj, M.; Grabowska, K.; Pryzmont, M. Sex-Related Analgesic Effects of Opioid-Based Anesthesia and Low-Opioid Anesthesia with Non-Opioid Postoperative Analgesia—A Clinical Observational Study. J. Clin. Med. 2025, 14, 2163. [Google Scholar] [CrossRef]
- Toleska, M.; Dimitrovski, A.; Dimitrovska, N. Comparison among opioid-based, low opioid and opioid free anesthesia in colorectal oncologic surgery. Pril 2023, 44, 117–126. [Google Scholar] [CrossRef]
| LOA | OBA | |
|---|---|---|
| Category 1 (≤40 years old) | n = 4 | n = 6 |
| Age | 35 (26–40) | 35 (27–40) |
| BMI | 25.5 (22–29) | 23 (21–29) |
| BSA | 1.8 (1.68–2.03) | 1.81 (1.64–1.96) |
| Duration of surgery (min) | 40 (35–50) | 42 (38–55) |
| Duration of anaesthesia (min) | 54 (45–65) | 52 (45–70) |
| Category 2 (41–60 years old) | n = 17 | n = 19 |
| Age | 49 (41–60) | 51 (41–60) |
| BMI | 26 (18–34) | 28 (19–41) |
| BSA | 1.8 (1.56–2.07) | 1.86 (1.54–2.14) |
| Duration of surgery (min) | 43 (35–52) | 45 (38–60) |
| Duration of anaesthesia (min) | 55 (45–75) | 56 (48–70) |
| Category 3 (>60 years old) | n = 18 | n = 12 |
| Age | 68 (63–85) | 68 (63–81) |
| BMI | 29.5 (20–38) | 31 (24–38) |
| BSA | 1.86 (1.61–2.21) | 1.85 (1.63–2.35) |
| Duration of surgery (min) | 45 (38–65) | 50 (40–68) |
| Duration of anaesthesia (min) | 55 (45–76) | 60 (50–75) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosciuczuk, U.; Talalaj, M.; Rynkiewicz-Szczepanska, E. Trajectories of Pain in Low-Opioid and Opioid-Based Postoperative Analgesia in Older Patients—Perioperative Clinical Study. J. Clin. Med. 2025, 14, 4416. https://doi.org/10.3390/jcm14134416
Kosciuczuk U, Talalaj M, Rynkiewicz-Szczepanska E. Trajectories of Pain in Low-Opioid and Opioid-Based Postoperative Analgesia in Older Patients—Perioperative Clinical Study. Journal of Clinical Medicine. 2025; 14(13):4416. https://doi.org/10.3390/jcm14134416
Chicago/Turabian StyleKosciuczuk, Urszula, Marcin Talalaj, and Ewa Rynkiewicz-Szczepanska. 2025. "Trajectories of Pain in Low-Opioid and Opioid-Based Postoperative Analgesia in Older Patients—Perioperative Clinical Study" Journal of Clinical Medicine 14, no. 13: 4416. https://doi.org/10.3390/jcm14134416
APA StyleKosciuczuk, U., Talalaj, M., & Rynkiewicz-Szczepanska, E. (2025). Trajectories of Pain in Low-Opioid and Opioid-Based Postoperative Analgesia in Older Patients—Perioperative Clinical Study. Journal of Clinical Medicine, 14(13), 4416. https://doi.org/10.3390/jcm14134416







