A Home-Based Interdisciplinary Intervention to Enhance Functionality in Oncology Patients: Results from a Clinical Trial
Abstract
1. Introduction
1.1. Primary Objective
1.2. Secondary Objectives
- 1.
- To analyse the impact of the Effort Re-education Programme on health-related quality of life, assessed using the EORTC QLQ-C30 questionnaire.
- 2.
- To evaluate the progression of patients’ general functional status throughout this study, using the Karnofsky Performance Scale.
- 3.
- To determine the effects of the programme on the ability to carry out instrumental activities of daily living, measured using the Lawton and Brody Scale.
- 4.
- To explore correlations between functionality, quality of life, general functional status, and level of autonomy at baseline, in order to identify potential clinical and sociodemographic factors associated with clinical outcomes.
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants and Sample
2.2.1. Target Population
2.2.2. Eligibility Criteria
- Inclusion Criteria:
- Any histopathological diagnosis of newly diagnosed or relapsed cancer as the reason for hospital admission.
- Hospitalisation in the Oncology Department of the University Hospital of Salamanca.
- Moderate to severe dependency: Barthel Index score between 20 and 55 points.
- Signed informed consent authorising voluntary participation.
- Exclusion Criteria:
- Cognitive impairment, defined as a score <24 on the Mini-Mental State Examination (MMSE).
- Patients with a history of lymphoma or severe cardiac involvement.
- Withdrawal Criteria:
- Death of the patient.
- Disease progression leading to a terminal condition.
- Hospitalisation at the time of home follow-up.
- Incomplete final assessment.
2.3. Sample Size Calculation
2.4. Randomisation and Blinding
2.5. Procedures and Data Collection
2.5.1. Assessment Schedule
- 1.
- Baseline assessment at hospital discharge (after inclusion and before randomisation).
- 2.
- Follow-up assessment at 15 days.
- 3.
- Final assessment one month after the baseline assessment.
2.5.2. Reporting of Results
2.6. Variables
2.6.1. Primary Variable
- Secondary Variables
- Health-related quality of life (HRQoL): This metric was measured using the EORTC QLQ-C30 questionnaire [19], which assesses multiple functional dimensions and cancer-related symptoms.
- Overall quality of life/functional status: This metric was assessed using the Karnofsky Performance Scale [20], with scores ranging from 0 (death) to 100 (normal activity, no evidence of disease).
- Instrumental activities of daily living (IADLs): This metric was assessed using the Lawton and Brody Scale [21], which rates the level of independence in tasks such as using the telephone, preparing meals, or managing finances.
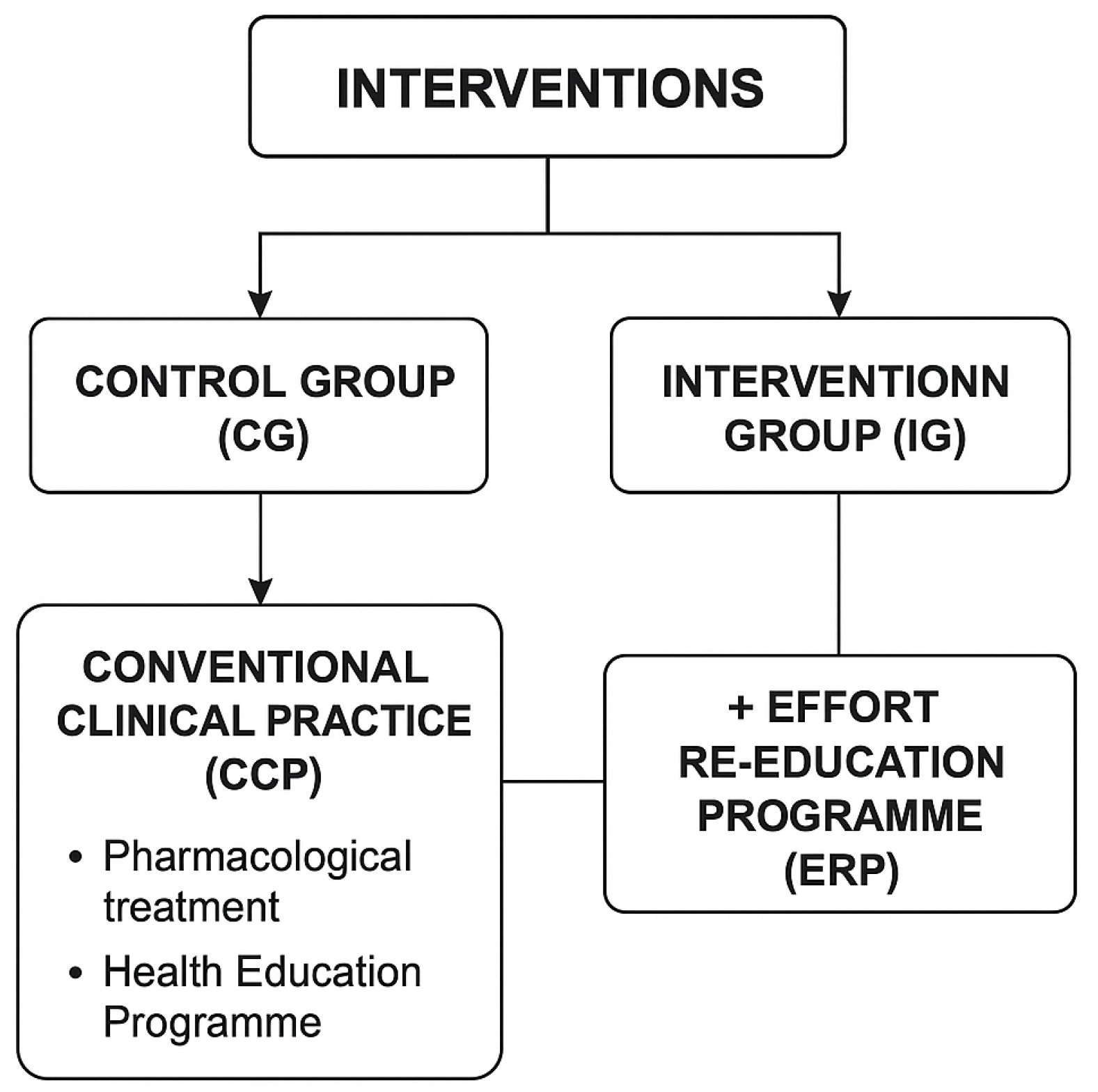
- Interventions
- A. Control Group (CG): Conventional Clinical Practice—Pharmacological Treatment + Health Education Programme
- B. Intervention Group (IG): Effort Re-education Programme to Improve Performance in Activities of Daily Living
- 1.
- Functional Re-education
- Direct intervention in activities of daily living (ADLs).
- Energy conservation techniques (ECTs).
- Sleep hygiene recommendations based on NCCN guidelines.
- 2.
- Prescription of assistive products and environmental adaptations
2.6.2. Visit Schedule
- Baseline visit: conducted before discharge and included full data collection and randomisation.
- Follow-up visits (15 days and 1 month): conducted at UDATO and followed the same structure as the baseline visit, except for sociodemographic data.
2.6.3. Data Analysis
2.6.4. Trial Registration and Scientific Rigor
3. Results
3.1. Baseline Sociodemographic and Clinical Characteristics
3.2. Intragroup Progression
3.3. Between-Group Comparative Analysis
3.4. Correlation Analysis
4. Discussion
Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NCCN | National Comprehensive Cancer Network. |
| CAUSA | University Hospital Complex of Salamanca. |
| UDATO | Teaching and Clinical Unit of Occupational Therapy. |
| HRQoL | Health-related quality of life. |
| IADLs | Instrumental activities of daily living. |
References
- Soerjomataram, I.; Bray, F. Planning for tomorrow: Global cancer incidence and the role of prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Emery, J.; Butow, P.; Lai-Kwon, J.; Nekhlyudov, L.; Rynderman, M.; Jefford, M. Management of common clinical problems experienced by survivors of cancer. Lancet 2022, 399, 1537–1550. [Google Scholar] [CrossRef] [PubMed]
- Amafah, J.; Temedie-Asogwa, T.; Atta, J.A.; Al Zoubi, M.A.M. The Impacts of Treatment Summaries on Patient-Centered Communication and Quality of Care for Cancer Survivors. Int. J. Med All Body Heal. Res. 2023, 4. Unpublished manuscript. [Google Scholar] [CrossRef]
- Julià-Torras, J.; Almeida Felipe, J.M.; Gándara Del Castillo, Á.; González-Barboteo, J.; Forero, D.; Alegre, S.; Cuervo-Pinna, M.Á.; Serna, J.; Muñoz-Unceta, N.; Alonso-Babarro, A.; et al. Prevalence, Clinical Characteristics, and Management of Episodic Dyspnea in Advanced Lung Cancer Outpatients: A Multicenter Nationwide Study—The INSPIRA-DOS Study. J. Palliat. Med. 2022, 25, 1197–1207. [Google Scholar] [CrossRef]
- Lucas-Ruano, D.; Sanchez-Gomez, C.; Rihuete-Galve, M.I.; Garcia-Martin, A.; Fonseca-Sanchez, E.; Fernández-Rodríguez, E.J. Descriptive Study on the Relationship between Dyspnea, Physical Performance, and Functionality in Oncology Patients. Healthcare 2024, 12, 1675. [Google Scholar] [CrossRef]
- Lo, S.B.; Ruprecht, A.L.; Post, K.E.; Eche-Ugwu, I.J.; Cooley, M.E.; Temel, J.S.; Greer, J.A. Dyspnea-Related Dimensions and Self-Efficacy: Associations with Well-Being in Advanced Lung Cancer. J. Pain. Symptom Manage. 2024, 67, 366–374. [Google Scholar] [CrossRef]
- Ortiz-Rubio, A.; Torres-Sánchez, I.; Cabrera-Martos, I.; López-López, L.; Rodríguez-Torres, J.; Granados-Santiago, M.; Valenza, M.C. Respiratory disturbances in fibromyalgia: A systematic review and meta-analysis of case control studies. Expert. Rev. Respir. Med. 2021, 15, 1217–1227. [Google Scholar] [CrossRef]
- Elgayar, S.L. Effect of Aerobic Exercises on Lung Function in Women with Fibromyalgia: A Randomized Controlled Trial. J. Phys. Act Health 2025, 1, 1–9. [Google Scholar] [CrossRef]
- Hui, D.; Bohlke, K.; Bao, T.; Campbell, T.C.; Coyne, P.J.; Currow, D.C.; Gupta, A.; Leiser, A.L.; Mori, M.; Nava, S.; et al. Management of dyspnea in advanced cancer: ASCO guideline. J. Clin. Oncol. 2021, 39, 1389–1411. [Google Scholar] [CrossRef]
- Dans, M.; Kutner, J.S.; Agarwal, R.; Baker, J.N.; Bauman, J.R.; Beck, A.C.; Campbell, T.C.; Carey, E.C.; Case, A.A.; Dalal, S.; et al. NCCN Guidelines® Insights: Palliative Care, Version 2.2021: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Canc. Netw. 2021, 19, 780–788. [Google Scholar] [CrossRef]
- Wu, C.; Zheng, Y.; Duan, Y.; Lai, X.; Cui, S.; Xu, N.; Tang, C.; Lu, L. Nonpharmacological interventions for cancer-related fatigue: A systematic review and Bayesian network meta-analysis. Worldviews Evid. Based Nurs. 2019, 16, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Winters-Stone, K.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.; Matthews, C.; Ligibel, J.; Gerber, L.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Ormel, H.L.; van der Schoot, G.G.F.; Sluiter, W.J.; Jalving, M.; Gietema, J.A.; Walenkamp, A.M.E. Predictors of adherence to exercise interventions during and after cancer treatment: A systematic review. Psychooncology 2018, 27, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Bickenbach, J.; Rubinelli, S.; Sabariego, C.; Stucki, G. The Learning Rehabilitation System: Strengthening an intersectoral strategy to improve functioning of an ageing population. Health Policy 2023, 135, 104866. [Google Scholar] [CrossRef]
- Sheeran, P.; Abraham, C.; Jones, K.; Villegas, M.E.; Avishai, A.; Symes, Y.R.; Ellinger, H.; Miles, E.; Gates, K.M.; Wright, C.E.; et al. Promoting physical activity among cancer survivors: Meta-analysis and meta-CART analysis of randomized controlled trials. Health Psychol. 2019, 38, 467–482. [Google Scholar] [CrossRef]
- Turner, R.R.; Steed, L.; Quirk, H.; Greasley, R.U.; Saxton, J.M.; Taylor, S.J.; Rosario, D.J.; A Thaha, M.; Bourke, L. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst. Rev. 2018, 9, CD010192. [Google Scholar] [CrossRef]
- Yang, D.D.; Hausien, O.; Aqeel, M.; Klonis, A.; Foster, J.; Renshaw, D.; Thomas, R. Physical activity levels and barriers to exercise referral among patients with cancer. Patient Educ. Couns. 2017, 100, 1402–1407. [Google Scholar] [CrossRef]
- Barros, V.d.S.; Bassi-Dibai, D.; Guedes, C.L.R.; Morais, D.N.; Coutinho, S.M.; Simões, G.d.O.; Mendes, L.P.; Leal, P.d.C.; Dibai-Filho, A.V. Barthel Index is a valid and reliable tool to measure the functional independence of cancer patients in palliative care. BMC Palliat. Care 2022, 21, 124. [Google Scholar]
- Cocks, K.; Wells, J.R.; Johnson, C.; Schmidt, H.; Koller, M.; Oerlemans, S.; Velikova, G.; Pinto, M.; Tomaszewski, K.A.; Aaronson, N.K.; et al. Content validity of the EORTC quality of life questionnaire QLQ-C30 for use in cancer. Eur. J. Cancer 2023, 178, 128–138. [Google Scholar] [CrossRef]
- Frappaz, D.; Bonneville-Levard, A.; Ricard, D.; Carrie, S.; Schiffler, C.; Xuan, K.H.; Weller, M. Assessment of Karnofsky (KPS) and WHO (WHO-PS) performance scores in brain tumour patients: The role of clinician bias. Support. Care Cancer 2021, 29, 1883–1891. [Google Scholar] [CrossRef]
- Soo, W.K.; King, M.; Pope, A.; Steer, C.; Devitt, B.; Chua, S.; Parente, P.; Davis, I.D.; Dārziņš, P. The Elderly Functional Index (ELFI), a patient-reported outcome measure of functional status in patients with cancer: A multicentre, prospective validation study. Lancet Healthy Longev. 2021, 2, e24–e33. [Google Scholar] [CrossRef] [PubMed]
- Fernández Rodríguez, E.J.; Rihuete Galve, M.I.; Cruz Hernández, J.J. Impact of a comprehensive functional rehabilitation programme on the quality of life of the oncological patient with dyspnoea. Med. Clin. 2021, 157, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Y.; Yin, Y.; Cao, H.; Lu, H.; Li, Y.; Xie, J. Effects of transitional care interventions on quality of life in people with lung cancer: A systematic review and meta-analysis. J. Clin. Nurs. 2024, 33, 1976–1994. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Deng, T.; Long, Y.; Li, J.; Yang, J.; Hu, Y.; Lu, T.; Luo, X.; Suen, L.K.P.; Chen, S. The effect of a hybrid structured pulmonary rehabilitation education program for patients with lung cancer with a high risk of postoperative pulmonary complications: A quasi-experimental study. Eur. J. Oncol. Nurs. 2024, 71, 102655. [Google Scholar] [CrossRef]
- Chen, K.; Yang, D.; Li, F.; Gao, L.; Tian, Y.; Xu, B.; Xu, X.; Xu, Q.; Cao, J. Changes in the symptom clusters of elderly patients with lung cancer over the course of postoperative rehabilitation and their correlation with frailty and quality of life: A longitudinal study. Eur. J. Oncol. Nurs. 2023, 67, 102388. [Google Scholar] [CrossRef]
- Lakkadsha, T.M.; Yadav, V.; Jain, M.; Lalwani, S.; Saifee, S.; Kaderi, A.S.A. Palliative Care as an Adjunct to Standard Pulmonary Rehabilitation: A Pathway to Improving Functional Independence & Quality of Life in a Patient with Lung Cancer. Cureus 2022, 14, e28580. [Google Scholar]
- Calvo-Paniagua, J.; Díaz-Arribas, M.J.; Valera-Calero, J.A.; Gallardo-Vidal, M.I.; Fernández-De-Las-Peñas, C.; López-De-Uralde-Villanueva, I.; del Corral, T.; Plaza-Manzano, G.; Martinuzzi, A. A tele-health primary care rehabilitation program improves self-perceived exertion in COVID-19 survivors experiencing Post-COVID fatigue and dyspnea: A quasi-experimental study. PLoS ONE 2022, 17, e0271802. [Google Scholar] [CrossRef]
- Manocchio, N.; Ljoka, C.; Buttarelli, L.; Giordan, L.; Sorbino, A.; Foti, C. Early motor and respiratory re-education in patients hospitalized for COVID-19. Adv. Rehabil. 2025, 39, 29–45. [Google Scholar] [CrossRef]
| Variables | IG (N = 33) | CG (N = 32) | |
|---|---|---|---|
| Age | 66.24 (±12.15) | 64.59 (±14.9) | |
| Sex | Male | 63.6% | 59.4% |
| Female | 36.4% | 40.6% | |
| Pathological diagnosis | Breast | 21.2% | 9.4% |
| Lung | 45.5% | 43.8% | |
| Digestive system | 6.1% | 9.4% | |
| Pancreas | 6.1% | 6.3% | |
| Prostate | 3% | 9.4% | |
| Other | 18.2% | 18.8% | |
| Number of oncology treatment lines | 3 (1) | 3 (4) | |
| Barthel | 58.12 (±19.78) | 55.03 (±21.34) | |
| EORTC QLQ-C30 | 58.40 (±14.25) | 59.90 (±13.95) | |
| Karnofsky | 60.00 (±10.23) | 62.50 (±11.12) | |
| Lawton–Brody | 3.10 (±1.52) | 3.32 (±1.67) | |
| Hospitalised time | 11 days | 12 days | |
| Metastasis | Yes | 75.8% | 81.2% |
| No | 24.2% | 18.8% | |
| Hospital readmission | Yes | 3% | 37.5% |
| No | 97% | 62.5% | |
| Variables | Intervention Group (IG) (n = 33) | Control Group (CG) (n = 32) | p-Value | Cohen’s d | ||
|---|---|---|---|---|---|---|
| Basal Assessment (T0) | Final Assessment (T2) | Basal Assessment (T0) | Final Assessment (T2) | |||
| Barthel | 58.12 (±19.78) | 76.45 (±17.62) | 55.03 (±21.34) | 61.22 (±20.08) | <0.001 | 0.730 |
| EORTC QLQ-C30 | 58.40 (±14.25) | 74.80 (±13.71) | 59.90 (±13.95) | 66.50 (±14.02) | <0.001 | 0.720 |
| Karnofsky | 60.00 (±10.23) | 78.75 (±9.87) | 62.50 (±11.12) | 68.10 (±10.55) | <0.001 | 1.190 |
| Lawton Brody | 3.10 (±1.48) | 5.88 (±1.60) | 3.32 (±1.52) | 4.10 (±1.67) | <0.001 | 0.880 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Rodríguez, E.J.; Sánchez-Gómez, C.; Rihuete-Galve, M.I.; Fonseca-Sánchez, E.; Cruz-Hernández, J.J. A Home-Based Interdisciplinary Intervention to Enhance Functionality in Oncology Patients: Results from a Clinical Trial. J. Clin. Med. 2025, 14, 4417. https://doi.org/10.3390/jcm14134417
Fernández-Rodríguez EJ, Sánchez-Gómez C, Rihuete-Galve MI, Fonseca-Sánchez E, Cruz-Hernández JJ. A Home-Based Interdisciplinary Intervention to Enhance Functionality in Oncology Patients: Results from a Clinical Trial. Journal of Clinical Medicine. 2025; 14(13):4417. https://doi.org/10.3390/jcm14134417
Chicago/Turabian StyleFernández-Rodríguez, Eduardo José, Celia Sánchez-Gómez, Maria Isabel Rihuete-Galve, Emilio Fonseca-Sánchez, and Juan Jesús Cruz-Hernández. 2025. "A Home-Based Interdisciplinary Intervention to Enhance Functionality in Oncology Patients: Results from a Clinical Trial" Journal of Clinical Medicine 14, no. 13: 4417. https://doi.org/10.3390/jcm14134417
APA StyleFernández-Rodríguez, E. J., Sánchez-Gómez, C., Rihuete-Galve, M. I., Fonseca-Sánchez, E., & Cruz-Hernández, J. J. (2025). A Home-Based Interdisciplinary Intervention to Enhance Functionality in Oncology Patients: Results from a Clinical Trial. Journal of Clinical Medicine, 14(13), 4417. https://doi.org/10.3390/jcm14134417





