Treatment of Ochronotic Osteoarthropathy and the Evaluation of Selected Lower Limb Muscle Properties, Including the Patellar Tendon: A Case Report and Mini Literature Review
Abstract
1. Introduction
1.1. Alkaptonuria—Definition, Causes and Epidemiology

1.2. Pathogenesis
1.3. Alkaptonuria—Musculoskeletal System
2. Case Description
2.1. Before Surgery
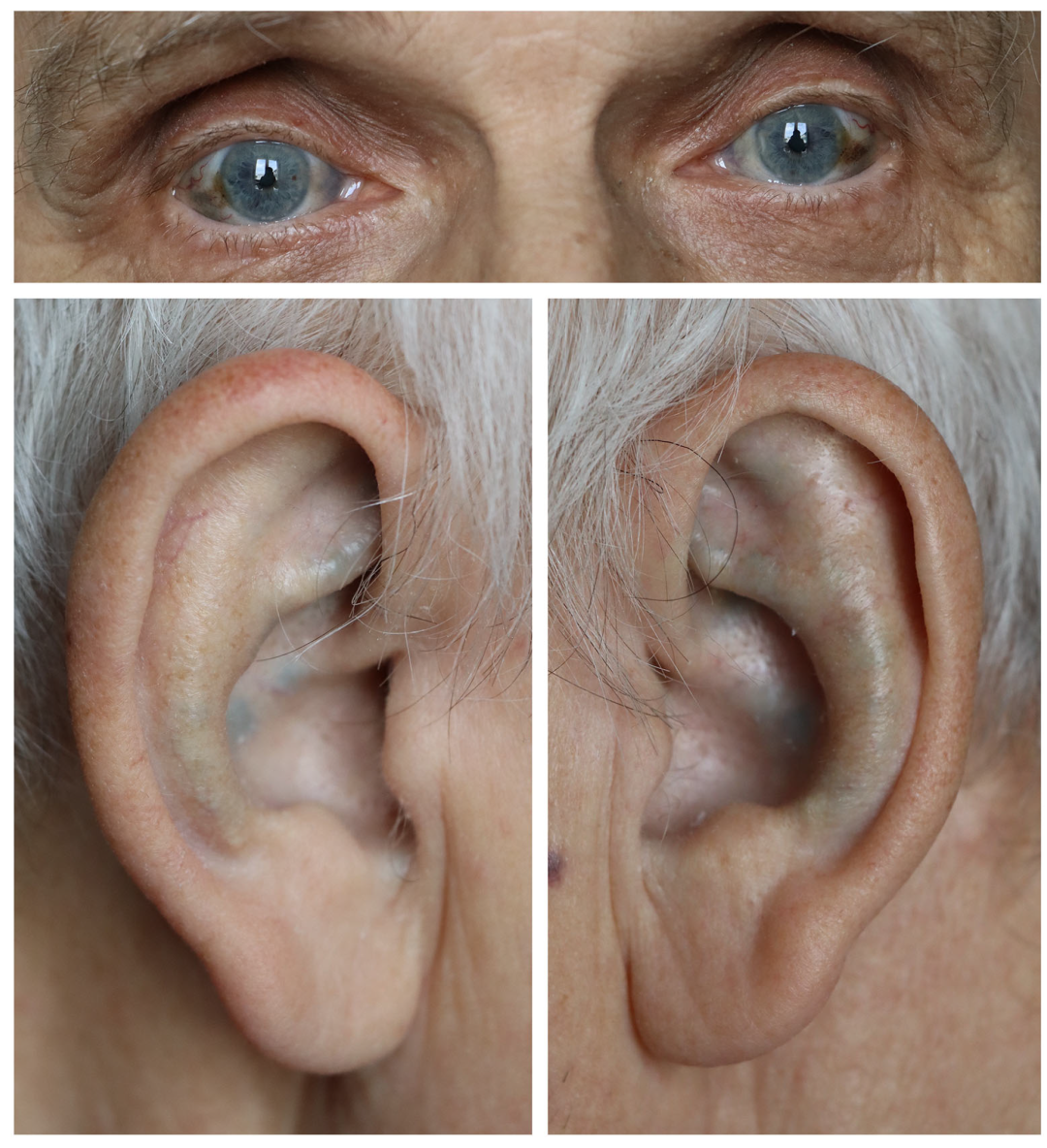
2.1.1. Interview and Clinical Examination
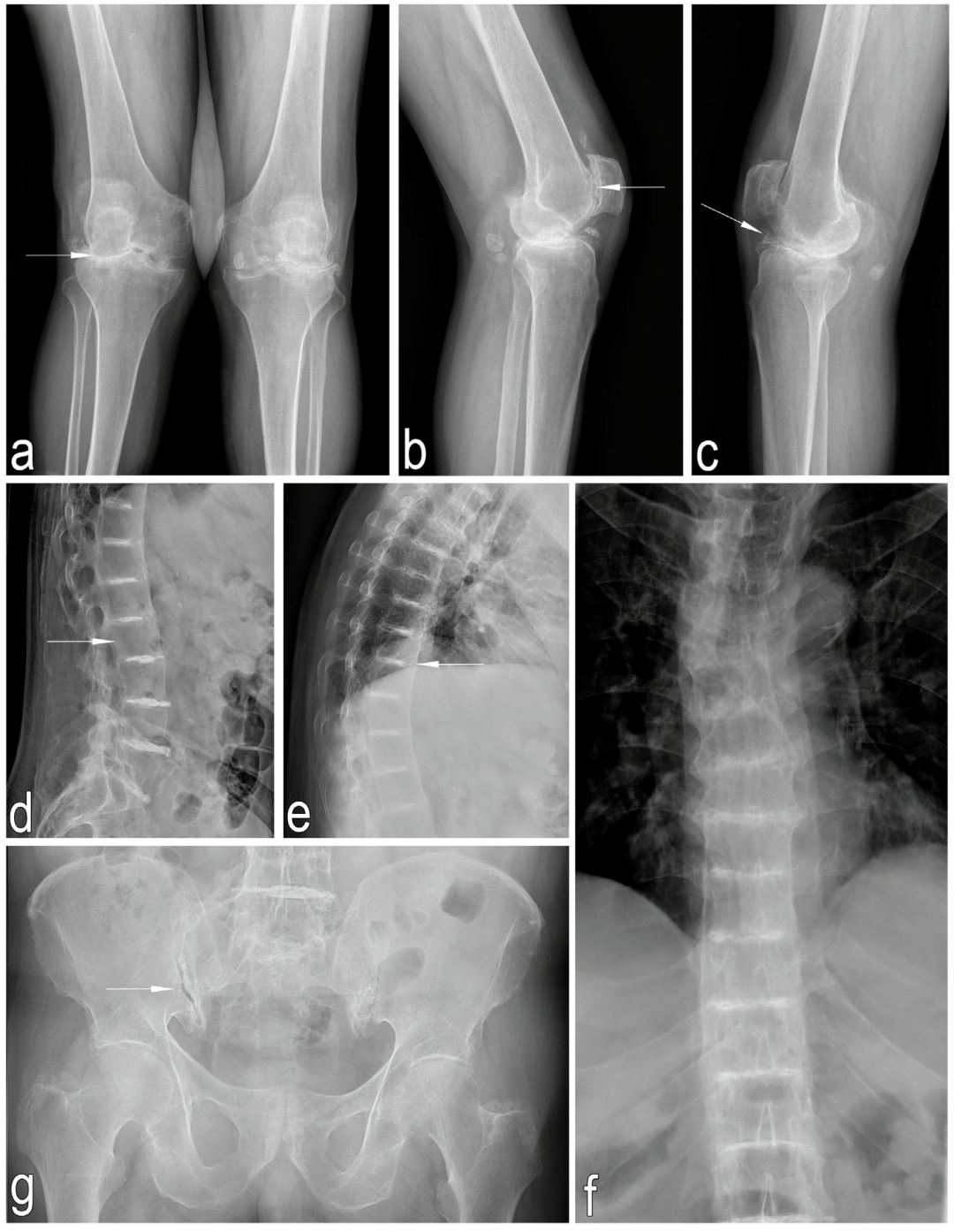
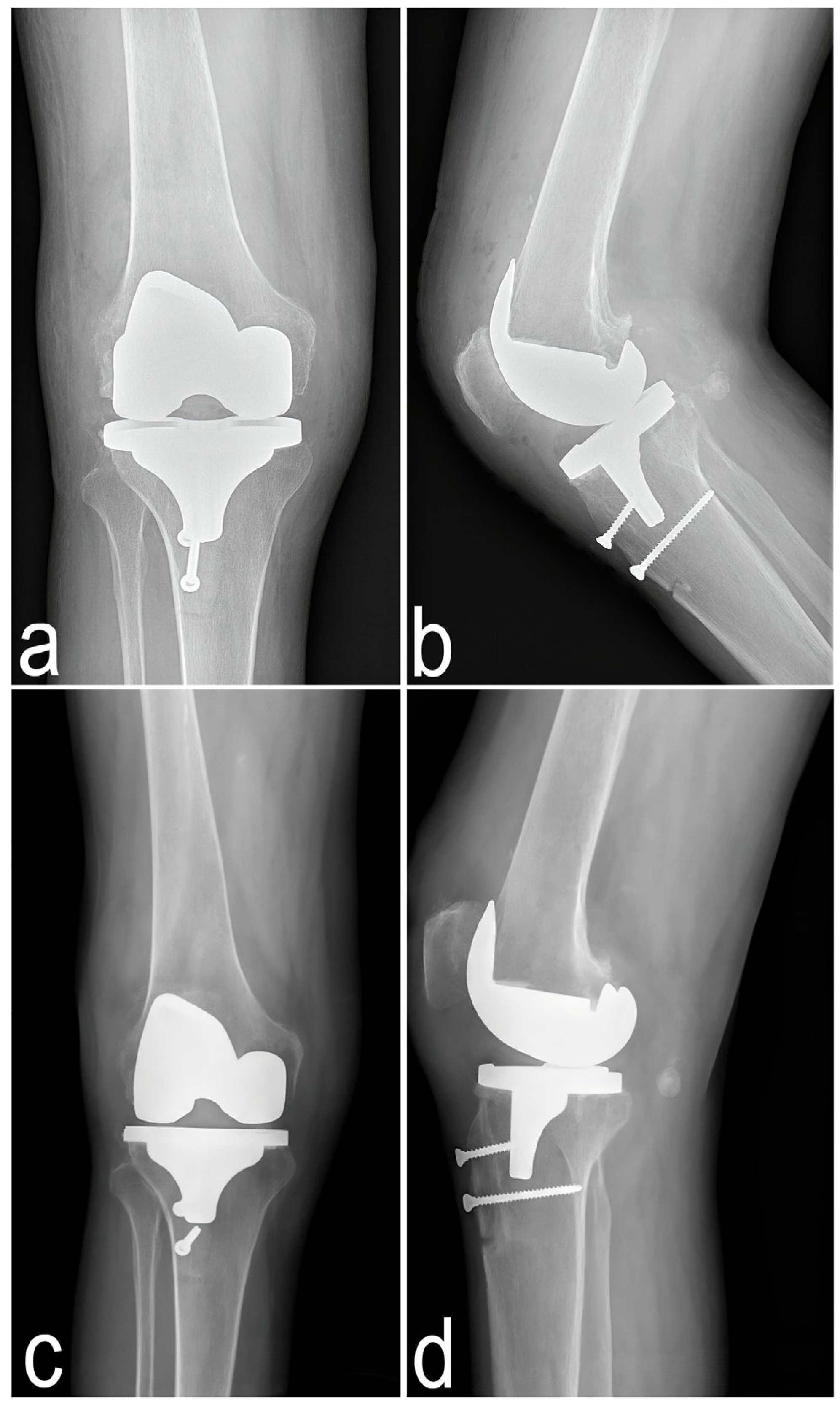
2.1.2. Imaging Studies
2.1.3. Laboratory Tests
2.1.4. Densitometric Examination
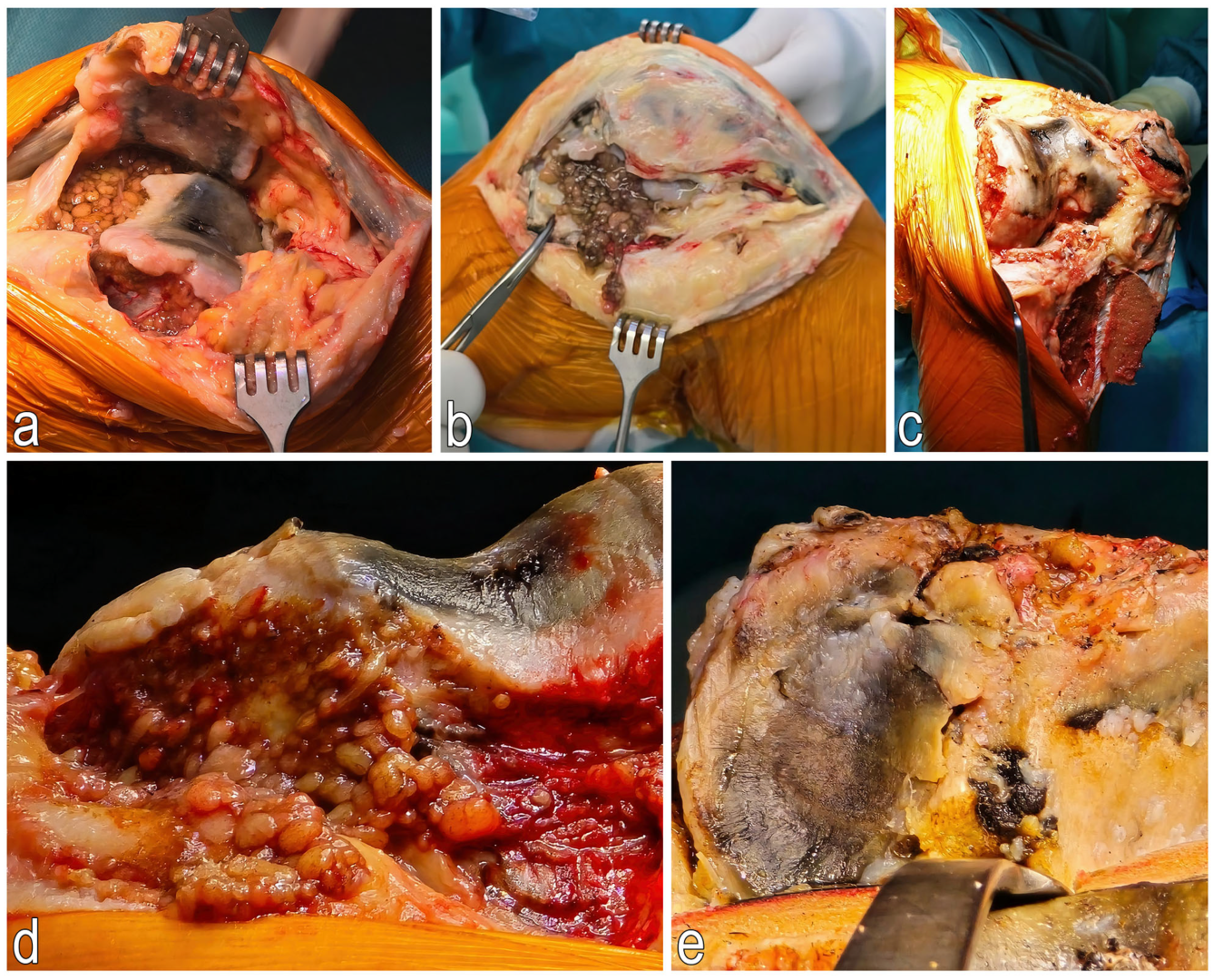
2.2. Surgical Treatment
2.2.1. Postoperative Procedure
2.2.2. Postoperative Rehabilitation
2.3. Additional Tests
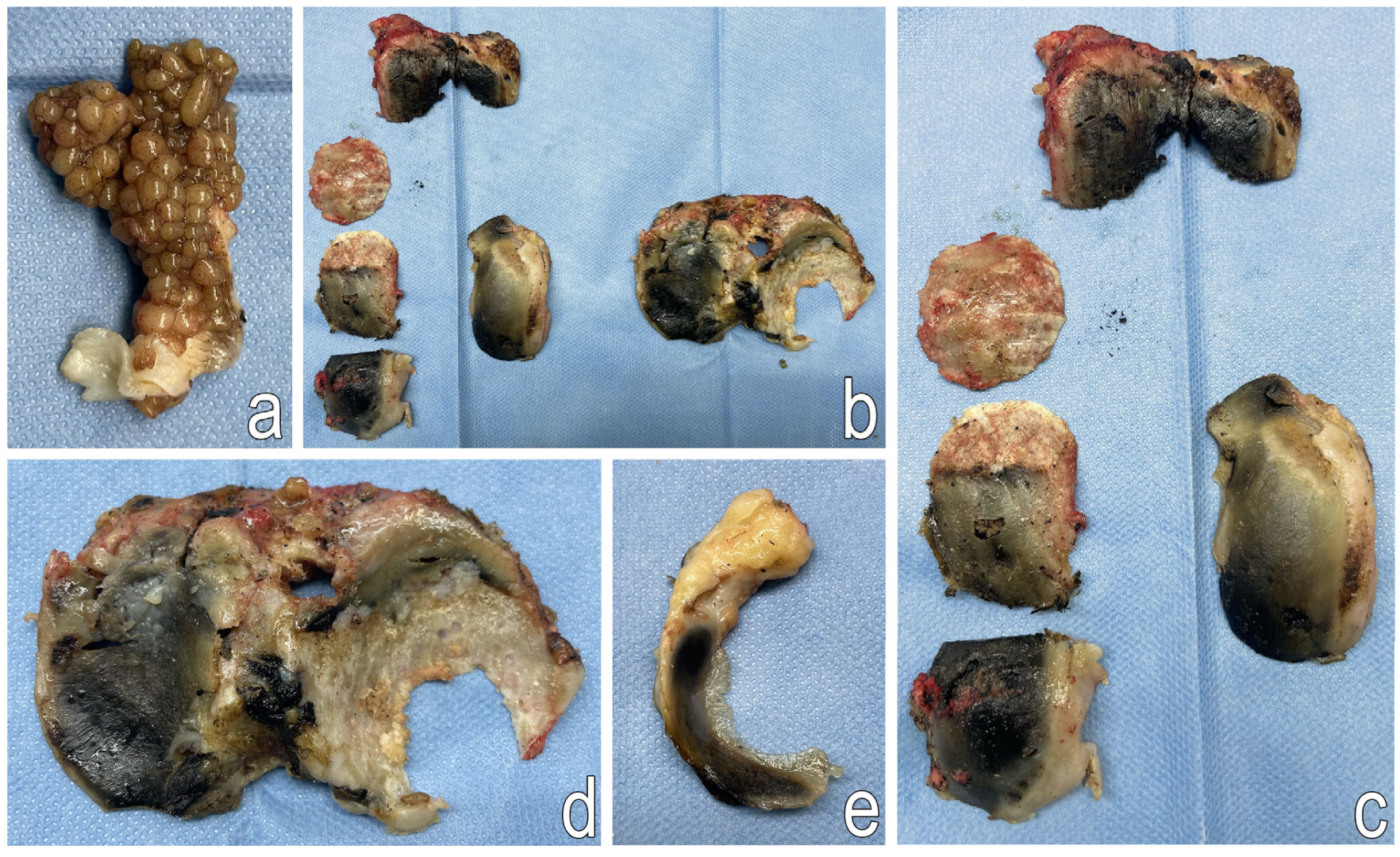
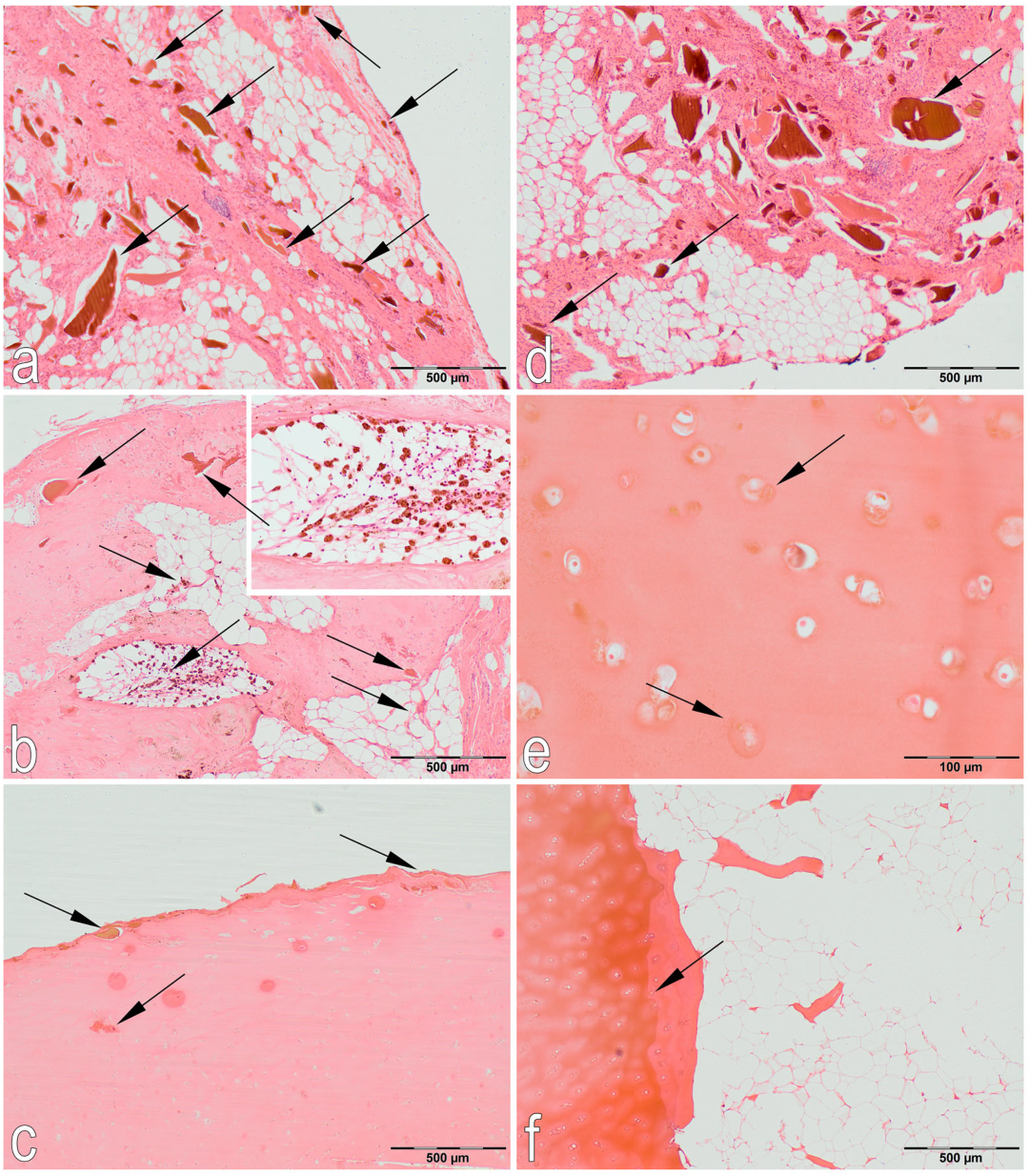
2.3.1. Histological Examination
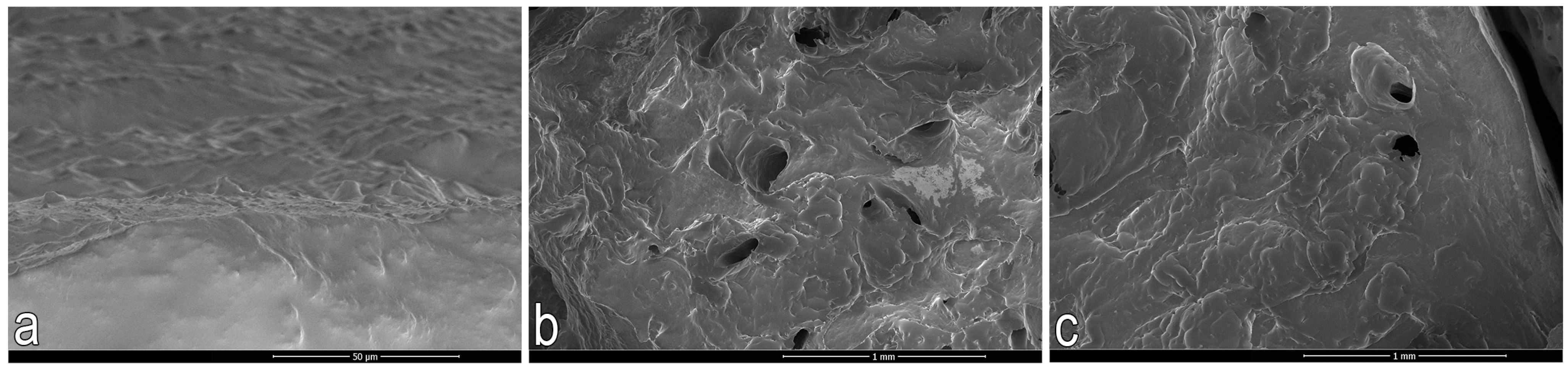
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Nano-CT
2.4. Follow-Up Examination 3 Months After Total Right Knee Arthroplasty
2.4.1. Clinical Examination
2.4.2. Imaging Examination
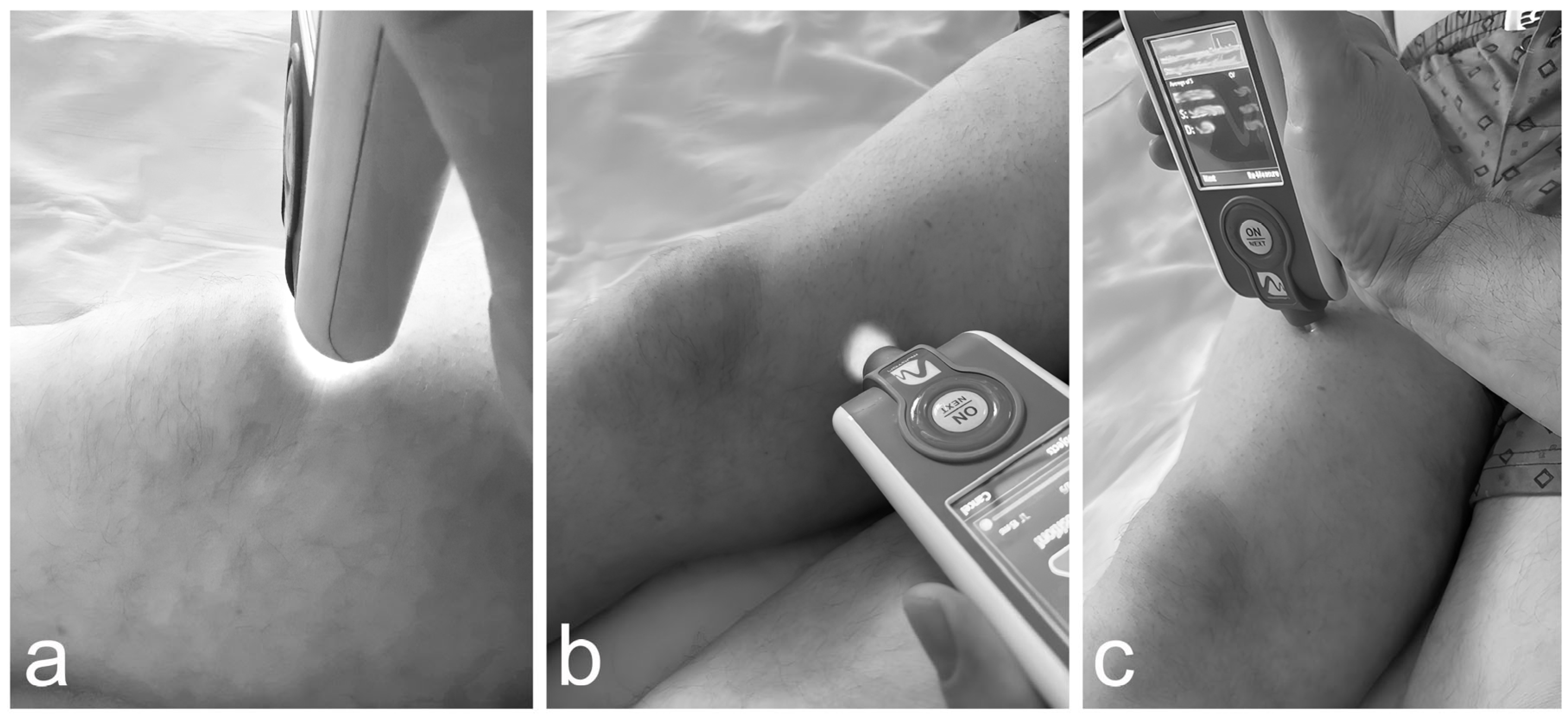
2.5. Innovative Applications of the MyotonPRO Examination for the Assessment of Muscle Condition Before and After Knee Arthroplasty in a Patient with Alkaptonuria
2.5.1.Results
Rectus Femoris
Vastus Medialis
Patellar Tendon
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKU | Alkaptonuria |
| HGA | Homogentisic acid |
| HGO | Homogentisate 1,2-dioxygenase |
| VAS | Visual Analog Scale |
| WOMAC | Western Ontario and McMaster Universities Index of Osteoarthritis |
| ASA | American Society of Anesthesiologists |
| AP | Anteroposterior |
| ACL | Anterior cruciate ligament |
| CPM | Continuous Passive Motion |
| HE | Hematoxylin and eosin staining |
| COMP | Cartilage oligomeric matrix protein |
| ICH | Immunohistochemical |
| SEM | Scanning electron microscopy |
| BS/BV | Specific bone surface |
| BV/TV | Percent bone volume |
| Tb.Th | Trabecular thickness |
| Tb.Sp | Trabecular separation |
| Tb.N | Trabecular number |
| SMI | Structure model index |
| DA | Degree of anisotropy |
| FD | Fractal dimension |
| Conn.Dn | Connectivity density |
| BMI | Bone mineral density |
| F | Frequency |
| S | Stiffness |
| D | Elasticity (logarithmic decrement) |
| R | Relaxation time |
| C | Creep |
| RANKL | receptor activator of nuclear factor kappa-B |
| OPG | osteoprotegerin |
| KOA | Knee osteoarthritis |
References
- Fernández-Cañón, J.M.; Granadino, B.; Beltrán-Valero de Bernabé, D.; Renedo, M.; Fernández-Ruiz, E.; Peñalva, M.A.; Rodríguez de Córdoba, S. The Molecular Basis of Alkaptonuria. Nat. Genet. 1996, 14, 19–24. [Google Scholar] [CrossRef]
- Maheshwor, T.; Yu, J.; Lee, W.; Islam, M.F.; Yoon, H.-R. Determination of Homogentisic Acid in Human Plasma by GC-MS for Diagnosis of Alkaptonuria. Anal. Sci. Technol. 2015, 28, 323–330. [Google Scholar]
- Zatková, A.; de Bernabé, D.B.; Poláková, H.; Zvarík, M.; Feráková, E.; Bosák, V.; Ferák, V.; Kádasi, L.; de Córdoba, S.R. High Frequency of Alkaptonuria in Slovakia: Evidence for the Appearance of Multiple Mutations in HGO Involving Different Mutational Hot Spots. Am. J. Hum. Genet. 2000, 67, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.A.; Dillon, J.P.; Ranganath, L.R. Development of an Effective Therapy for Alkaptonuria—Lessons for Osteoarthritis. Rheumatol. Immunol. Res. 2021, 2, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Mannoni, A.; Selvi, E.; Lorenzini, S.; Giorgi, M.; Airó, P.; Cammelli, D.; Andreotti, L.; Marcolongo, R.; Porfirio, B. Alkaptonuria, Ochronosis, and Ochronotic Arthropathy. Semin. Arthritis Rheum. 2004, 33, 239–248. [Google Scholar] [CrossRef]
- Gil, J.A.; Wawrzynski, J.; Waryasz, G.R. Orthopedic Manifestations of Ochronosis: Pathophysiology, Presentation, Diagnosis, and Management. Am. J. Med. 2016, 129, 536.e1–536.e6. [Google Scholar] [CrossRef]
- Ranganath, L.R.; Norman, B.P.; Gallagher, J.A. Ochronotic Pigmentation Is Caused by Homogentisic Acid and Is the Key Event in Alkaptonuria Leading to the Destructive Consequences of the Disease-A Review. J. Inherit. Metab. Dis. 2019, 42, 776–792. [Google Scholar] [CrossRef]
- Wu, K.; Bauer, E.; Myung, G.; Fang, M.A. Musculoskeletal Manifestations of Alkaptonuria: A Case Report and Literature Review. Eur. J. Rheumatol. 2019, 6, 98–101. [Google Scholar] [CrossRef]
- Milella, M.S.; Geminiani, M.; Trezza, A.; Visibelli, A.; Braconi, D.; Santucci, A. Alkaptonuria: From Molecular Insights to a Dedicated Digital Platform. Cells 2024, 13, 1072. [Google Scholar] [CrossRef] [PubMed]
- Zatkova, A.; Ranganath, L.; Kadasi, L. Alkaptonuria: Current Perspectives. Appl. Clin. Genet. 2020, 13, 37–47. [Google Scholar] [CrossRef]
- Ventura-Ríos, L.; Hernández-Díaz, C.; Gutiérrez-Pérez, L.; Bernal-González, A.; Pichardo-Bahena, R.; Cedeño-Garcidueñas, A.L.; Pineda, C. Ochronotic Arthropathy as a Paradigm of Metabolically Induced Degenerative Joint Disease. A Case-Based Review. Clin. Rheumatol. 2016, 35, 1389–1395. [Google Scholar] [CrossRef]
- Taylor, A.M.; Boyde, A.; Wilson, P.J.M.; Jarvis, J.C.; Davidson, J.S.; Hunt, J.A.; Ranganath, L.R.; Gallagher, J.A. The Role of Calcified Cartilage and Subchondral Bone in the Initiation and Progression of Ochronotic Arthropathy in Alkaptonuria. Arthritis Rheum. 2011, 63, 3887–3896. [Google Scholar] [CrossRef]
- Aliberti, G.; Pulignano, I.; Pisani, D.; Rocchietti March, M.; Del Porto, F.; Proietta, M. Bisphosphonate Treatment in Ochronotic Osteoporotic Patients. Clin. Rheumatol. 2007, 26, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Zatkova, A.; Olsson, B.; Ranganath, L.R.; Imrich, R. Analysis of the Phenotype Differences in Siblings with Alkaptonuria. Metabolites 2022, 12, 990. [Google Scholar] [CrossRef]
- Latham, N.; Liu, C. Strength Training in Older Adults: The Benefits for Osteoarthritis. Clin. Geriatr. Med. 2010, 26, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Shorter, E.; Sannicandro, A.J.; Poulet, B.; Goljanek-Whysall, K. Skeletal Muscle Wasting and Its Relationship With Osteoarthritis: A Mini-Review of Mechanisms and Current Interventions. Curr. Rheumatol. Rep. 2019, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Alnahdi, A.H.; Zeni, J.A.; Snyder-Mackler, L. Muscle Impairments in Patients with Knee Osteoarthritis. Sports Health 2012, 4, 284–292. [Google Scholar] [CrossRef]
- Lettner, J.; Królikowska, A.; Ramadanov, N.; Oleksy, Ł.; Hakam, H.T.; Becker, R.; Prill, R. Evaluating the Reliability of MyotonPro in Assessing Muscle Properties: A Systematic Review of Diagnostic Test Accuracy. Medicina 2024, 60, 851. [Google Scholar] [CrossRef]
- Lieber, R.L.; Binder-Markey, B. Biochemical and Structural Basis of the Passive Mechanical Properties of Whole Skeletal Muscle. J. Physiol. 2021, 599, 3809–3823. [Google Scholar] [CrossRef]
- Ge, J.-S.; Chang, T.-T.; Zhang, Z.-J. Reliability of Myotonometric Measurement of Stiffness in Patients with Spinal Cord Injury. Med. Sci. Monit. 2020, 26, e924811. [Google Scholar] [CrossRef]
- Chen, G.; Wu, J.; Chen, G.; Lu, Y.; Ren, W.; Xu, W.; Xu, X.; Wu, Z.; Guan, Y.; Zheng, Y.; et al. Reliability of a Portable Device for Quantifying Tone and Stiffness of Quadriceps Femoris and Patellar Tendon at Different Knee Flexion Angles. PLoS ONE 2019, 14, e0220521. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Feng, Y.N.; Zhang, H.Q.; Li, Y.P.; Zhu, Y.; Zhang, Z.J. Assessing the Viscoelastic Properties of Upper Trapezius Muscle: Intra- and Inter-Tester Reliability and the Effect of Shoulder Elevation. J. Electromyogr. Kinesiol. 2018, 43, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-T.; Zhu, Y.-C.; Li, Z.; Li, F.; Li, Y.-P.; Guo, J.-Y.; Wang, X.-Q.; Zhang, Z.-J. Modulation in the Stiffness of Specific Muscles of the Quadriceps in Patients With Knee Osteoarthritis and Their Relationship With Functional Ability. Front. Bioeng. Biotechnol. 2021, 9, 781672. [Google Scholar] [CrossRef]
- Morgan, G.E.; Martin, R.; Williams, L.; Pearce, O.; Morris, K. Objective Assessment of Stiffness in Achilles Tendinopathy: A Novel Approach Using the MyotonPRO. BMJ Open Sport. Exerc. Med. 2018, 4, e000446. [Google Scholar] [CrossRef] [PubMed]
- Ohko, H.; Ota, S.; Imai, Y.; Kawasaki, S. Limiting Factors of Knee Flexion Angle and Inferior Patellar Mobility in Patients with Knee Osteoarthritis. Osteoarthr. Cartil. 2020, 28, S162–S163. [Google Scholar] [CrossRef]
- Shen, X.; Wang, S.; Chen, J.; Li, J.; Li, C.; Xiang, R.; Zhao, C.; Xu, X. Inter-Rater Reliability and Test-Retest Reliability of the Foot Posture Index (FPI-6) for Assessing Static Foot Posture in Elderly Female Patients with Knee Osteoarthritis and Its Association with Quadriceps Muscle Tone and Stiffness. Front. Bioeng. Biotechnol. 2024, 12, 1385986. [Google Scholar] [CrossRef]
- Li, J.; Wu, Z.; Lu, B.; Li, C.; Wang, S.; Zhang, J.; Shen, X.; Xiang, R.; Chen, J.; Jiang, T.; et al. The Differences in Parameters in Ultrasound Imaging and Biomechanical Properties of the Quadriceps Femoris with Unilateral Knee Osteoarthritis in the Elderly: A Preliminary Observational Study. Clin. Interv. Aging 2024, 19, 1479–1491. [Google Scholar] [CrossRef]
- Murillo, B.C.; Johnson, A.J.; Peterson, J.A.; Cruz-Almeida, Y. The Relationship Between Muscle Mechanical Properties And Mechanical Pain Sensitivity In Older Adults With Knee Osteoarthritis. J. Pain 2023, 24, 48. [Google Scholar] [CrossRef]
- Collins, K.H.; Lenz, K.L.; Pollitt, E.N.; Ferguson, D.; Hutson, I.; Springer, L.E.; Oestreich, A.K.; Tang, R.; Choi, Y.-R.; Meyer, G.A.; et al. Adipose Tissue Is a Critical Regulator of Osteoarthritis. Proc. Natl. Acad. Sci. USA 2021, 118, e2021096118. [Google Scholar] [CrossRef]
- Effect of Gender, Muscle Type and Skinfold Thickness on Myometric Parameters in Young People—PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8590390/ (accessed on 9 April 2025).
- Kara, A.; Celik, H.; Seker, A.; Sezer, H.B.; Kilinc, E.; Uzun, M. Ochronosis Diagnosed after Knee Arthroscopy. Int. J. Surg. Case Rep. 2014, 5, 497–499. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Zhang, Z.; Lu, X.; Zhang, H. Ochronotic Arthropathy Effectively Treated with Total Hip and Total Knee Arthroplasty: A Case Report. Front. Med. 2023, 10, 1212580. [Google Scholar] [CrossRef]
- Castagna, A.; Giombini, A.; Vinanti, G.; Massazza, G.; Pigozzi, F. Arthroscopic Treatment of Shoulder Ochronotic Arthropathy: A Case Report and Review of Literature. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 176–181. [Google Scholar] [CrossRef]
- Doganavsargil, B.; Pehlivanoglu, B.; Bicer, E.K.; Argin, M.; Bingul, K.B.; Sezak, M.; Kececi, B.; Coker, M.; Oztop, F. Black Joint and Synovia: Histopathological Evaluation of Degenerative Joint Disease Due to Ochronosis. Pathol.-Res. Pract. 2015, 211, 470–477. [Google Scholar] [CrossRef]
- Gowda, N.; Kumar, M.J.; Kumar, A.K. Black Hip: A Rare Case Treated by Total Hip Replacement. Ann. Saudi Med. 2013, 33, 368–371. [Google Scholar] [CrossRef]
- Harun, M.; Hayrettin, Y.; Serhat, M.; Cuneyt, M.; Fırat, F.; Ufuk, O. A Rare Cause of Arthropathy: An Ochronotic Patient with Black Joints. Int. J. Surg. Case Rep. 2014, 5, 554–557. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, X.; Shen, Z.; Wang, Y.; Wu, Z.; Chen, G.; Guan, Y.; Wu, J.; Jiang, T.; Wu, H.; et al. Comparison of the Asymmetries in Foot Posture and Properties of Gastrocnemius Muscle and Achilles Tendon Between Patients With Unilateral and Bilateral Knee Osteoarthritis. Front. Bioeng. Biotechnol. 2021, 9, 636571. [Google Scholar] [CrossRef]
- Paravlic, A.H.; Pisot, R.; Simunic, B. Muscle-specific changes of lower extremities in the early period after total knee arthroplasty: Insight from tensiomyography. J. Musculoskelet. Neuronal Interact. 2020, 20, 390–397. [Google Scholar]




| Subchondral Bone Plate | Bone Trabeculae | |
|---|---|---|
| BS/BV, 1/mm | 0.700 | 1.15 |
| BV/TV, % | 10.0 | 15.4 |
| Tb.Th, mm | 7.28 | 4.81 |
| Tb.Sp, mm | 20.6 | 16.5 |
| Tb.N, 1/mm | 0.014 | 0.032 |
| SMI, - | 4.35 | 3.03 |
| DA, - | 1.67 | 1.46 |
| FD, - | 2.06 | 2.28 |
| Conn.Dn, - | 0.006 | 0.020 |
| BMD, g/cm3 | 1.93 | 1.35 |
| Muscle/Ligament | Muscle Condition | Side | F, Hz | S, N/m | D, - | R, ms | C, - |
|---|---|---|---|---|---|---|---|
| before TKA right knee | |||||||
| Rectus femoris | Rest | Non-operated | 20.5 | 418 | 0.94 | 13.3 | 0.85 |
| Operated | 22.2 | 502 | 1.23 | 11.0 | 0.71 | ||
| Contraction | Non-operated | 22.5 | 545 | 1.19 | 9.7 | 0.63 | |
| Operated | 20.6 | 433 | 1.36 | 12.6 | 0.80 | ||
| Vastus medialis | Rest | Non-operated | 19.3 | 416 | 1.27 | 13.5 | 0.84 |
| Operated | 16.5 | 340 | 1.56 | 17.4 | 1.10 | ||
| Contraction | Non-operated | 21.0 | 412 | 1.50 | 12.6 | 0.80 | |
| Operated | 18.9 | 381 | 1.34 | 13.4 | 0.84 | ||
| Patellar tendon | Rest | Non-operated | 27.2 | 725 | 0.92 | 7.5 | 0.50 |
| Operated | 31.3 | 915 | 0.57 | 5.6 | 0.38 | ||
| Contraction | Non-operated | 38.5 | 1094 | 0.35 | 4.3 | 0.31 | |
| Operated | 30.8 | 904 | 0.77 | 5.9 | 0.41 | ||
| after TKA right knee | |||||||
| Rectus femoris | Rest | Non-operated | 21.8 | 454 | 1.12 | 12.0 | 0.77 |
| Operated | 20.7 | 444 | 1.03 | 11.9 | 0.76 | ||
| Contraction | Non-operated | 22.3 | 511 | 0.92 | 10.1 | 0.65 | |
| Operated | 20.7 | 448 | 1.01 | 11.7 | 0.74 | ||
| Vastus medialis | Rest | Non-operated | 19.1 | 445 | 1.36 | 12.5 | 0.80 |
| Operated | 19.0 | 393 | 1.16 | 14.1 | 0.90 | ||
| Contraction | Non-operated | 18.0 | 398 | 1.20 | 13.6 | 0.86 | |
| Operated | 18.0 | 398 | 1.20 | 13.6 | 0.86 | ||
| Patellar tendon | Rest | Non-operated | 25.9 | 716 | 0.95 | 7.7 | 0.51 |
| Operated | 29.5 | 810 | 0.72 | 6.7 | 0.46 | ||
| Contraction | Non-operated | 38.6 | 1027 | 0.56 | 5.7 | 0.49 | |
| Operated | 33.7 | 947 | 0.57 | 5.4 | 0.37 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarecki, J.; Tomczyk-Warunek, A.; Posturzyńska, A.; Warda, E.; Waśko, M.; Arciszewski, K.; Tomaszewska, E.; Muszyński, S.; Bieniaś, J.; Ostapiuk, M.; et al. Treatment of Ochronotic Osteoarthropathy and the Evaluation of Selected Lower Limb Muscle Properties, Including the Patellar Tendon: A Case Report and Mini Literature Review. J. Clin. Med. 2025, 14, 4413. https://doi.org/10.3390/jcm14134413
Jarecki J, Tomczyk-Warunek A, Posturzyńska A, Warda E, Waśko M, Arciszewski K, Tomaszewska E, Muszyński S, Bieniaś J, Ostapiuk M, et al. Treatment of Ochronotic Osteoarthropathy and the Evaluation of Selected Lower Limb Muscle Properties, Including the Patellar Tendon: A Case Report and Mini Literature Review. Journal of Clinical Medicine. 2025; 14(13):4413. https://doi.org/10.3390/jcm14134413
Chicago/Turabian StyleJarecki, Jaromir, Agnieszka Tomczyk-Warunek, Agnieszka Posturzyńska, Edward Warda, Marcin Waśko, Kamil Arciszewski, Ewa Tomaszewska, Siemowit Muszyński, Jarosław Bieniaś, Monika Ostapiuk, and et al. 2025. "Treatment of Ochronotic Osteoarthropathy and the Evaluation of Selected Lower Limb Muscle Properties, Including the Patellar Tendon: A Case Report and Mini Literature Review" Journal of Clinical Medicine 14, no. 13: 4413. https://doi.org/10.3390/jcm14134413
APA StyleJarecki, J., Tomczyk-Warunek, A., Posturzyńska, A., Warda, E., Waśko, M., Arciszewski, K., Tomaszewska, E., Muszyński, S., Bieniaś, J., Ostapiuk, M., Skrzypek, T., & Gągała, J. (2025). Treatment of Ochronotic Osteoarthropathy and the Evaluation of Selected Lower Limb Muscle Properties, Including the Patellar Tendon: A Case Report and Mini Literature Review. Journal of Clinical Medicine, 14(13), 4413. https://doi.org/10.3390/jcm14134413






