An Imaging-Based Marker to Refine Risk Stratification for Transcatheter Mitral Valve Replacement
Abstract
1. Introduction
2. Materials and Methods
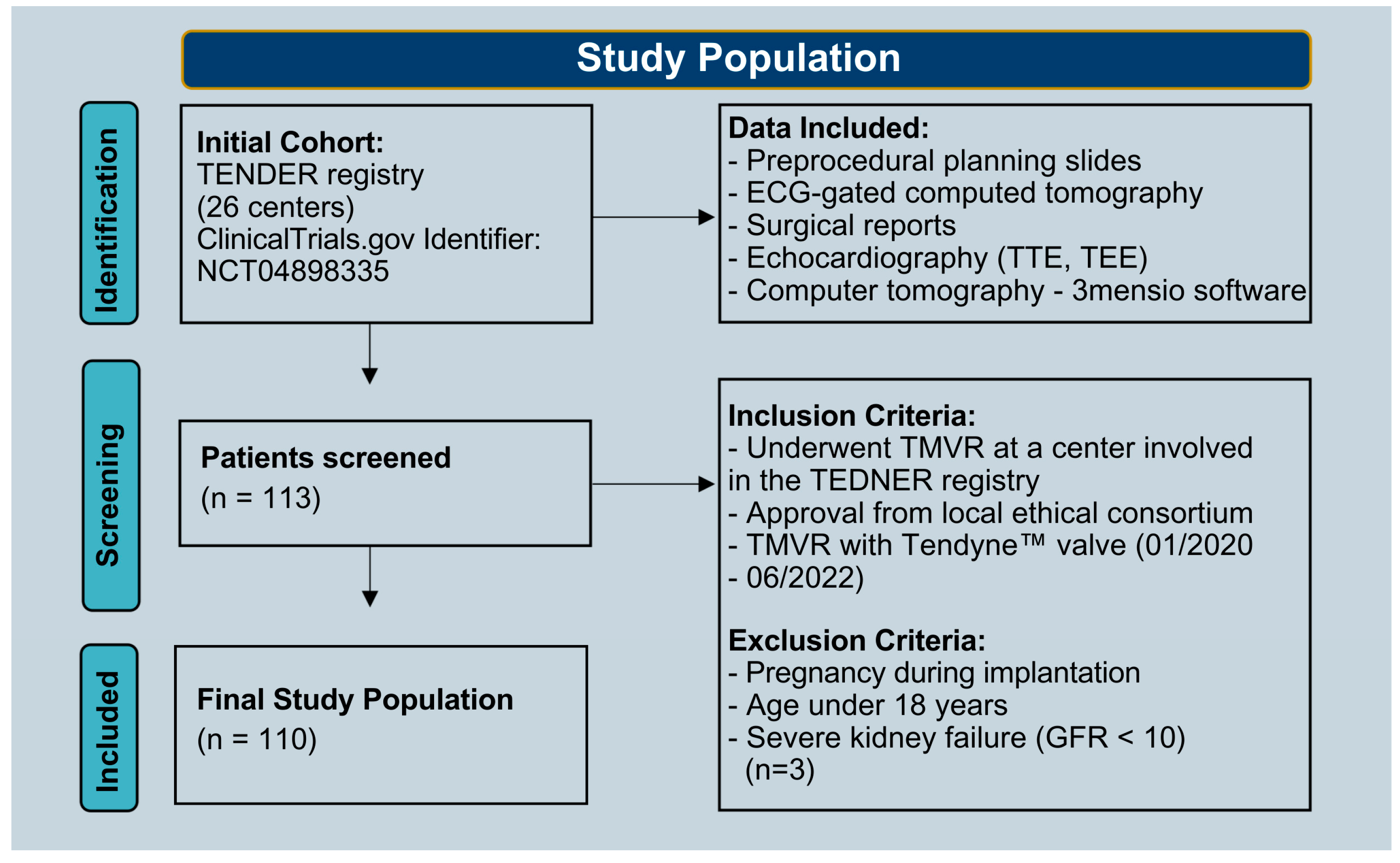
2.1. Study Population
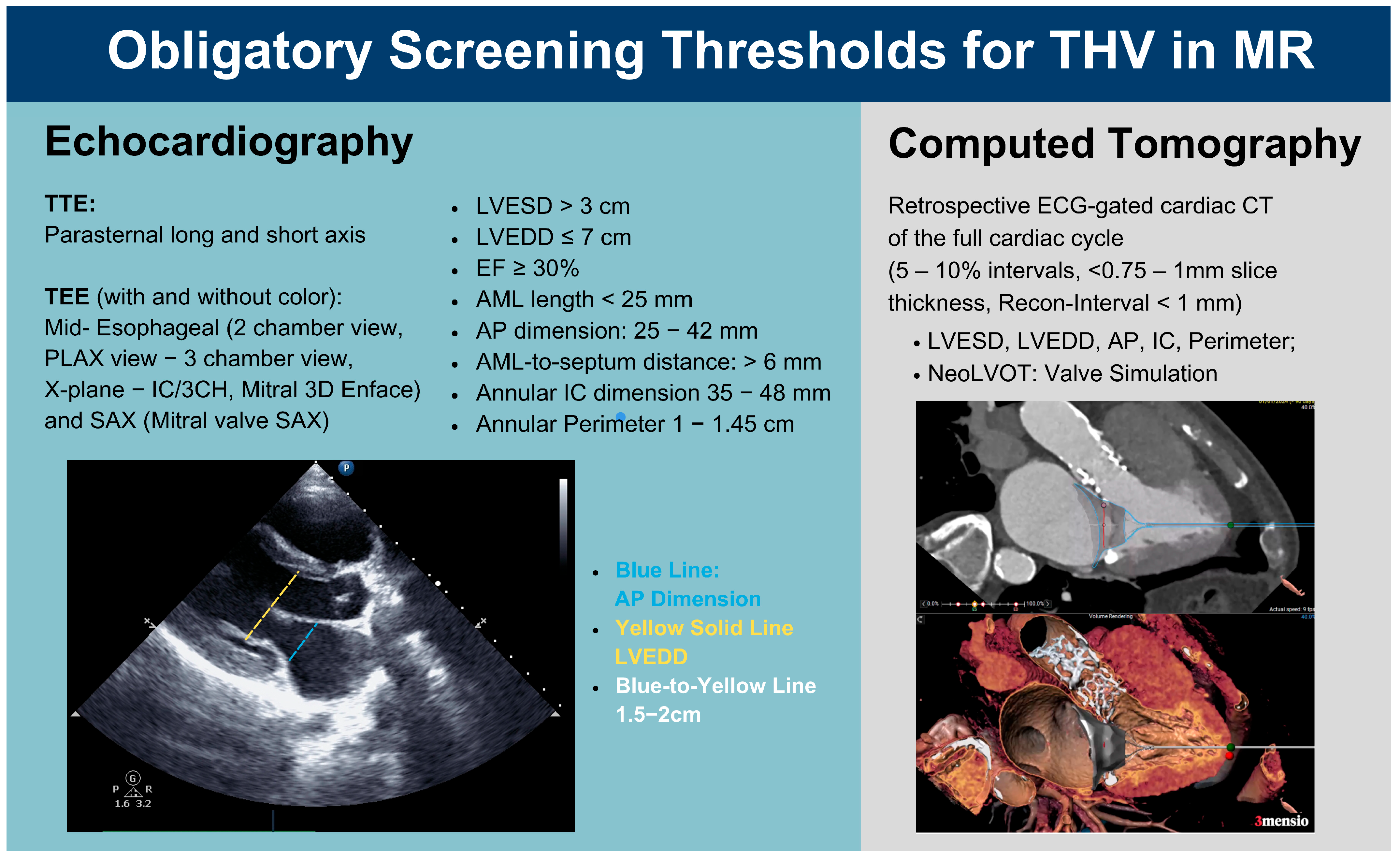
2.2. Preintervention Imaging
2.3. Statistical Analysis
3. Results
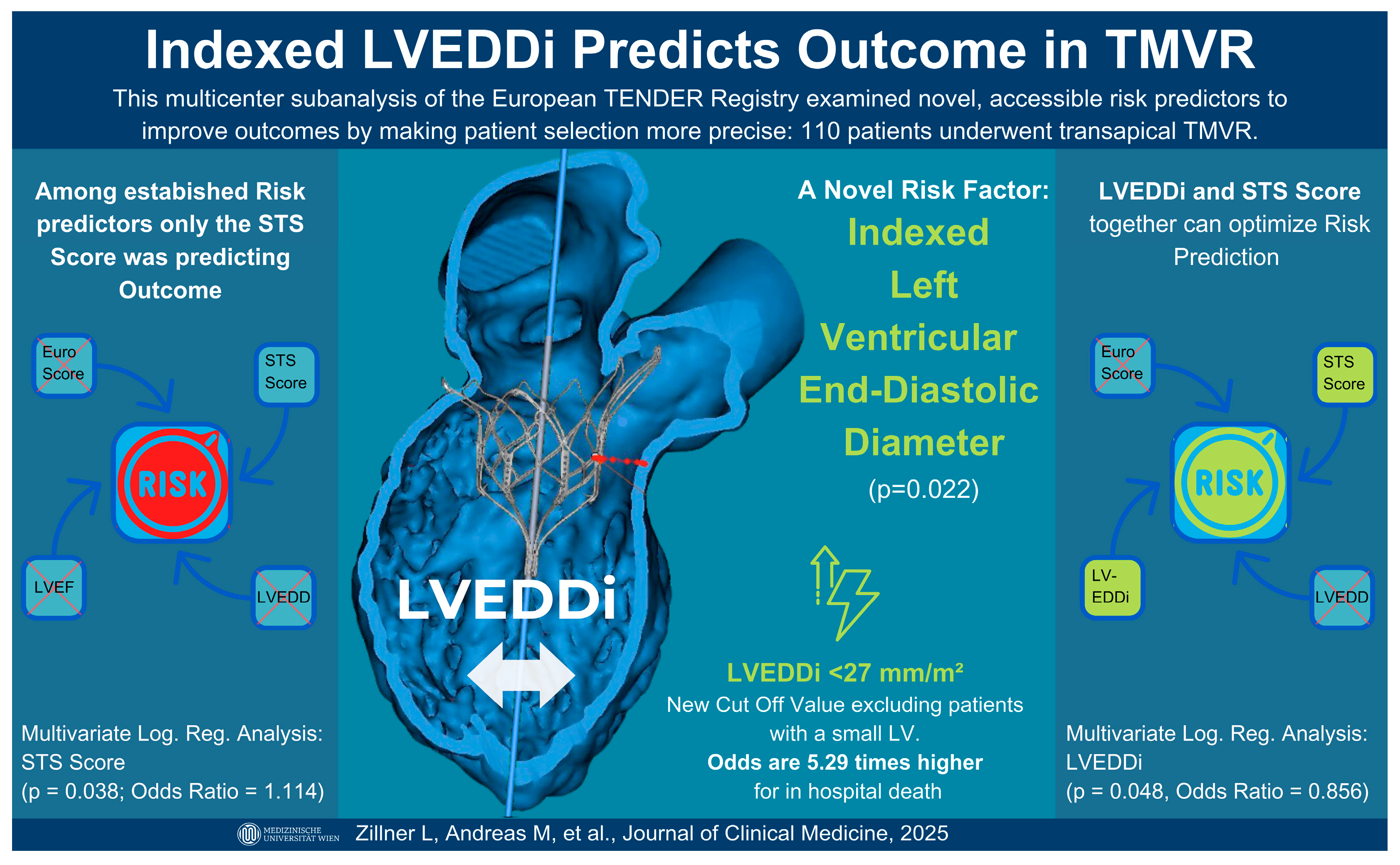
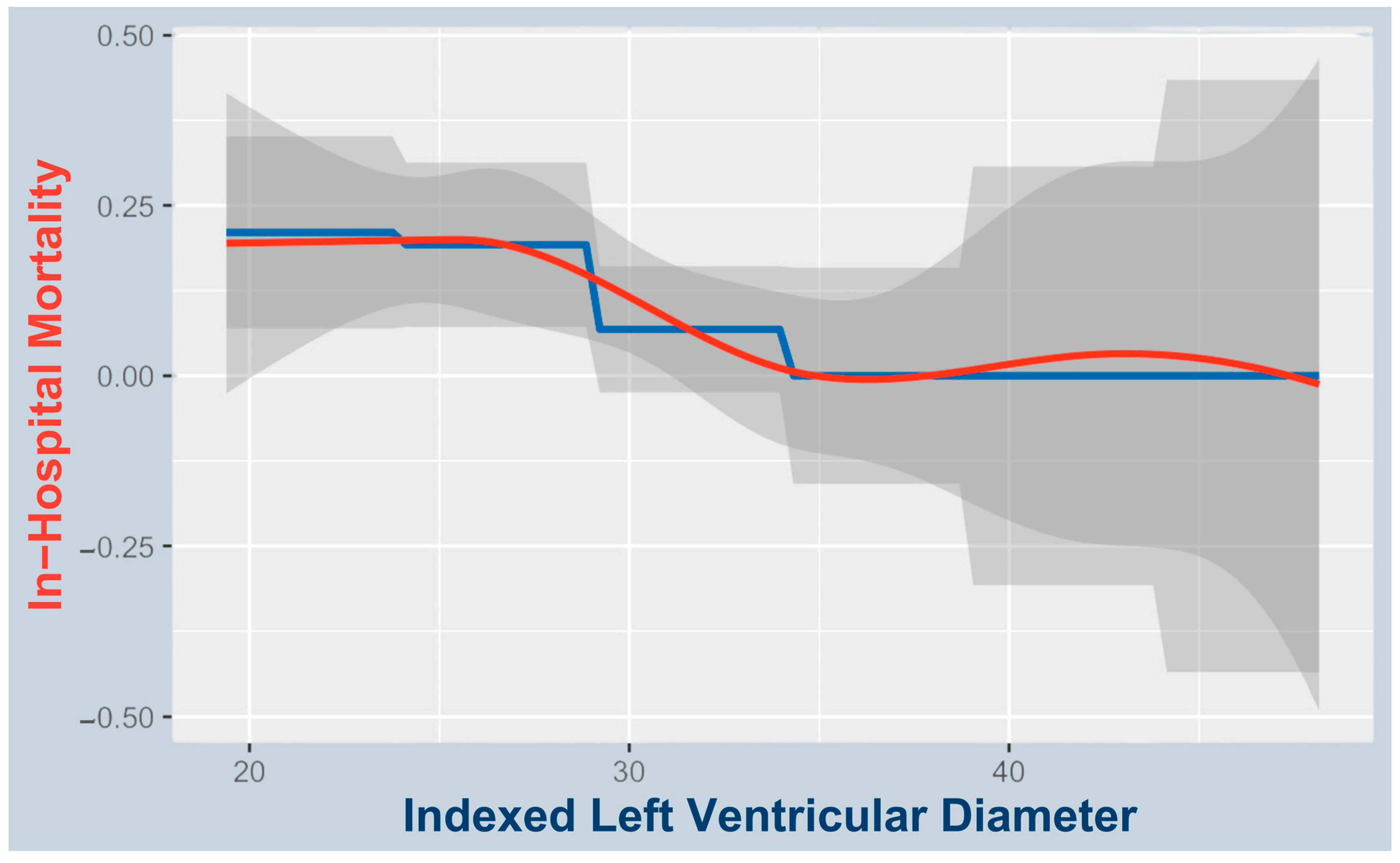
3.1. Predictors of In-Hospital Mortality
3.2. Causes of Death
3.3. LVEDDi’s Correlation to the STS Score
3.4. Predictors of In-Hospital Complications
3.5. Associations with AF and LVEDDi at Baseline
4. Discussion
4.1. Small LVEDDi as a Predictor of In-Hospital Mortality and Complications
4.2. LVEDDi as Indicator of AF at Baseline
4.3. Pathophysiologic Considerations Regarding a Smaller LVEDDi and Increased Mortality
4.3.1. Rapid Hemodynamic Compromise in Access Complication or Bleeding
4.3.2. Diastolic Dysfunction and Hypertrophy
4.4. LVEDDi and Its Correlation with the STS Score
4.5. What the Indexed LVEDDi Captures That the Absolute LVEDD Misses
4.6. A Small Ventricle Might Contribute to Late-Onset SAM
4.7. Future Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AML | Anterior Mitral Leaflet |
| AP | Anterior–Posterior |
| BSA | Body Surface Area |
| CI | Confidence Interval |
| COPD | Chronic Obstructive Pulmonary Disease |
| CT | Computed Tomography |
| ECG | Electrocardiogram |
| ECMO | Extracorporeal Membrane Oxygenation |
| EF | Ejection Fraction |
| GFR | Glomerular Filtration Rate |
| IC | Intercommissural |
| LA | Left Atrium |
| LV | Left Ventricle |
| LVEDD | Left Ventricular End-Diastolic Diameter |
| LVEDDi | Indexed Left Ventricular End-Diastolic Diameter |
| LVESD | Left Ventricular End-Systolic Diameter |
| LVOT | Left Ventricular Outflow Tract |
| LVOTO | Left Ventricular Outflow Tract Obstruction |
| MVARC | Mitral Valve Academic Research Consortium |
| MR | Mitral Regurgitation |
| MV | Mitral Valve |
| NeoLVOT | Neo Left Ventricular Outflow Tract |
| NYHA | New York Heart Association |
| OR | Odds Ratio |
| SAM | Systolic Anterior Motion |
| STS | Society of Thoracic Surgeons |
| TEE | Transesophageal Echocardiography |
| TEER | Transcatheter Edge-to-Edge Repair |
| THV | Transcatheter Heart Valve |
| TMVR | Transcatheter Mitral Valve Replacement |
| TTE | Transthoracic Echocardiography |
References
- Muller, D.W.M.; Sorajja, P.; Duncan, A.; Bethea, B.; Dahle, G.; Grayburn, P.; Babaliaros, V.; Guerrero, M.; Thourani, V.H.; Bedogni, F.; et al. 2-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients With Severe Symptomatic Mitral Regurgitation. J. Am. Coll. Cardiol. 2021, 78, 1847–1859. [Google Scholar] [CrossRef]
- Guerrero, M.; Urena, M.; Himbert, D.; Wang, D.D.; Eleid, M.; Kodali, S.; George, I.; Chakravarty, T.; Mathur, M.; Holzhey, D.; et al. 1-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients with Severe Mitral Annular Calcification. J. Am. Coll. Cardiol. 2018, 71, 1841–1853. [Google Scholar] [CrossRef]
- Duncan, A.; Daqa, A.; Yeh, J.; Davies, S.; Uebing, A.; Quarto, C.; Moat, N. Transcatheter Mitral Valve Replacement: Long-Term Outcomes of First-in-Man Experience with an Apically Tethered Device- a Case Series from a Single Centre. EuroIntervention 2017, 13, e1047–e1057. [Google Scholar] [CrossRef]
- Gössl, M.; Thourani, V.; Babaliaros, V.; Conradi, L.; Chehab, B.; Dumonteil, N.; Badhwar, V.; Rizik, D.; Sun, B.; Bae, R.; et al. Early Outcomes of Transcatheter Mitral Valve Replacement with the Tendyne System in Severe Mitral Annular Calcification. EuroIntervention 2022, 17, 1523–1531. [Google Scholar] [CrossRef]
- Sorajja, P.; Moat, N.; Badhwar, V.; Walters, D.; Paone, G.; Bethea, B.; Bae, R.; Dahle, G.; Mumtaz, M.; Grayburn, P.; et al. Initial Feasibility Study of a New Transcatheter Mitral Prosthesis: The First 100 Patients. J. Am. Coll. Cardiol. 2019, 73, 1250–1260. [Google Scholar] [CrossRef]
- Hausleiter, J. Tendyne European Experience Registry; Clinical Trials.gov: Bethesda, MD, USA, 2021.
- Wilde, N.; Tanaka, T.; Vij, V.; Sugiura, A.; Sudo, M.; Eicheler, E.; Silaschi, M.; Vogelhuber, J.; Bakhtiary, F.; Nickenig, G.; et al. Characteristics and Outcomes of Patients Undergoing Transcatheter Mitral Valve Replacement with the Tendyne System. Clin. Res. Cardiol. 2024, 113, 1–10. [Google Scholar] [CrossRef]
- Evans, J.S.; Huang, S.J.; McLean, A.S.; Nalos, M. Left Ventricular Outflow Tract Obstruction-Be Prepared! Anaesth. Intensive Care 2017, 45, 12–20. [Google Scholar] [CrossRef]
- Duncan, C.F.; Bowcock, E.; Pathan, F.; Orde, S.R. Mitral Regurgitation in the Critically Ill: The Devil Is in the Detail. Ann. Intensive Care 2023, 13, 67. [Google Scholar] [CrossRef]
- Kerbel, T.; Wild, M.G.; Hell, M.M.; Herkner, H.; Zillner, L.; Kuhn, E.W.; Rudolph, T.; Walther, T.; Conradi, L.; Zierer, A.; et al. Apical Access Management in Transapical Transcatheter Mitral Valve Replacement. Ann. Thorac. Surg. 2025, in press. [Google Scholar] [CrossRef]
- Stone, G.W.; Adams, D.H.; Abraham, W.T.; Kappetein, A.P.; Généreux, P.; Vranckx, P.; Mehran, R.; Kuck, K.-H.; Leon, M.B.; Piazza, N.; et al. Clinical Trial Design Principles and Endpoint Definitions for Transcatheter Mitral Valve Repair and Replacement: Part 2: Endpoint Definitions: A Consensus Document From the Mitral Valve Academic Research Consortium. J. Am. Coll. Cardiol. 2015, 66, 308–321. [Google Scholar] [CrossRef]
- Blanke, P.; Dvir, D.; Cheung, A.; Ye, J.; Levine, R.A.; Precious, B.; Berger, A.; Stub, D.; Hague, C.; Murphy, D.; et al. A Simplified D-Shaped Model of the Mitral Annulus to Facilitate CT-Based Sizing before Transcatheter Mitral Valve Implantation. J. Cardiovasc. Comput. Tomogr. 2014, 8, 459–467. [Google Scholar] [CrossRef]
- Reid, A.; Ben Zekry, S.; Turaga, M.; Tarazi, S.; Bax, J.J.; Wang, D.D.; Piazza, N.; Bapat, V.N.; Ihdayhid, A.R.; Cavalcante, J.L.; et al. Neo-LVOT and Transcatheter Mitral Valve Replacement: Expert Recommendations. JACC Cardiovasc. Imaging 2021, 14, 854–866. [Google Scholar] [CrossRef]
- Hahn, R.T.; Abraham, T.; Adams, M.S.; Bruce, C.J.; Glas, K.E.; Lang, R.M.; Reeves, S.T.; Shanewise, J.S.; Siu, S.C.; Stewart, W.; et al. Guidelines for Performing a Comprehensive Transesophageal Echocardiographic Examination: Recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J. Am. Soc. Echocardiogr. 2013, 26, 921–964. [Google Scholar] [CrossRef]
- Hell, M.M.; Wild, M.G.; Baldus, S.; Rudolph, T.; Treede, H.; Petronio, A.S.; Modine, T.; Andreas, M.; Coisne, A.; Duncan, A.; et al. Transapical Mitral Valve Replacement: 1-Year Results of the Real-World Tendyne European Experience Registry. JACC Cardiovasc. Interv. 2024, 17, 648–661. [Google Scholar] [CrossRef]
- Conradi, L.; Ludwig, S.; Sorajja, P.; Duncan, A.; Bethea, B.; Dahle, G.; Babaliaros, V.; Guerrero, M.; Thourani, V.; Dumonteil, N.; et al. Clinical Outcomes and Predictors of Transapical Transcatheter Mitral Valve Replacement: The Tendyne Expanded Clinical Study. EuroIntervention 2024, 20, e887–e897. [Google Scholar] [CrossRef]
- Fukui, M.; Sorajja, P.; Gössl, M.; Bae, R.; Lesser, J.R.; Sun, B.; Duncan, A.; Muller, D.; Cavalcante, J.L. Left Ventricular Remodeling After Transcatheter Mitral Valve Replacement With Tendyne: New Insights From Computed Tomography. JACC Cardiovasc. Interv. 2020, 13, 2038–2048. [Google Scholar] [CrossRef]
- Duncan, A.; Dahle, G.; Conradi, L.; Dumonteil, N.; Wang, J.; Shah, N.; Sun, B.; Sorajja, P.; Ailawadi, G.; Rogers, J.H.; et al. Multicenter Clinical Management Practice to Optimize Outcomes Following Tendyne Transcatheter Mitral Valve Replacement. Struct. Heart 2022, 6, 100025. [Google Scholar] [CrossRef]
- Ludwig, S.; Perrin, N.; Coisne, A.; Ben Ali, W.; Weimann, J.; Duncan, A.; Akodad, M.; Scotti, A.; Kalbacher, D.; Bleiziffer, S.; et al. Clinical Outcomes of Transcatheter Mitral Valve Replacement: Two-Year Results of the CHOICE-MI Registry. EuroIntervention 2023, 19, 512–525. [Google Scholar] [CrossRef]
- Ludwig, S.; Kalbacher, D.; Ali, W.B.; Weimann, J.; Adam, M.; Duncan, A.; Webb, J.G.; Windecker, S.; Orban, M.; Giannini, C.; et al. Transcatheter Mitral Valve Replacement or Repair for Secondary Mitral Regurgitation: A Propensity Score-Matched Analysis. Eur. J. Heart Fail. 2023, 25, 399–410. [Google Scholar] [CrossRef]
- Kasa, G.; Teis, A.; De Raffele, M.; Cediel, G.; Juncà, G.; Lupón, J.; Santiago-Vacas, E.; Codina, P.; Bayés-Genis, A.; Delgado, V. Prognostic Value of Left Atrioventricular Coupling Index in Heart Failure. Eur. Heart J. Cardiovasc. Imaging 2025, 26, 610–617. [Google Scholar] [CrossRef]
- Mauriello, A.; Correra, A.; Ascrizzi, A.; Del Vecchio, G.E.; Benfari, G.; Ilardi, F.; Lisi, M.; Malagoli, A.; Mandoli, G.E.; Pastore, M.C.; et al. Relationship Between Left Atrial Strain and Atrial Fibrillation: The Role of Stress Echocardiography. Diagnostics 2025, 15, 7. [Google Scholar] [CrossRef]
- Wolsk, E.; Kaye, D.M.; Komtebedde, J.; Shah, S.J.; Borlaug, B.A.; Burkhoff, D.; Kitzman, D.W.; Cleland, J.G.; Hasenfuß, G.; Hassager, C.; et al. Determinants and Consequences of Heart Rate and Stroke Volume Response to Exercise in Patients with Heart Failure and Preserved Ejection Fraction. Eur. J. Heart Fail. 2021, 23, 754–764. [Google Scholar] [CrossRef]
- Heward, S.J.; Shams, P.; Widrich, J. Coronary Perfusion Pressure. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Miličić, D.; Jakuš, N.; Fabijanović, D. Microcirculation and Heart Failure. Curr. Pharm. Des. 2018, 24, 2954–2959. [Google Scholar] [CrossRef]
- Dhont, S.; van den Acker, G.; van Loon, T.; Verbrugge, F.H.; Verwerft, J.; Deferm, S.; Churchill, T.W.; Mullens, W.; Lumens, J.; Bertrand, P.B. Mitral Regurgitation in Heart Failure with Preserved Ejection Fraction: The Interplay of Valve, Ventricle, and Atrium. Eur. J. Heart Fail. 2024, 26, 974–983. [Google Scholar] [CrossRef]
- STS ACSD Operative Risk Calculator. Available online: https://acsdriskcalc.research.sts.org/ (accessed on 23 May 2025).
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Støylen, A.; Mølmen, H.E.; Dalen, H. Importance of Length and External Diameter in Left Ventricular Geometry. Normal Values from the HUNT Study. Open Heart 2016, 3, e000465. [Google Scholar] [CrossRef]
- Slama, M.; Tribouilloy, C.; Maizel, J. Left Ventricular Outflow Tract Obstruction in ICU Patients. Curr. Opin. Crit. Care 2016, 22, 260–266. [Google Scholar] [CrossRef]
- Vahl, T.P.; Grogan, A.; Cheng, Y.; Yi, G.; von Oepen, R.; Khalique, O.K.; Wallace, D.T.; Modine, T.; Granada, J.F. Experimental Evaluation of a Novel Percutaneous Transseptal Catheter-Based Mitral Valve Replacement Technology. Circ. Cardiovasc. Interv. 2019, 12, e008002. [Google Scholar] [CrossRef]
| Baseline Characteristics | In-Hospital Survival (n = 98) | In-Hospital Death (n = 12) | p-Value |
|---|---|---|---|
| BMI (kg/m2) | 26.15 ± 5.07 | 27.09 ± 3.28 | 0.53 |
| Age (years) | 77 (12) | 79.5 (12) | 0.23 |
| Male | 54 (55.1) | 8 (66.67) | 0.45 |
| Female | 44 (44.9) | 4 (33.33) | 0.45 |
| EuroScore II (%) | 6.3 (6.8) | 8.45 (7.08) | 0.16 |
| STS | 5.8 (6.4) | 7.65 (10.03) | 0.02 |
| NT-pro-BNP (pg/mL) | 3578 (5374) | 3843 (11392) | 0.94 |
| GFR (mL/min) | 42 (31) | 37.5 (19.8) | 0.14 |
| sPAP (mmHg) | 51.68 ± 14.76 | 58.27 ± 18.51 | 0.18 |
| NYHA Class II | 18 (18.37) | 0 | 0.26 |
| NYHA Class III | 72 (73.47) | 11 (91.67) | 0.26 |
| NYHA Class IV | 8 (8.16) | 1 (8.33) | 0.26 |
| Heart Failure Hospitalization | 60 (62.5) | 10 (90.9) | 0.06 |
| Atrial Fibrillation | 62 (63.27) | 7 (58.33) | 0.74 |
| COPD | 16 (16.33) | 4 (33.33) | 0.15 |
| Prior Stroke | 14 (14.29) | 1 (8.33) | 0.57 |
| Coronary Artery Disease | 59 (60.2) | 10 (83.33) | 0.12 |
| Prior Myocardial Infarction | 17 (17.53) | 4 (33.33) | 0.19 |
| Prior Mitral Valve Intervention | 9 (9.18) | 0 | 0.27 |
| Prior Mitral Valve Surgery | 3 (3.06) | 0 | 0.54 |
| Primary MI | 41 (41.84) | 5 (41.67) | 0.97 |
| Secondary MI | 35 (35.71) | 4 (33.33) | 0.97 |
| Mixed MI | 22 (22.45) | 3 (25.00) | 0.97 |
| Echo + CT Baseline | |||
| 2D Echocardiographic Parameters: | |||
| LVEF (%) | 50 (17) | 50 (19) | 0.17 |
| LVEDD (mm) | 55.28 ± 8.06 | 50.83 ± 8.04 | 0.07 |
| LVEDDi (mm/m2) | 30.37 ± 5.58 | 26.42 ± 3.76 | 0.02 |
| TAPSE < 1 (cm) | 33 (35.87) | 4 (36.36) | 0.97 |
| Tricuspid Regurgitation III–IV | 23 (23.71) | 5 (41.67) | 0.18 |
| (ECG Gated) 2D-CT Parameters: | |||
| Diameter of the Apex (mm) | 5.89 ± 2.09 | 5.33 ± 2.18 | 0.38 |
| Apex to Surgical Apex (mm) | 2.5 (2.2) | 2.5 (1.6) | 0.71 |
| LAD Distance (mm) | 17.24 ± 8.45 | 17.18 ± 9.62 | 0.98 |
| Ant.-Post. Oversize (%) | 6 (7.7) | 7.8 (19.5) | 0.26 |
| Intercommissural Oversize (%) | 18.2 (8.5) | 13 (8.3) | 0.41 |
| A2 Clearing Systole (mm) | 10.6 (5.35) | 11.8 (1.7) | 0.63 |
| End systolic Neo-LVOT (mm2) | 380 (169.65) | 364.2 (30.85) | 0.88 |
| A2 Clearing Diastole (mm) | 11.7 (6.4) | 9.6 (6.95) | 0.35 |
| End diastolic Neo-LVOT (mm2) | 431.5 (228) | 310 (215.4) | 0.26 |
| AMVL length (mm) | 21.7 (4.3) | 20.6 (9.9.) | 0.42 |
| Left Atrium height (mm) | 67 ± 9.78 | 64.68 ± 9.6 | 0.44 |
| B | S.E. | p-Value | Exp(B) (Odds Ratio) | 95% CI for Exp(B) | |
|---|---|---|---|---|---|
| Clinical Characteristics | |||||
| EuroSCORE II (%) | 0.029 | 0.045 | 0.515 | 1.030 | 0.943–1.124 |
| STS Score (%) | 0.110 | 0.052 | 0.036 | 1.116 | 1.007–1.236 |
| NT-pro-BNP (pg/mL) | 0.000 | 0.000 | 0.907 | 1.000 | 1.000–1.000 |
| GFR (mL/min) | −0.024 | 0.017 | 0.159 | 0.976 | 0.943–1.010 |
| sPAP (mmHg) | 0.026 | 0.020 | 0.181 | 1.027 | 0.988–1.067 |
| 2D Echocardiographic Parameters: | |||||
| LVEF (%) | −0.037 | 0.029 | 0.209 | 0.964 | 0.91–1.021 |
| LVEDD (mm) | −0.073 | 0.042 | 0.080 | 0.930 | 0.857–1.009 |
| LVEDDI (mm/m2) | −0.159 | 0.069 | 0.022 | 0.853 | 0.745–0.977 |
| t-Test: | No In-Hospital Complications (n = 43) | In-Hospital Complications (n = 66) | p-Value |
|---|---|---|---|
| LVEDD | 57.34 ± 8.06 | 53.16 ± 7.87 | 0.008 |
| LVEDDi (mm/m2) | 32.10 ± 6.14 | 26.42 ± 3.76 | <0.001 |
| Logistic Regression: | OR (95%-CI) | p-value | |
| LVEDD | 0.929 (0.881–0.975) | 0.006 | |
| LVEDDi (mm/m2) | 0.867 (0.796–0.946) | <0.001 |
| Major Complications | Total (n) | In-Hospital Mortality (n) | p-Value |
|---|---|---|---|
| Patients | 110 | 10 | |
| Major Access Complication (= Life-Threatening Bleeding) | 8 | 4 | <0.001 |
| Bleeding grade 2, 3, or 5 | 23 | 6 | 0.004 |
| Sepsis | 11 | 6 | <0.001 |
| ECMO | 4 | 3 | <0.001 |
| Stroke | |||
| -nondisabling | 2 | 1 | 0.211 |
| -disabling | 1 | 0 | |
| LVOTO | 9 | 3 | 0.025 |
| Acute Kidney Injury | 23 | 5 | 0.011 |
| Acute Dialysis | 2 | 1 | 0.035 |
| Reintervention (any cause) | 4 | 0 | 0.46 |
| Full Sternotomy | 1 | 1 | 0.004 |
| Myocardial Infarction | 1 | 0 | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zillner, L.; Wild, M.G.; Hell, M.M.; Herkner, H.; Kuhn, E.W.; Rudolph, T.; Walther, T.; Conradi, L.; Zierer, A.; Maisano, F.; et al. An Imaging-Based Marker to Refine Risk Stratification for Transcatheter Mitral Valve Replacement. J. Clin. Med. 2025, 14, 4412. https://doi.org/10.3390/jcm14134412
Zillner L, Wild MG, Hell MM, Herkner H, Kuhn EW, Rudolph T, Walther T, Conradi L, Zierer A, Maisano F, et al. An Imaging-Based Marker to Refine Risk Stratification for Transcatheter Mitral Valve Replacement. Journal of Clinical Medicine. 2025; 14(13):4412. https://doi.org/10.3390/jcm14134412
Chicago/Turabian StyleZillner, Liliane, Mirjam G. Wild, Michaela M. Hell, Harald Herkner, Elmar W. Kuhn, Tanja Rudolph, Thomas Walther, Lenard Conradi, Andreas Zierer, Francesco Maisano, and et al. 2025. "An Imaging-Based Marker to Refine Risk Stratification for Transcatheter Mitral Valve Replacement" Journal of Clinical Medicine 14, no. 13: 4412. https://doi.org/10.3390/jcm14134412
APA StyleZillner, L., Wild, M. G., Hell, M. M., Herkner, H., Kuhn, E. W., Rudolph, T., Walther, T., Conradi, L., Zierer, A., Maisano, F., Russo, M., Rosati, F., Colli, A., Piñón, M., Reineke, D., Aphram, G., Kerbel, T., Dubois, C., Hausleiter, J., ... Andreas, M. (2025). An Imaging-Based Marker to Refine Risk Stratification for Transcatheter Mitral Valve Replacement. Journal of Clinical Medicine, 14(13), 4412. https://doi.org/10.3390/jcm14134412





