Clinical and Biochemical Characteristics of Pseudohypoaldosteronism Type 1 with and Without Genetic Mutations: A Literature Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
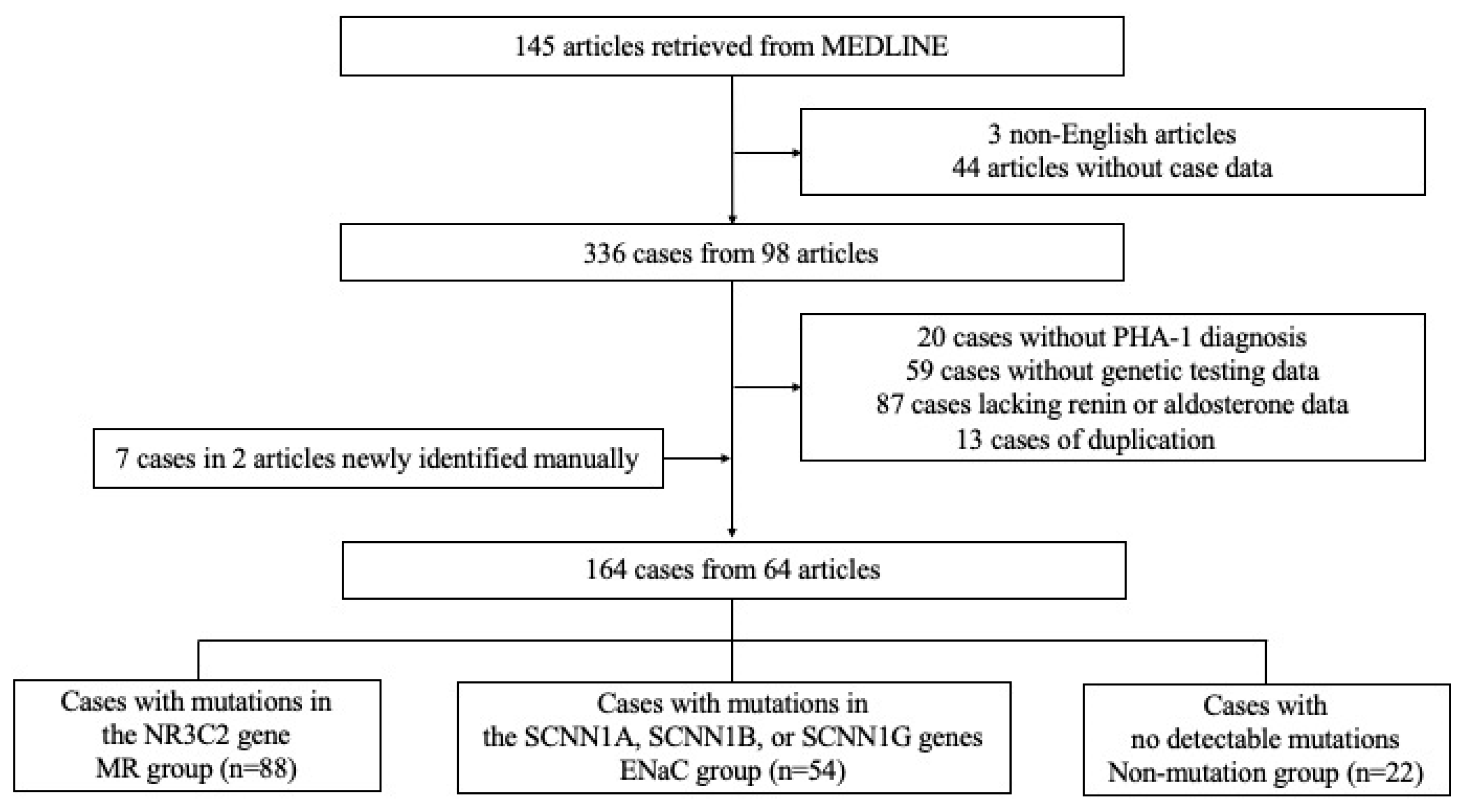
3.1. Study Subsection
3.2. Comparison Among MR, ENaC, and Non-Mutation Groups
3.3. Comparison Between Truncating and Non-Truncating Variants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PHA-1 | Pseudohypoaldosteronism type 1 |
| PHA-2 | Pseudohypoaldosteronism type 2 |
| MR | Mineralocorticoid receptor |
| ENaC | Epithelial sodium channel |
| Na | Sodium |
| K | Potassium |
| Cl | Chloride |
| NaCl | Sodium chloride |
| PRA | Plasma renin activity |
| ARC | Active renin concentration |
| ARR | Aldosterone/renin ratio |
References
- Cheek, D.B.; Perry, J.W. A salt wasting syndrome in infancy. Arch. Dis. Child. 1958, 33, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Zennaro, M.C.; Hubert, E.L.; Fernandes-Rosa, F.L. Aldosterone resistance: Structural and functional considerations and new perspectives. Mol. Cell. Endocrinol. 2012, 350, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Attia, N.A.; Marzouk, Y.I. Pseudohypoaldosteronism in a Neonate Presenting as Life-Threatening Hyperkalemia. Case Rep. Endocrinol. 2016, 2016, 6384697. [Google Scholar] [CrossRef] [PubMed]
- Rajpoot, S.K.; Maggi, C.; Bhangoo, A. Pseudohypoaldosteronism in a neonate presenting as life-threatening arrhythmia. Endocrinol. Diabetes Metab. Case Rep. 2014, 2014, 130077. [Google Scholar] [CrossRef]
- Pathare, G.; Hoenderop, J.G.; Bindels, R.J.; San-Cristobal, P. A molecular update on pseudohypoaldosteronism type II. Am. J. Physiol. Renal Physiol. 2013, 305, F1513–F1520. [Google Scholar] [CrossRef]
- Tajima, T.; Morikawa, S.; Nakamura, A. Clinical features and molecular basis of pseudohypoaldosteronism type 1. Clin. Pediatr. Endocrinol. 2017, 26, 109–117. [Google Scholar] [CrossRef]
- Bogdanovic, R.; Stajic, N.; Putnik, J.; Paripovic, A. Transient type 1 pseudo-hypoaldosteronism: Report on an eight-patient series and literature review. Pediatr. Nephrol. 2009, 24, 2167–2175. [Google Scholar] [CrossRef]
- Dirlewanger, M.; Huser, D.; Zennaro, M.C.; Girardin, E.; Schild, L.; Schwitzgebel, V.M. A homozygous missense mutation in SCNN1A is responsible for a transient neonatal form of pseudohypoaldosteronism type 1. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E467–E473. [Google Scholar] [CrossRef][Green Version]
- Kim, S.J.; Park, D.; Jang, W.; Lee, J. A Neonate with Autosomal Dominant Pseudohypoaldosteronism Type 1 Due to a Novel Microdeletion of the NR3C2 Gene at 4q31.23. Children 2021, 8, 1090. [Google Scholar] [CrossRef]
- Crnkovic Cuk, M.; Kovacevic, A.; Zaja, O.; Pozgaj Sepec, M.; Roic, G.; Valent Moric, B.; Trutin, I. Transient Pseudohypoaldosteronism Secondary to Urinary Tract Infection in a Male Infant with Unilateral Hydronephrosis Due to Primary Obstructive Megaureter: A Case Report. Acta Clin. Croat. 2022, 61, 717–721. [Google Scholar] [CrossRef]
- Martinerie, L.; Viengchareun, S.; Delezoide, A.L.; Jaubert, F.; Sinico, M.; Prevot, S.; Boileau, P.; Meduri, G.; Lombes, M. Low renal mineralocorticoid receptor expression at birth contributes to partial aldosterone resistance in neonates. Endocrinology 2009, 150, 4414–4424. [Google Scholar] [CrossRef] [PubMed]
- Martinerie, L.; Pussard, E.; Foix-L’Helias, L.; Petit, F.; Cosson, C.; Boileau, P.; Lombes, M. Physiological partial aldosterone resistance in human newborns. Pediatr. Res. 2009, 66, 323–328. [Google Scholar] [CrossRef]
- Fujioka, K.; Nakasone, R.; Nishida, K.; Ashina, M.; Sato, I.; Nozu, K. Neonatal Pseudohypoaldosteronism Type-1 in Japan. J. Clin. Med. 2022, 11, 5135. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, K.; Kyriazopoulou Panagiotopoulou, S.; McRae, J.F.; Darbandi, S.F.; Knowles, D.; Li, Y.I.; Kosmicki, J.A.; Arbelaez, J.; Cui, W.; Schwartz, G.B.; et al. Predicting Splicing from Primary Sequence with Deep Learning. Cell 2019, 176, 535–548.e24. [Google Scholar] [CrossRef]
- Teruyama, K.; Naruse, M.; Tsuiki, M.; Kobayashi, H. Novel chemiluminescent immunoassay to measure plasma aldosterone and plasma active renin concentrations for the diagnosis of primary aldosteronism. J. Hum. Hypertens. 2022, 36, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P.; Shaw, S.G.; Nicod, J.; Saner, E.; Nussberger, J. Active renin versus plasma renin activity to define aldosterone-to-renin ratio for primary aldosteronism. J. Hypertens. 2004, 22, 377–381. [Google Scholar] [CrossRef]
- Adachi, M.; Nagahara, K.; Ochi, A.; Toyoda, J.; Muroya, K.; Mizuno, K. Acid-Base Imbalance in Pseudohypoaldosteronism Type 1 in Comparison With Type IV Renal Tubular Acidosis. J. Endocr. Soc. 2022, 6, bvac147. [Google Scholar] [CrossRef]
- Reincke, M.; Bancos, I.; Mulatero, P.; Scholl, U.I.; Stowasser, M.; Williams, T.A. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. 2021, 9, 876–892. [Google Scholar] [CrossRef]
- Riepe, F.G. Clinical and molecular features of type 1 pseudohypoaldosteronism. Horm. Res. 2009, 72, 1–9. [Google Scholar] [CrossRef]
- Delforge, X.; Kongolo, G.; Cauliez, A.; Braun, K.; Haraux, E.; Buisson, P. Transient pseudohypoaldosteronism: A potentially severe condition affecting infants with urinary tract malformation. J. Pediatr. Urol. 2019, 15, 265.e1–265.e7. [Google Scholar] [CrossRef]
- Hatta, Y.; Nakamura, A.; Hara, S.; Kamijo, T.; Iwata, J.; Hamajima, T.; Abe, M.; Okada, M.; Ushio, M.; Tsuyuki, K.; et al. Clinical and molecular analysis of six Japanese patients with a renal form of pseudohypoaldosteronism type 1. Endocr. J. 2013, 60, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Riepe, F.G.; Finkeldei, J.; de Sanctis, L.; Einaudi, S.; Testa, A.; Karges, B.; Peter, M.; Viemann, M.; Grotzinger, J.; Sippell, W.G.; et al. Elucidating the underlying molecular pathogenesis of NR3C2 mutants causing autosomal dominant pseudohypoaldosteronism type 1. J. Clin. Endocrinol. Metab. 2006, 91, 4552–4561. [Google Scholar] [CrossRef] [PubMed]
- Gopal-Kothandapani, J.S.; Doshi, A.B.; Smith, K.; Christian, M.; Mushtaq, T.; Banerjee, I.; Padidela, R.; Ramakrishnan, R.; Owen, C.; Cheetham, T.; et al. Phenotypic diversity and correlation with the genotypes of pseudohypoaldosteronism type 1. J. Pediatr. Endocrinol. Metab. 2019, 32, 959–967. [Google Scholar] [CrossRef]
- Adachi, M.; Asakura, Y.; Muroya, K.; Tajima, T.; Fujieda, K.; Kuribayashi, E.; Uchida, S. Increased Na reabsorption via the Na-Cl cotransporter in autosomal recessive pseudohypoaldosteronism. Clin. Exp. Nephrol. 2010, 14, 228–232. [Google Scholar] [CrossRef]
- Hanukoglu, A.; Edelheit, O.; Shriki, Y.; Gizewska, M.; Dascal, N.; Hanukoglu, I. Renin-aldosterone response, urinary Na/K ratio and growth in pseudohypoaldosteronism patients with mutations in epithelial sodium channel (ENaC) subunit genes. J. Steroid Biochem. Mol. Biol. 2008, 111, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Edelheit, O.; Hanukoglu, I.; Gizewska, M.; Kandemir, N.; Tenenbaum-Rakover, Y.; Yurdakok, M.; Zajaczek, S.; Hanukoglu, A. Novel mutations in epithelial sodium channel (ENaC) subunit genes and phenotypic expression of multisystem pseudohypoaldosteronism. Clin. Endocrinol. 2005, 62, 547–553. [Google Scholar] [CrossRef]
| MR (n = 88) | ENaC (n = 54) | Non-Mutation (n = 22) | p-Value | |
|---|---|---|---|---|
| Male sex (%) | 47 (53%) | 23 (43%) | 11 (50%) | 0.45 |
| Preterm birth | 8 (9%) | 6 (11%) | 2 (9%) | 0.93 |
| Presence of secondary factors | 6 (7%) | 3 (6%) | 3 (14%) | 0.49 |
| Presentation within 28 days after birth a,b | 57 (65%) | 45(83%) | 13 (59%) | 0.03 |
| Presentation within 3 months after birth a,b | 72 (82%) | 53 (98%) | 18 (82%) | <0.01 |
| Presentation within 12 months after birth | 87 (99%) | 54 (100%) | 22 (100%) | >0.99 |
| Sodium supplementation | 81 (92%) | 52 (96%) | 19 (86%) | 0.25 |
| Serum sodium levels (mEq/L) a | 127.5 (113.3–140.0) n = 82 | 124.0 (105.0–135.0) n = 54 | 126.0 (114.3–134.0) n = 21 | <0.01 |
| Serum potassium levels (mEq/L) a,b | 6.3 (4.9–10.5) n = 79 | 9.0 (5.1–13.0) n = 53 | 6.53 (4.9–11.4) n = 21 | <0.01 |
| Plasma renin activity (PRA, ng/mL/h) | 100.2 (3.1–2,531,400) n = 42 | 140 (5.36–6,410,000) n = 27 | 38.8 (0.5–430) n = 11 | 0.14 |
| Active renin concentration (ARC, pg/mL) a,c | 2838 (101–323,000) n = 22 | 396 (21–104,200) n = 16 | 116 (6.6–18,000) n = 9 | <0.01 |
| Serum aldosterone concentrations (pg/mL) c | 9170 (368.0–45,600) n = 72 | 6440 (209.1–45,745) n = 48 | 3585 (234.3–34,402) n = 22 | 0.02 |
| Aldosterone/renin ratio (pmol/L per ng/mL/h) | 289.8 (0.0–39,104.5) n = 36 | 188.2 (0.2–1924.5) n = 26 | 300.0 (60.0–5548.0) n = 11 | 0.28 |
| Aldosterone/renin ratio (pmol/ng) | 9.8 (0.1–148.7) n = 18 | 33.8 (0.1–302.9) n = 15 | 98.5 (0.1–1131.7) n = 9 | 0.06 |
| Truncating | Non-Truncating | p-Value | |
|---|---|---|---|
| MR Group | |||
| Na (mEq/L) | 127 (113.3–140, n = 60) | 128 (114–136, n = 17) | 0.76 |
| K (mEq/L) | 6.3 (5.1–10.5, n = 60) | 6.7 (4.9–9.7, n = 14) | 0.14 |
| Plasma renin activity (PRA, ng/mL/h) | 74 (6.858–2,531,400, n = 31) | 200 (65.4–15,176, n = 9) | 0.04 |
| Active renin concentration (ARC, pg/mL) | 3000 (101–322,899, n = 15) | 1460 (1266–52,668, n = 5) | 0.67 |
| Aldosterone (pg/mL) | 9170 (1153–45,600, n = 54) | 7592 (368–38,530, n = 14) | 0.91 |
| Aldosterone/renin ratio (pmol/L per ng/mL/h) | 321.6 (0.0–4674.2, n = 27) | 72.9 (1.9–1083.8, n = 8) | 0.24 |
| Aldosterone/renin ratio (pmol/ng) | 5.0 (0.1–78.3, n = 13) | 11.0 (8.6–21.9, n = 3) | 0.61 |
| ENaC Group | |||
| Na (mEq/L) | 123 (105–135, n = 37) | 125 (106–133.4, n = 15) | 0.18 |
| K (mEq/L) | 9.3 (5.1–13, n = 36) | 7.7 (5.4–10, n = 15) | <0.01 |
| Plasma renin activity (PRA, ng/mL/h) | 84.5 (5.36–192,000, n = 22) | 140 (13.39–6,410,000, n = 3) | 0.78 |
| Active renin concentration (ARC, pg/mL) | 1092 (160.3–104,200, n = 8) | 187.1 (21–96,900, n = 8) | <0.05 |
| Aldosterone (pg/mL) | 6168 (209.1–45,745, n = 32) | 8560 (806–20,880, n = 14) | 0.46 |
| Aldosterone/renin ratio (pmol/L per ng/mL/h) | 259.0 (0.2–1924.5, n = 22) | 428.7 (187.4–669.9, n = 2) | 0.59 |
| Aldosterone/renin ratio (pmol/ng) | 21.0 (0.1–184.3, n = 7) | 95.5 (0.1–302.9, n = 8) | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakata, Y.; Nagano, C.; Imagawa, Y.; Shirai, K.; Masuda, Y.; Kido, T.; Ashina, M.; Nozu, K.; Fujioka, K. Clinical and Biochemical Characteristics of Pseudohypoaldosteronism Type 1 with and Without Genetic Mutations: A Literature Review. J. Clin. Med. 2025, 14, 4408. https://doi.org/10.3390/jcm14134408
Nakata Y, Nagano C, Imagawa Y, Shirai K, Masuda Y, Kido T, Ashina M, Nozu K, Fujioka K. Clinical and Biochemical Characteristics of Pseudohypoaldosteronism Type 1 with and Without Genetic Mutations: A Literature Review. Journal of Clinical Medicine. 2025; 14(13):4408. https://doi.org/10.3390/jcm14134408
Chicago/Turabian StyleNakata, Yuki, China Nagano, Yukihito Imagawa, Keisuke Shirai, Yu Masuda, Takumi Kido, Mariko Ashina, Kandai Nozu, and Kazumichi Fujioka. 2025. "Clinical and Biochemical Characteristics of Pseudohypoaldosteronism Type 1 with and Without Genetic Mutations: A Literature Review" Journal of Clinical Medicine 14, no. 13: 4408. https://doi.org/10.3390/jcm14134408
APA StyleNakata, Y., Nagano, C., Imagawa, Y., Shirai, K., Masuda, Y., Kido, T., Ashina, M., Nozu, K., & Fujioka, K. (2025). Clinical and Biochemical Characteristics of Pseudohypoaldosteronism Type 1 with and Without Genetic Mutations: A Literature Review. Journal of Clinical Medicine, 14(13), 4408. https://doi.org/10.3390/jcm14134408






