Atypical Lead Pathway Leading to Vocal Cord Paralysis and Tracheostomy Following Pacemaker Implantation
Abstract
1. Introduction
2. Imaging Studies
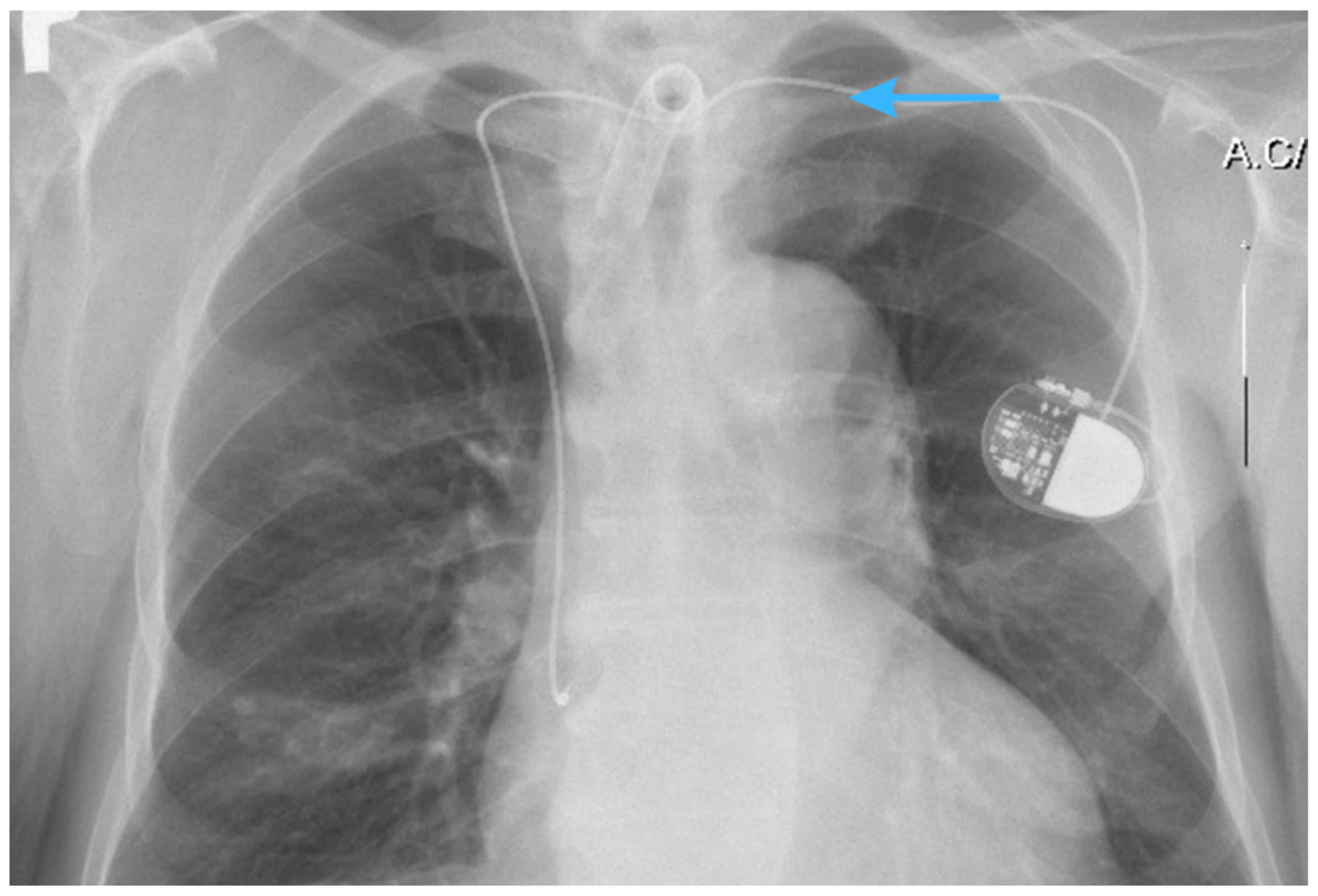
2.1. X-Ray Imaging
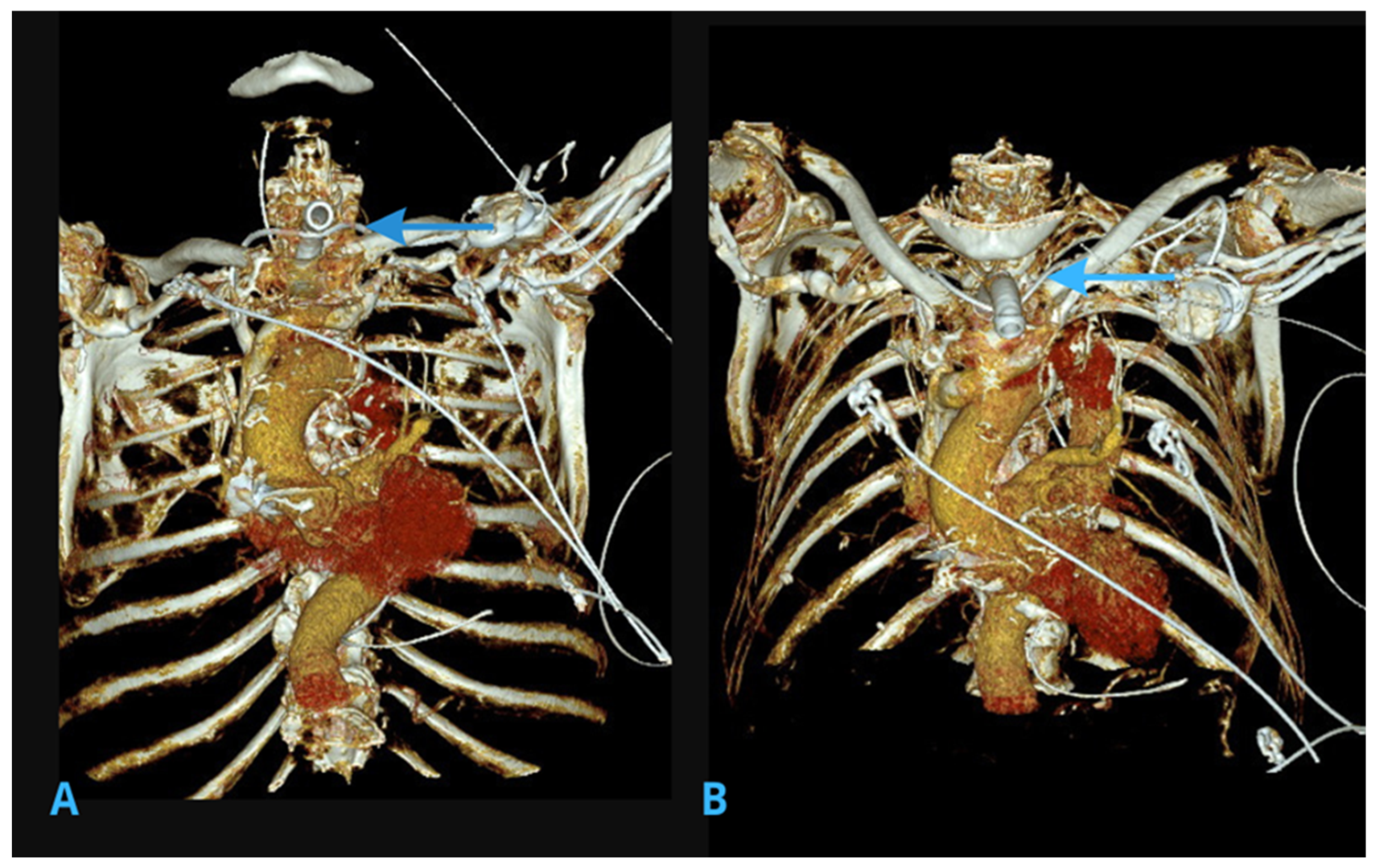
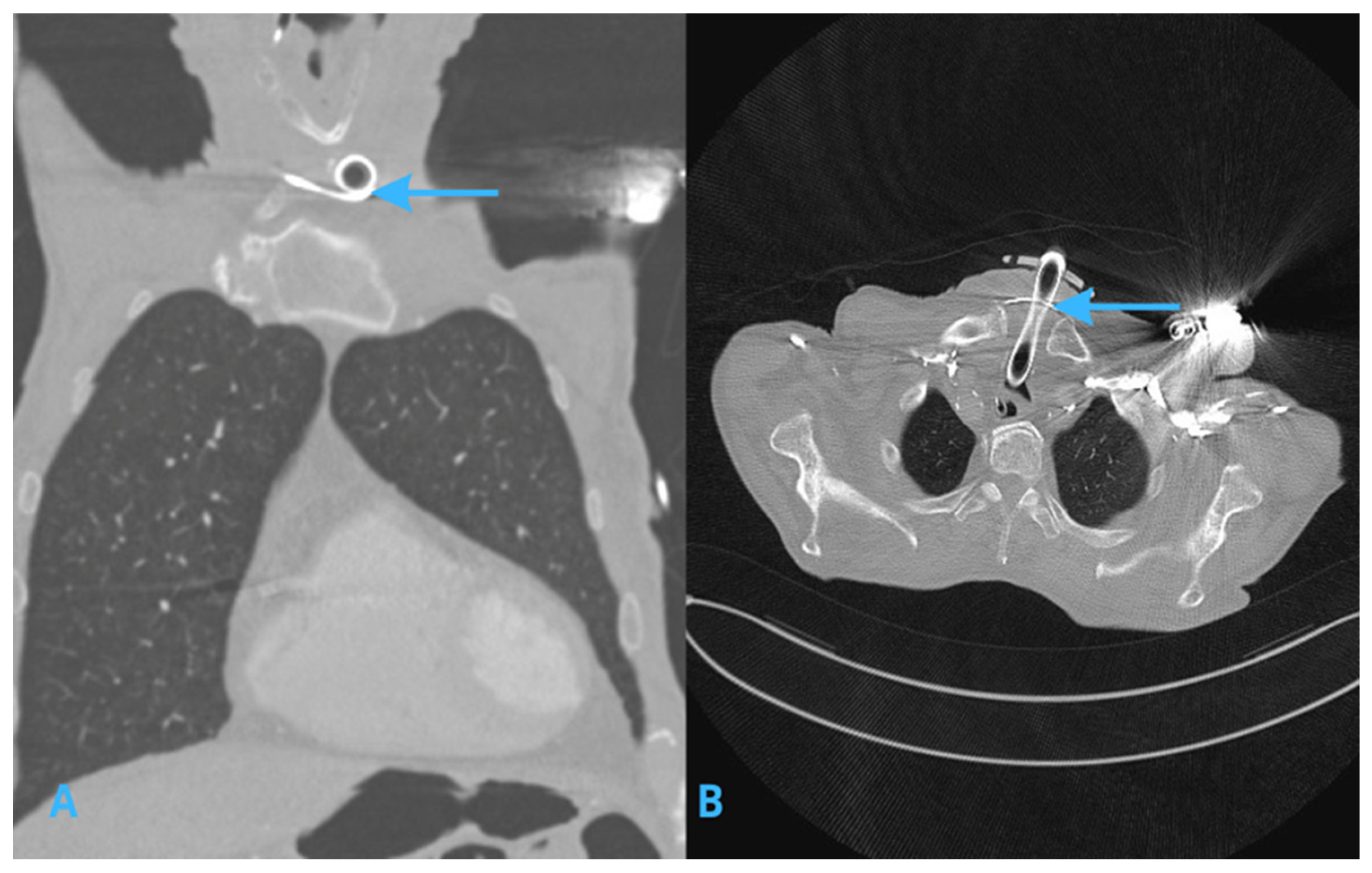
2.2. Computed Tomography (CT) Scans of the Chest
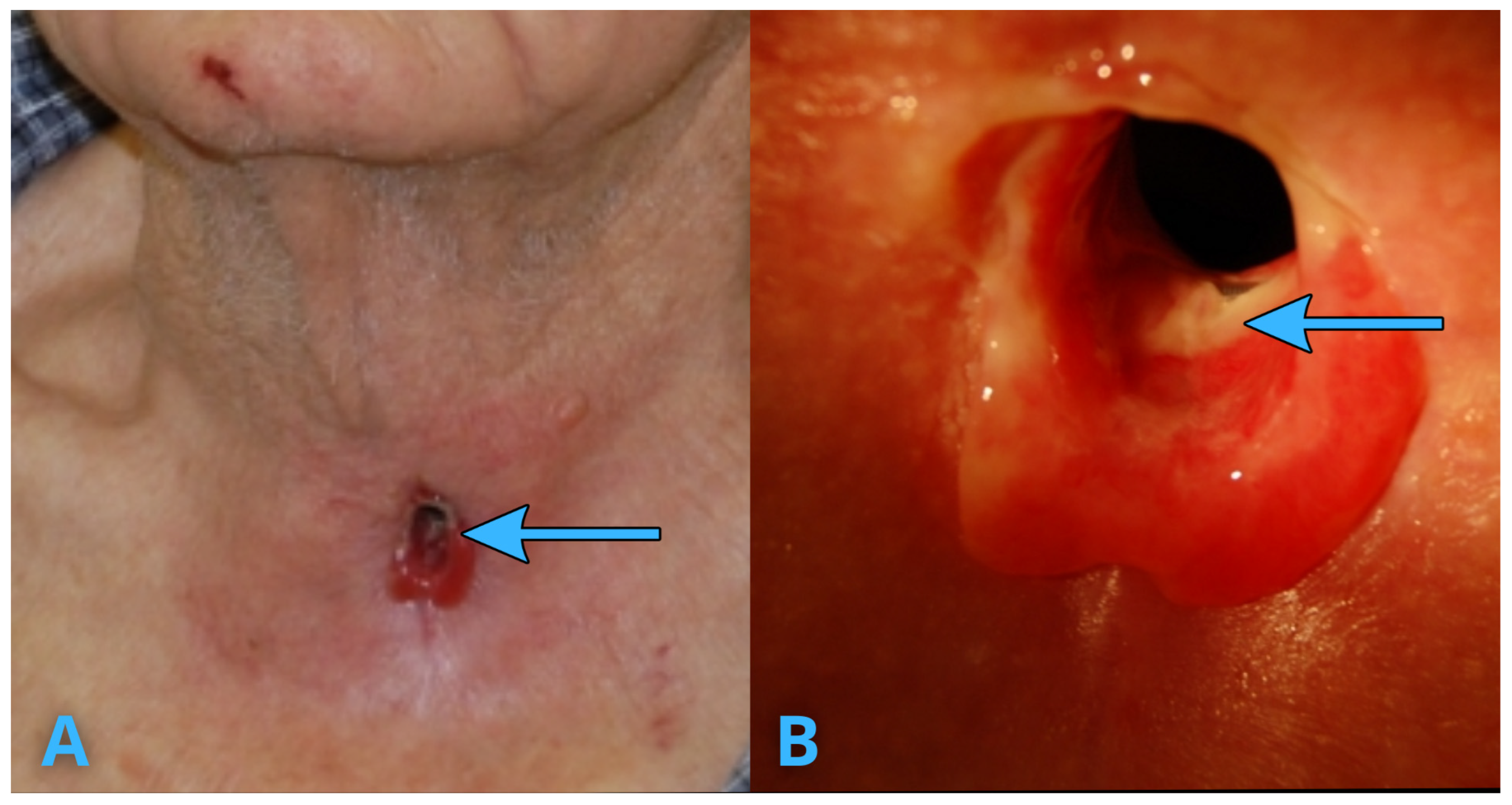
3. Infection Risk and Management
4. Discussion
4.1. Persistent Left Superior Vena Cava (PLSVC)
4.2. Collateral Venous Pathways and Tortuous Veins
4.3. Lead Migration and Complications
4.4. A Practical Approach to Diagnostic Imaging
4.5. AI-Assisted Imaging in Preventing Complications of CIED Implantation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Zheng, C.; Wang, P.; Wang, D.; Huang, B.; Li, G.; Hu, H.; Yang, Z.; Duan, X.; Zheng, S.; et al. Assessment of Cardiac Lead Perforation: Comparison Among Chest Radiography, Transthoracic Echocardiography and Electrocardiography-gated Contrast-enhanced Cardiac CT. Eur. Radiol. 2019, 29, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Balabanoff, C.; Gaffney, C.E.; Ghersin, E.; Okamoto, Y.; Carrillo, R.; Fishman, J.E. Radiographic and electrocardiography-gated non contrast cardiac CT assessment of lead perforation: Modality comparison and interobserver agreement. J. Cardiovasc. Comput. Tomogr. 2014, 8, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Kulbacki, M.; Segen, J.; Chaczko, Z.; Kulbacki, M.; Nikodem, J.; Klempous, R.; Bąk, A.; Rozenblit, J.; Zyśko, D.; Hrymniak, B.; et al. Medical Protocols and AI-Driven Algorithms for Enhanced Monitoring of Cardiac Implantable Electronic Devices. In Computer Aided Systems Theory—EUROCAST 2024; Quesada-Arencibia, A., Affenzeller, M., Moreno-Díaz, R., Eds.; Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2025; Volume 15173. [Google Scholar] [CrossRef]
- Pontillo, D.; Patruno, N. Persistent left superior vena cava and pacemaker implantation. World J. Cardiol. 2013, 5, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Maytin, M.; Epstein, L.M. The challenges of transvenous lead extraction. Heart 2011, 97, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Zohar, Y.; Buller, N.; Shvilly, Y. Recurrent laryngeal nerve paralysis during transvenous insertion of a permanent endocardial pacemaker. Ann. Otol. Rhinol. Laryngol. 1993, 102, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Culp, J.M.; Patel, G. Recurrent Laryngeal Nerve Injury. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560832/ (accessed on 20 March 2025).
- Khera, R.; Oikonomou, E.K.; Nadkarni, G.N.; Morley, J.R.; Wiens, J.; Butte, A.J.; Topol, E.J. Transforming Cardiovascular Care with Artificial Intelligence: From Discovery to Practice: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2024, 84, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Hartsock, I.; Rasool, G. Vision-language models for medical report generation and visual question answering: A review. Front. Artif. Intell. 2024, 7, 1430984. [Google Scholar] [CrossRef] [PubMed]
- Olawade, D.B.; Aderinto, N.; Olatunji, G.; Kokori, E.; David-Olawade, A.C.; Hadi, M. Advancements and applications of artificial intelligence in cardiology: Current trends and future prospects. J. Med. Surg. Public Health 2024, 3, 100109. [Google Scholar] [CrossRef]
- Jacob, S.; Shahzad, M.A.; Maheshwari, R.; Panaich, S.S.; Aravindhakshan, R. Cardiac rhythm device identification algorithm using X-rays: CaRDIA-X. Heart Rhythm 2011, 8, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, M.L.; Rozenblit, J.W. Learning Using Anti-Training with Sacrificial Data. J. Mach. Learn. Res. 2016, 17, 1–42. [Google Scholar]
- Copperman, Y.J.; Beiser, M.; Samuel, Y.; Laniado, S.; Shanon, E. Recurrent laryngeal nerve paralysis following pacemaker introduction. Pacing Clin. Electrophysiol. 1982, 5, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Gianni, C.; Elchouemi, M.; Helmy, R.; Spinetta, L.; La Fazia, V.M.; Pierucci, N.; Asfour, I.; Della Rocca, D.G.; Mohanty, S.; Bassiouny, M.A.; et al. Safety and feasibility of same-day discharge following uncomplicated transvenous lead extraction. J. Cardiovasc. Electrophysiol. 2024, 35, 278–287. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagielski, D.; Jacków-Nowicka, J.; Hrymniak, B.; Kulbacki, M.; Bladowska, J. Atypical Lead Pathway Leading to Vocal Cord Paralysis and Tracheostomy Following Pacemaker Implantation. J. Clin. Med. 2025, 14, 4395. https://doi.org/10.3390/jcm14134395
Jagielski D, Jacków-Nowicka J, Hrymniak B, Kulbacki M, Bladowska J. Atypical Lead Pathway Leading to Vocal Cord Paralysis and Tracheostomy Following Pacemaker Implantation. Journal of Clinical Medicine. 2025; 14(13):4395. https://doi.org/10.3390/jcm14134395
Chicago/Turabian StyleJagielski, Dariusz, Jagoda Jacków-Nowicka, Bruno Hrymniak, Marek Kulbacki, and Joanna Bladowska. 2025. "Atypical Lead Pathway Leading to Vocal Cord Paralysis and Tracheostomy Following Pacemaker Implantation" Journal of Clinical Medicine 14, no. 13: 4395. https://doi.org/10.3390/jcm14134395
APA StyleJagielski, D., Jacków-Nowicka, J., Hrymniak, B., Kulbacki, M., & Bladowska, J. (2025). Atypical Lead Pathway Leading to Vocal Cord Paralysis and Tracheostomy Following Pacemaker Implantation. Journal of Clinical Medicine, 14(13), 4395. https://doi.org/10.3390/jcm14134395




