Recurrence Pattern of Left Upper Lobectomies and Trisegmentectomies: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
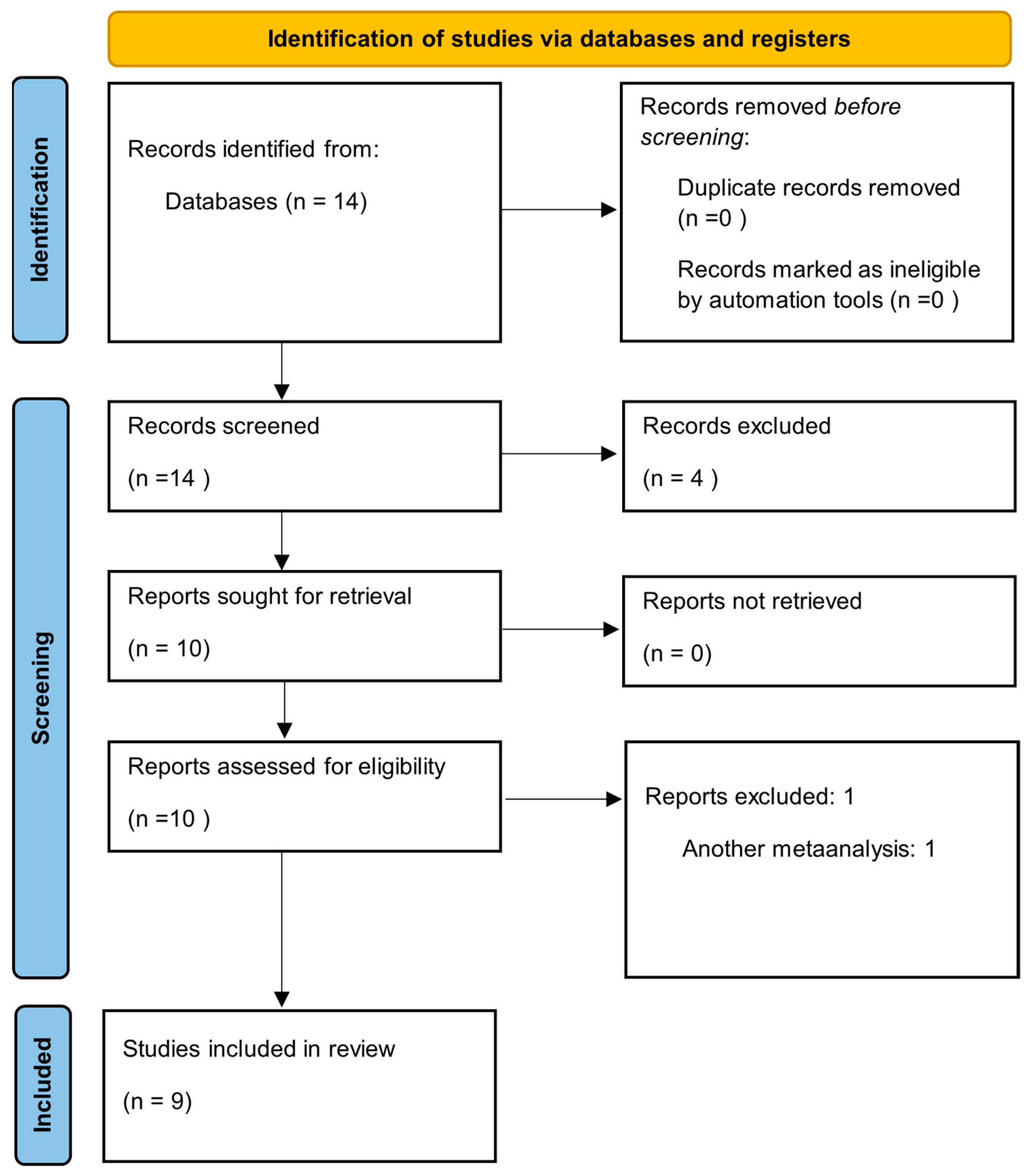
2.2. Identifying Relevant Studies
2.3. Study Selection
2.4. Quality Assessment
- Was the cohort recruited in an acceptable manner?
- Was the exposure accurately measured to minimize bias?
- Was the outcome accurately measured to minimize bias?
- Have the authors identified all important confounding factors?
- How precise are the results?
- Was the follow-up of subjects sufficiently long and complete?
2.5. Data Retrieval
- Descriptive data: country, year of publication, sample size, mean age, sex distribution, and histological subtypes.
- Methodological data: strategies to reduce bias (e.g., propensity score matching), and the number of patients lost to follow-up.
- Outcome data: morbidity, local recurrence, distant recurrence, and overall recurrence. Recurrence was classified as local or distant; when studies reported mixed recurrences, these cases were included in both categories.
2.6. Measuring the Outcomes
2.7. Statistical Analysis
3. Results
3.1. Methodological Quality
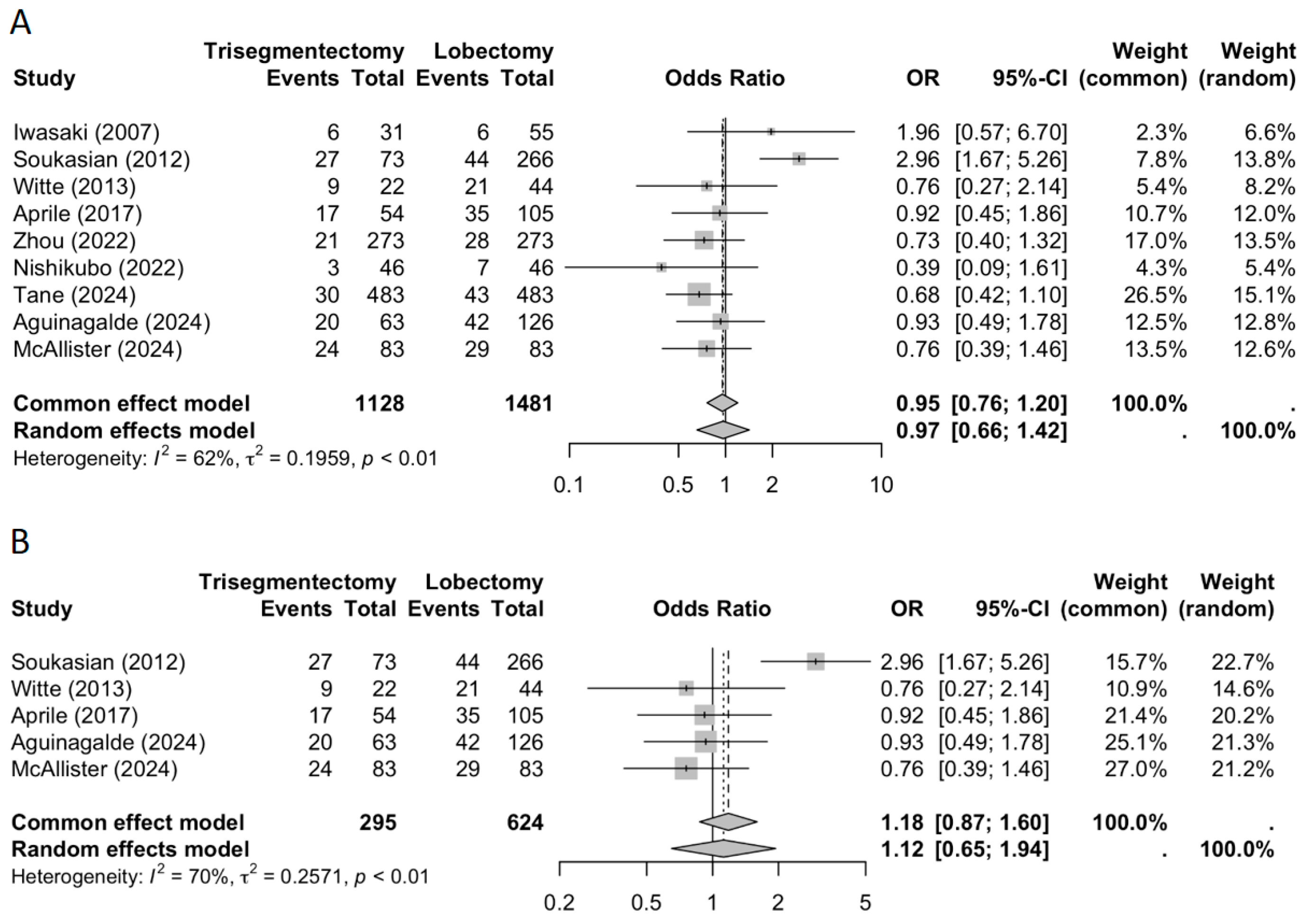
3.2. Measured Outcomes
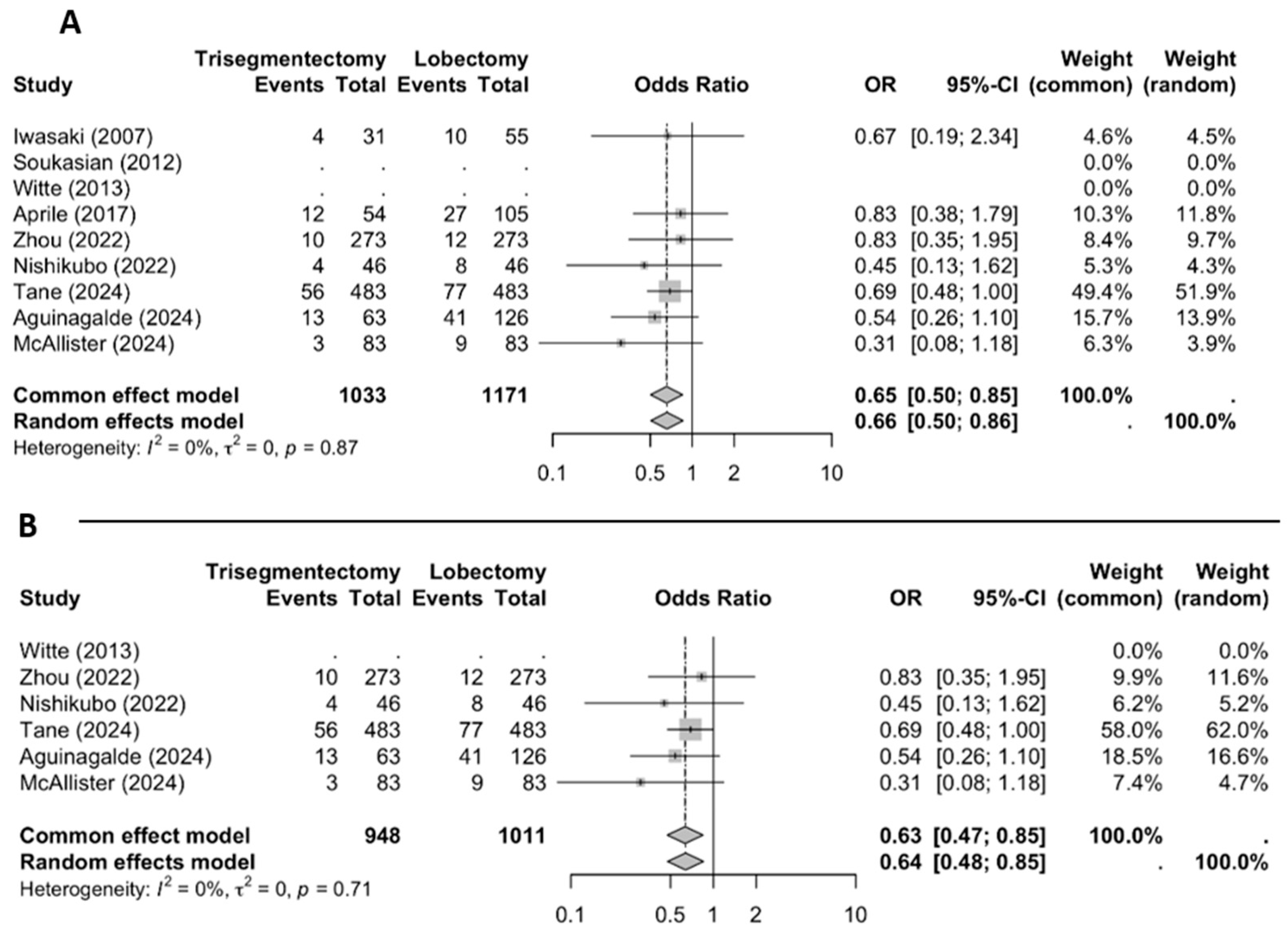
3.2.1. Locoregional Recurrence
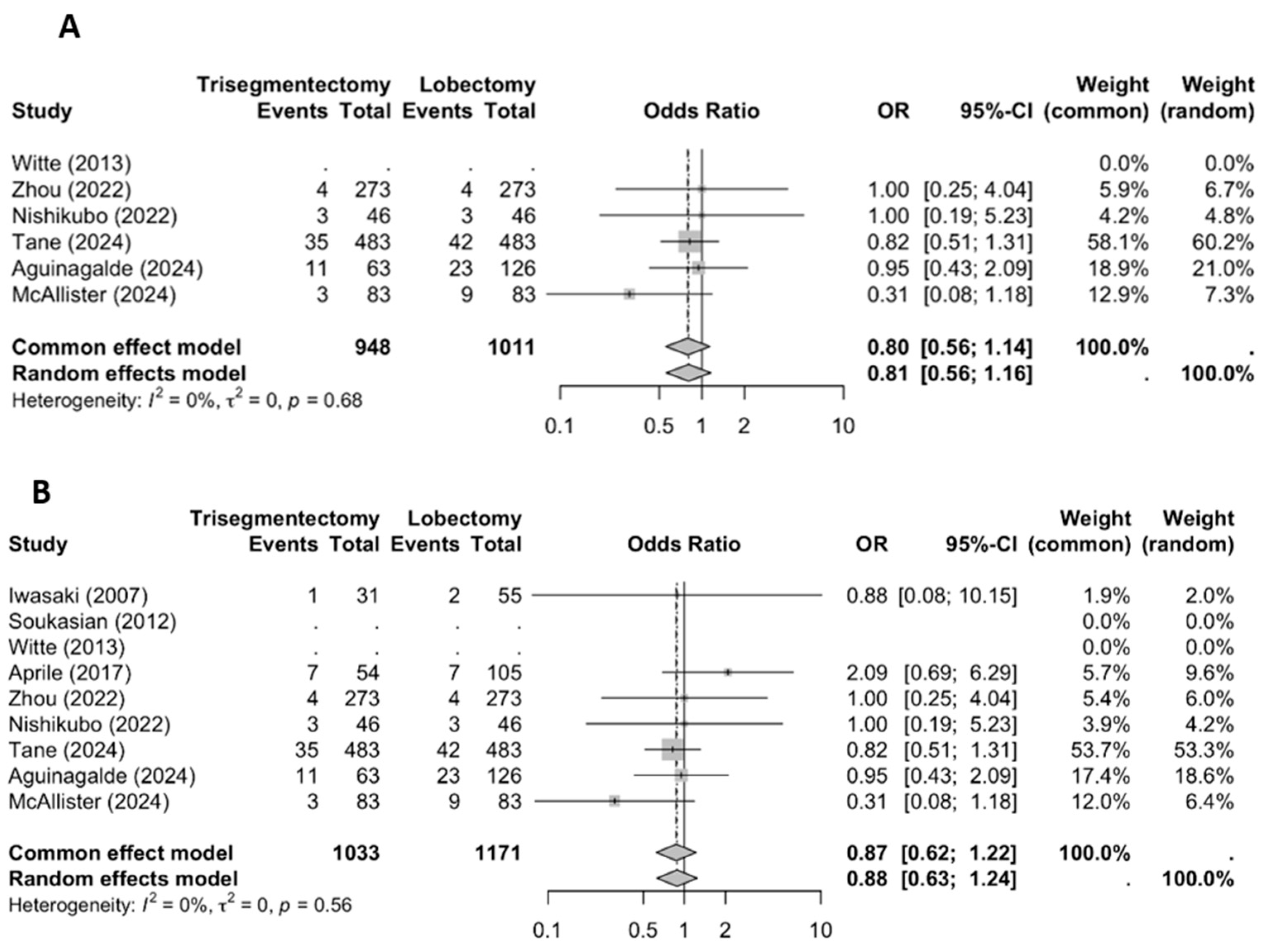
3.2.2. Distant Recurrence
3.2.3. Overall Recurrence
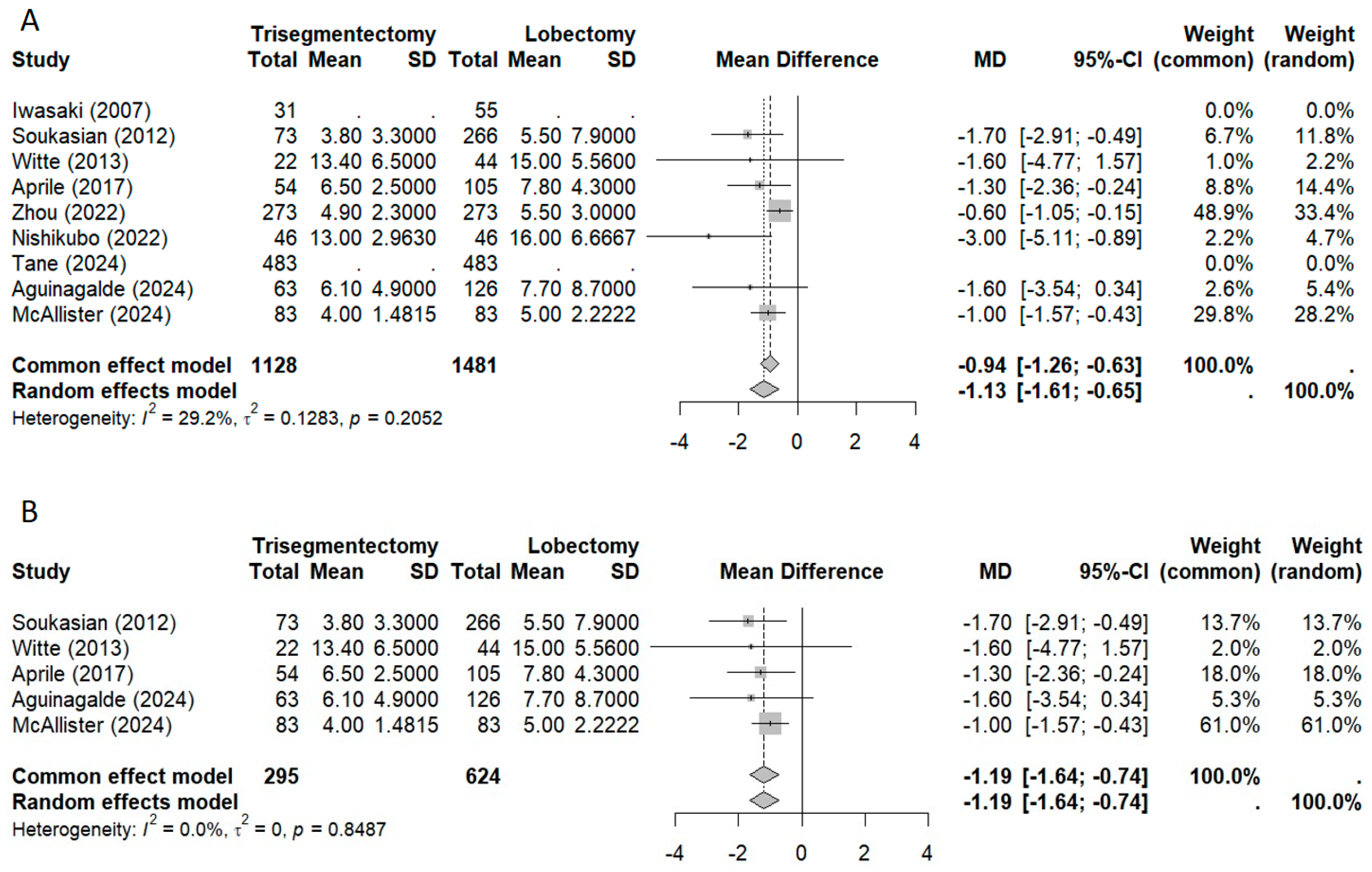
3.2.4. Morbidity and Length of Stay
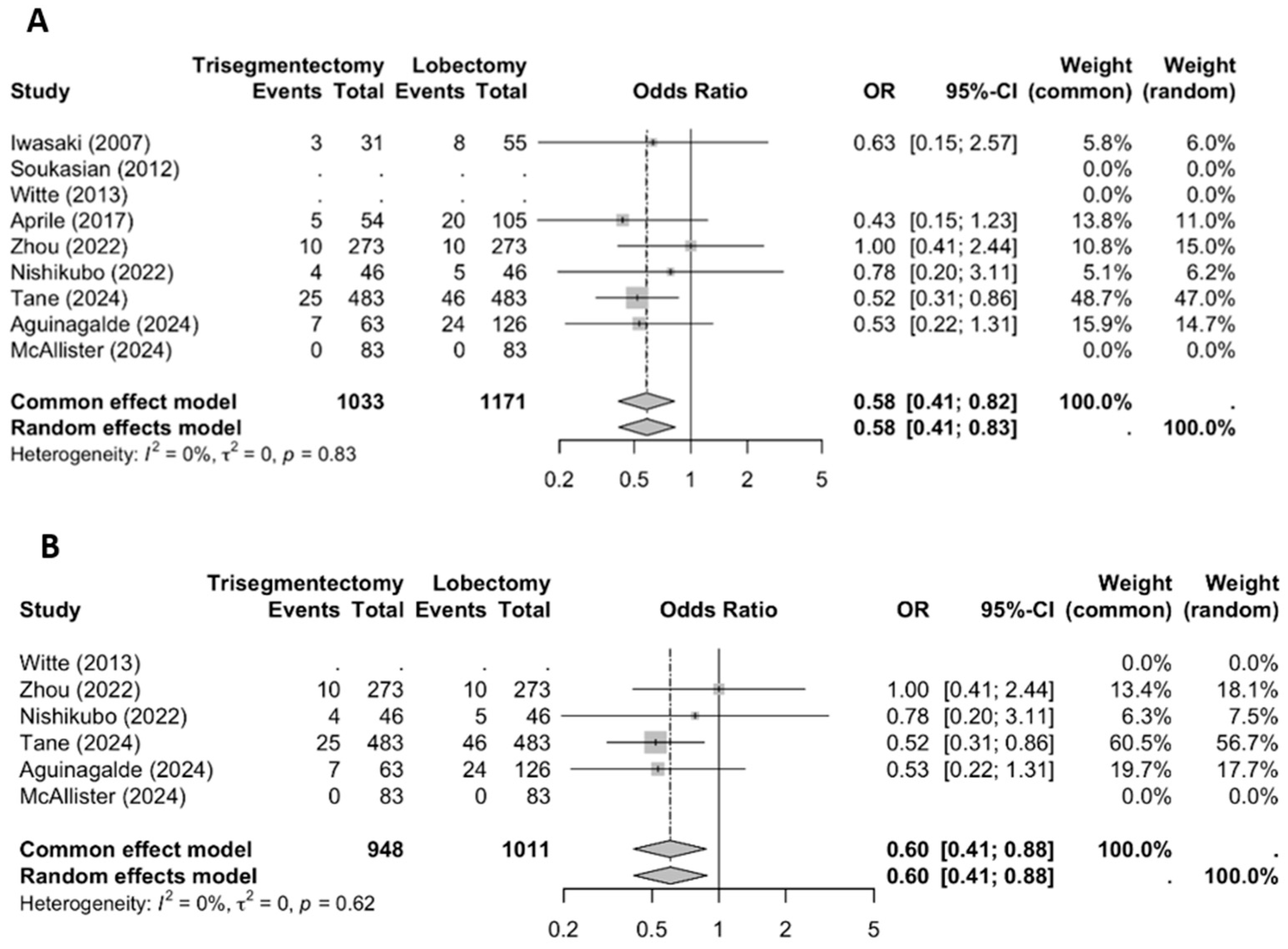
3.3. Sensitivity Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ginsberg, R.J.; Lawrence, V.R.; Lung Cancer Study Group. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann. Thorac. Surg. 1995, 60, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Hamanaka, W.; Hamada, T.; Hiratsuka, M.; Yamamoto, S.; Shiraishi, T.; Shirakusa, T. Comparison between a case-matched analysis of left upper lobe trisegmentectomy and left upper lobectomy for small size lung cancer located in the upper division. Thorac. Cardiovasc. Surg. 2007, 55, 454–457. [Google Scholar] [CrossRef]
- Soukiasian, H.J.; Hong, E.; McKenna, R.J., Jr. Video-assisted thoracoscopic trisegmentectomy and left upper lobectomy provide equivalent survivals for stage IA and IB lung cancer. J. Thorac. Cardiovasc. Surg. 2012, 144, S23–S26. [Google Scholar] [CrossRef] [PubMed]
- Witte, B.; Wolf, M.; Hillebrand, H.; Huertgen, M. Split-lobe resections versus lobectomy for lung carcinoma of the left upper lobe: A pair-matched case–control study of clinical and oncological outcomes. Eur. J. Cardiothorac. Surg. 2014, 45, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Aprile, V.; Bertoglio, P.; Dini, P.; Palmiero, G.; Mussi, A.; Ambrogi, M.C.; Lucchi, M. Is left upper lobectomy always worthwhile for early stage lung cancer? A comparison between left upper lobectomy, trisegmentectomy, and lingulectomy. J. Surg. Oncol. 2018, 117, 618–624. [Google Scholar] [CrossRef]
- Zhou, B.; Xu, X.; Dai, J.; Guo, Y.; Jin, K.; Zhu, Y.; Wang, H.; Jiang, G. Propensity-matched comparison of VATS left upper trisegmentectomy and lobectomy. Ann. Thorac. Surg. 2022, 114, 1007–1014. [Google Scholar] [CrossRef]
- Bayfield, N.G.; Bibo, L.; Wang, E.; Edelman, J. Left upper lobe multi-segmentectomy versus lobectomy for early-stage lung cancer: A meta-analysis. Heart Lung Circ. 2023, 32, 596–603. [Google Scholar] [CrossRef]
- Nishikubo, M.; Tane, S.; Kimura, K.; Shimizu, N.; Kitamura, Y.; Nishio, W. Comparison of oncological outcomes between trisegmentectomy and lobectomy for non-small cell lung cancer in the left upper division. J. Thorac. Dis. 2022, 14, 4614–4623. [Google Scholar] [CrossRef]
- Tane, S.; Okami, J.; Maniwa, Y.; Shintani, Y.; Ito, H.; Ohtsuka, T.; Toyooka, S.; Mori, T.; Watanabe, S.-I.; Chida, M.; et al. Clinical outcomes of left upper segmentectomy vs. lobectomy for early non-small-cell lung cancer: A nationwide database study in Japan. Surg. Today 2024, 54, 1162–1172. [Google Scholar] [CrossRef]
- Aguinagalde, B.; Ferrer-Bonsoms, J.A.; López, I.; Lizarbe, I.A.; Fernández-Monge, A.; Recuero, J.; Royo, I.; Embún, R. Comparison of 5-year survival and disease recurrence after trisegmentectomy or left upper lobectomy: A propensity score analysis of the national GEVATS database. Arch. Bronconeumol. 2024, 60, 705–713. [Google Scholar] [CrossRef]
- McAllister, M.A.; Herrera-Zamora, J.; Barcelos, R.R.; Leo, R.; Sugarbaker, E.A.; Singh, A.; Mazzola, E.; Figueroa, P.A.U.; Jaklitsch, M.T.; Swanson, S.J. Left Upper Lobectomy vs Trisegmentectomy for Lung Cancer: A Propensity Score–Matched Comparison. Ann. Thorac. Surg. 2025, 119, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Miyamoto, E.; Ohsumi, Y.; Gotoh, M.; Matsuoka, T.; Kobayashi, M.; Omasa, M.; Okumura, N. Comparison of survival between lobectomy and trisegmentectomy for clinical stage T1c-2aN0M0 non-small cell lung cancer in the left upper segment of the lung. Updates Surg. 2025, 77, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Blumenthaler, A.N.; Hofstetter, W.L.; Mehran, R.J.; Rajaram, R.; Rice, D.C.; Roth, J.A.; Sepesi, B.; Swisher, S.G.; Vaporciyan, A.A.; Walsh, G.L.; et al. Preoperative maximum standardized uptake value associated with recurrence risk in early lung cancer. Ann. Thorac. Surg. 2022, 113, 1835–1844. [Google Scholar] [CrossRef]
- Shigefuku, S.; Ito, H.; Miura, J.; Kikuchi, A.; Isaka, T.; Adachi, H.; Nakayama, H.; Ikeda, N. Prognostic significance of the maximum standardized uptake value on the prognosis of clinical stage IA lung adenocarcinoma based on the 8th edition TNM classification. Ann. Surg. Oncol. 2023, 30, 830–838. [Google Scholar] [CrossRef]
- Sainani, K.L. Propensity scores: Uses and limitations. PM R 2012, 4, 693–697. [Google Scholar] [CrossRef]
- Soukiasian, H.J.; McKenna, R.J., Jr. Minimally invasive VATS left upper lobe apical trisegmentectomy. Ann. Cardiothorac. Surg. 2014, 3, 194–196. [Google Scholar]
- Wei, S.; Guo, C.; He, J.; Tan, Q.; Mei, J.; Yang, Z.; Liu, C.; Pu, Q.; Ma, L.; Yuan, Y.; et al. Effect of Vein-First vs Artery-First Surgical Technique on Circulating Tumor Cells and Survival in Patients With Non-Small Cell Lung Cancer: A Randomized Clinical Trial and Registry-Based Propensity Score Matching Analysis. JAMA Surg. 2019, 154, e190972. [Google Scholar] [CrossRef]
- Duan, X.; Yang, Z.; Hao, X.; Zhou, S.; Liu, Z.; Zhang, K.; Cui, Y. Early ligation of the pulmonary vein can reduce the dissemination of shed tumor cells during thoracoscopic lobectomy. J. Thorac. Cardiovasc. Surg. 2022, 164, 1623–1635. [Google Scholar] [CrossRef]
- Kurusu, Y.; Yamashita, J.; Hayashi, N.; Mita, S.; Fujino, N.; Ogawa, M. The sequence of vessel ligation affects tumor release into the circulation. J. Thorac. Cardiovasc. Surg. 1998, 116, 107–113. [Google Scholar] [CrossRef]
- Huang, K.L.; Deng, H.Y.; Fan, M.; Zheng, Q.; Lin, S.; Zhu, D.; Zhou, Q. The sequence of pulmonary vessels ligation during lobectomy for non-small cell lung cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2021, 47, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Kamigaichi, A.; Mimae, T.; Amioka, J.; Aoki, G.; Yoshimura, K.; Kawamoto, N.; Tsubokawa, N.; Miyata, Y.; Okada, M. Segmentectomy preserves better immune-nutritional status than lobectomy in patients with early-stage lung cancer. Eur. J. Cardiothorac. Surg. 2023, 63, ezad019. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, K.; Notsuda, H.; Onodera, K.; Watanabe, T.; Watanabe, Y.; Suzuki, T.; Hirama, T.; Oishi, H.; Niikawa, H.; Okada, Y. Prognostic value of perioperative changes in the prognostic nutritional index in patients with surgically resected non-small cell lung cancer. Surg. Today 2024, 54, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef]

| Author | Year | Country | Design | Surgery | Stage | Adenoca | Female |
|---|---|---|---|---|---|---|---|
| Iwasaki | 2007 | Japan | RAoPD | LUL vs. TRI | Stage IA | %75.6 | %43 |
| Soukasian | 2012 | EEUU | RAoPD | LUL vs. TRI | Stage I | ----- | ------ |
| Witte | 2013 | Germany | Retrospective | LUL vs. TRI or lingula | pStage I-IIIA | %34.8 | %27.3 |
| Aprile | 2017 | Italy | RAoPD | LUL vs. TRI or lingula | Stage IA-IIA | %61.6 | %20.8 |
| Zhou | 2022 | China | RAoHR | LUL vs. TRI | cStage I | %96.3 | %61.3 |
| Nishikubo | 2022 | Japan | RAoHR | LUL vs. TRI | Stage I-IIB | %71.7 | %31.5 |
| Tane | 2024 | Japan | RAoPD | LUL vs. TRI | cStage I | %87.8 | %49.5 |
| Aguinagalde | 2024 | Spain | RAoPD | LUL vs. TRI | All | ------- | -------- |
| McAllister | 2024 | EEUU | RAoPD | LUL vs. TRI | Stage cIA-IIA | %78.2 | %65.5 |
| Author | Patients | PM | Variables for PM | Quality | Issues |
|---|---|---|---|---|---|
| Iwasaki | 86 | No | - | Low | No comparable groups |
| Soukasian | 339 | No | - | Low | No comparable groups |
| Witte | 66 | Yes | Histology, pN, tumor diameter, age | Medium | TRI and lingulectomy are mixed |
| Aprile | 159 | No | - | Low | No comparable groups |
| Zhou | 546 | Yes | Age, sex, CT appearance, pathologic type, tumor size, pathologic stage | High | |
| Nishikubo | 92 | Yes | Age, sex, smoking history, histology, SUVmax, cT, PFT, GGO. | High | |
| Tane | 966 | Yes | Age, sex, PS, CEA, PFT, tumor size | Medium | The number of patients for each type of left upper lobe segmentectomy is not specified |
| Aguinagalde | 189 | Yes | Tumor size, tumor density, surgical access, cN, pN, pTNM, histological subtype, PFT, stroke, diabetes | High | |
| McAllister | 166 | Yes | Age, sex, CCI, PFT, smoking history, cT | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguinagalde, B.; Ferrer-Bonsoms, J.A.; López, I.; Lizarbe, J.A.; Fernandez-Monge, A.; Mainer, M.; Embun, R.; Zabaleta, J. Recurrence Pattern of Left Upper Lobectomies and Trisegmentectomies: Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 4385. https://doi.org/10.3390/jcm14124385
Aguinagalde B, Ferrer-Bonsoms JA, López I, Lizarbe JA, Fernandez-Monge A, Mainer M, Embun R, Zabaleta J. Recurrence Pattern of Left Upper Lobectomies and Trisegmentectomies: Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(12):4385. https://doi.org/10.3390/jcm14124385
Chicago/Turabian StyleAguinagalde, Borja, Juan A. Ferrer-Bonsoms, Iker López, Jon Ander Lizarbe, Arantza Fernandez-Monge, Maria Mainer, Raul Embun, and Jon Zabaleta. 2025. "Recurrence Pattern of Left Upper Lobectomies and Trisegmentectomies: Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 12: 4385. https://doi.org/10.3390/jcm14124385
APA StyleAguinagalde, B., Ferrer-Bonsoms, J. A., López, I., Lizarbe, J. A., Fernandez-Monge, A., Mainer, M., Embun, R., & Zabaleta, J. (2025). Recurrence Pattern of Left Upper Lobectomies and Trisegmentectomies: Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(12), 4385. https://doi.org/10.3390/jcm14124385