A Multicentre, Double-Blind, Randomised, Non-Inferiority Trial of a Novel Single-Injection Intra-Articular HMDA-Cross-Linked Hyaluronate Gel for Knee Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Trial Oversight
2.3. Randomisation and Masking
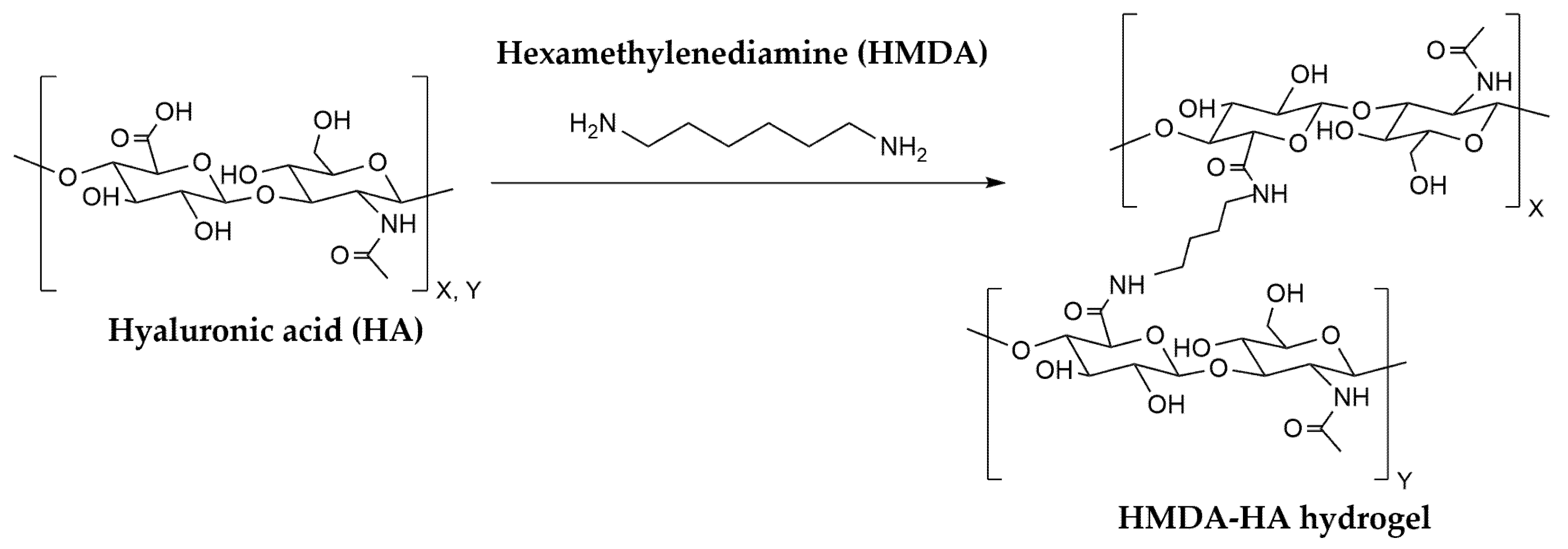
2.4. Study Intervention
2.5. Assessments and Outcomes
2.6. Statistical Analysis
3. Results
3.1. Patient Disposition
3.2. Efficacy Outcomes
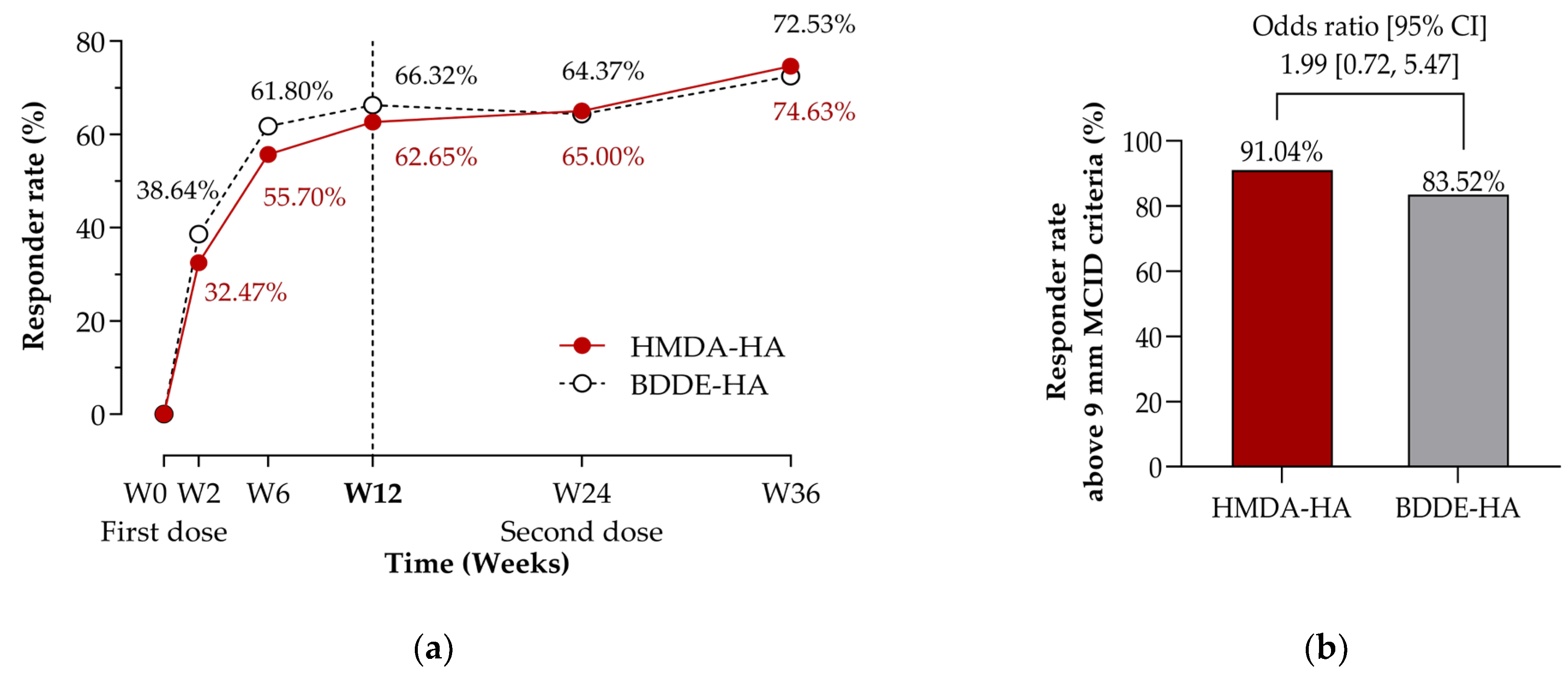
3.2.1. Primary Efficacy Endpoint
3.2.2. Secondary Efficacy Endpoints
3.3. Safety Outcomes
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADR | adverse drug reaction |
| AE | adverse event |
| ANCOVA | analysis of covariance |
| BDDE | 1,4-butanedioldiglycidylether |
| BDDE-HA | 1,4-butanedioldiglycidylether cross-linked hyaluronic acid hydrogel |
| BMI | body mass index |
| CI | confidence interval |
| COVID-19 | coronavirus disease 2019 |
| DVS | divinyl sulfone |
| ECG | electrocardiogram |
| ESCEO | European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases |
| EOS | end-of-study |
| FAS | full analysis set |
| GCP | Good Clinical Practice |
| GMP | Good Manufacturing Practice |
| HMDA | hexamethylenediamine |
| HMDA-HA | hexamethylenediamine cross-linked hyaluronic acid hydrogel |
| Hz | hertz |
| IAHA | intra-articular hyaluronic acid |
| ICH | International Council for Harmonisation of technical requirements for pharmaceuticals for human use |
| IGA | investigator global assessment |
| IRB | institutional review board |
| KL grade | Kellgren and Lawrence grade |
| LOCF | last observation carried forward |
| LS Mean | least squares mean |
| MedDRA | Medical Dictionary for Regulatory Activities |
| NOAEL | no-observed-adverse-effect level |
| NSAID | non-steroidal anti-inflammatory drug |
| OA | osteoarthritis |
| OMERACT-OARSI | Outcome Measures for Rheumatology Committee and Osteoarthritis Research Society International Standing Committee for Clinical Trials Response Criteria Initiative |
| Pa | pascal |
| PBS | phosphate buffered saline |
| PGA | patient global assessment |
| PPS | per-protocol set |
| PT | preferred term |
| RCT | randomised controlled trial |
| SADR | serious adverse drug reaction |
| SAE | serious adverse event |
| SAP | statistical analysis plan |
| SOC | system organ class |
| SD | standard deviation |
| SE | standard error |
| TCA | tricarboxylic acid |
| TEAE | treatment emergent adverse event |
| VAS | visual analogue scale |
| WBP | weight-bearing pain |
| WOMAC | Western Ontario and McMaster Universities Osteoarthritis |
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Bruyere, O.; Honvo, G.; Veronese, N.; Arden, N.K.; Branco, J.; Curtis, E.M.; Al-Daghri, N.M.; Herrero-Beaumont, G.; Martel-Pelletier, J.; Pelletier, J.P.; et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin. Arthritis Rheum. 2019, 49, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Sarabia, F.; Coronel, P.; Collantes, E.; Navarro, F.J.; de la Serna, A.R.; Naranjo, A.; Gimeno, M.; Herrero-Beaumont, G.; group, A.S. A 40-month multicentre, randomised placebo-controlled study to assess the efficacy and carry-over effect of repeated intra-articular injections of hyaluronic acid in knee osteoarthritis: The AMELIA project. Ann. Rheum. Dis. 2011, 70, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Natov, N.S.; Obadan, I.E.; Price, L.L.; Schmid, C.H.; McAlindon, T.E. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: A systematic review and meta-analysis. Arthritis Rheum. 2009, 61, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, N.; Campbell, J.; Robinson, V.; Gee, T.; Bourne, R.; Wells, G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst. Rev. 2006, 2006, CD005321. [Google Scholar] [CrossRef] [PubMed]
- Evaniew, N.; Simunovic, N.; Karlsson, J. Cochrane in CORR(R): Viscosupplementation for the treatment of osteoarthritis of the knee. Clin. Orthop. Relat. Res. 2014, 472, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Moreland, L.W. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: Mechanisms of action. Arthritis Res. Ther. 2003, 5, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Manjoo, A.; Fierlinger, A.; Niazi, F.; Nicholls, M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: A systematic review. BMC Musculoskelet. Disord. 2015, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.D.; Smith, G.N., Jr.; Simon, L.S. Intraarticular injection of hyaluronan as treatment for knee osteoarthritis: What is the evidence? Arthritis Rheum. 2000, 43, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, U.; Tolmachev, V.; Kairemo, K.; Astrom, G.; Jonsson, E.; Lundqvist, H. Elimination of stabilised hyaluronan from the knee joint in healthy men. Clin. Pharmacokinet. 2002, 41, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, S.; Wang, J.; Chen, Y.; Li, H.; Li, J.P.; Kan, Y.; Zhang, T. Methods for determining the structure and physicochemical properties of hyaluronic acid and its derivatives: A review. Int. J. Biol. Macromol. 2024, 282, 137603. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Kim, J.S.; Hahn, S.K. A Novel Degradation Controlled Hyaluronic Acid Derivatives. Key Eng. Mater. 2007, 342–343, 525–528. [Google Scholar] [CrossRef]
- Kennedy, G. Toxicity of Hexamethylenediamine (HMDA). Drug Chem. Toxicol. 2005, 28, 15–33. [Google Scholar] [CrossRef]
- Yeom, J.; Bhang, S.H.; Kim, B.S.; Seo, M.S.; Hwang, E.J.; Cho, I.H.; Park, J.K.; Hahn, S.K. Effect of cross-linking reagents for hyaluronic acid hydrogel dermal fillers on tissue augmentation and regeneration. Bioconjug Chem. 2010, 21, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Product Overview and Prescribing Information for Hyalflex® (Sodium Hyaluronate Gel Cross-Linked Hexamethylenediamine). Available online: https://nedrug.mfds.go.kr/pbp/CCBBB01/getItemDetailCache?cacheSeq=202401722 (accessed on 3 June 2025). (In Korean)
- Edsman, K.; Hjelm, R.; Lärkner, H.; Nord, L.I.; Karlsson, A.; Wiebensjö, Å.; Höglund, A.U.; Kenne, A.H.; Näsström, J. Intra-articular Duration of Durolane™ after Single Injection into the Rabbit Knee. Cartilage 2011, 2, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.E.; Dursema, H.D.; Pollak, C.T.; Skrabut, E.M. Clearance kinetics of a hylan-based viscosupplement after intra-articular and intravenous administration in animal models. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.R.; Kimpton, W.G.; Pierscionek, B.K.; Cahill, R.N. The kinetics of hyaluronan in normal and acutely inflamed synovial joints: Observations with experimental arthritis in sheep. Semin. Arthritis Rheum. 1993, 22, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Brorson, T.; Skarping, G.; Sandstrom, J.F.; Stenberg, M. Biological monitoring of isocyanates and related amines. I. Determination of 1,6-hexamethylene diamine (HDA) in hydrolysed human urine after oral administration of HDA. Int. Arch. Occup. Environ. Health 1990, 62, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Callery, P.S.; Egorin, M.J.; Geelhaar, L.A.; Nayar, M.S. Identification of metabolites of the cell-differentiating agent hexamethylene bisacetamide in humans. Cancer Res. 1986, 46, 4900–4903. [Google Scholar] [PubMed]
- Levy, J.H.; Koster, A.; Quinones, Q.J.; Milling, T.J.; Key, N.S. Antifibrinolytic Therapy and Perioperative Considerations. Anesthesiology 2018, 128, 657–670. [Google Scholar] [CrossRef] [PubMed]
- De Boulle, K.; Glogau, R.; Kono, T.; Nathan, M.; Tezel, A.; Roca-Martinez, J.X.; Paliwal, S.; Stroumpoulis, D. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatol. Surg. 2013, 39, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Sakhaii, P.; Bohorc, B.; Olpp, T.; Mohnicke, M.; Rieke-Zapp, J.; Dhal, P.K. Radio frequency gradient enhanced diffusion-edited semi-solid state NMR spectroscopy for detailed structural characterization of chemically modified hyaluronic acid hydrogels. Sci. Rep. 2024, 14, 28612. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.M.; Seo, M.S.; Hwang, E.J.; Cho, I.H.; Hahn, S.K.; Sohn, U.D. Improved synthesis of hyaluronic acid hydrogel and its effect on tissue augmentation. J. Biomater. Appl. 2012, 27, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Short, R.D.; Johannsen, F.R.; Schardein, J.L. A two-generation reproduction study in rats receiving diets containing hexamethylenediamine. Fundam. Appl. Toxicol. 1991, 16, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Balazs, E.A.; Watson, D.; Duff, I.F.; Roseman, S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum. 1967, 10, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, D.; McKinley, G.; Scott, R.D.; Spector, M. Rheology of joint fluid in total knee arthroplasty patients. J. Orthop. Res. 2002, 20, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Watterson, J.R.; Esdaile, J.M. Viscosupplementation: Therapeutic mechanisms and clinical potential in osteoarthritis of the knee. J. Am. Acad. Orthop. Surg. 2000, 8, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Praest, B.M.; Greiling, H.; Kock, R. Assay of synovial fluid parameters: Hyaluronan concentration as a potential marker for joint diseases. Clin. Chim. Acta 1997, 266, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, H.-H.; Lindenhayn, K.; Walther, H.-U. Das Synovia-Volumen gesunder und arthrotischer menschlicher Kniegelenke. Z. Orthopädie Ihre Grenzgeb. 1996, 134, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.W.; Park, Y.B.; Choi, C.H.; Kyung, H.S.; Lee, J.H.; Yoo, J.D.; Yoo, J.H.; Choi, C.H.; Kim, C.W.; Kim, H.C.; et al. Efficacy and safety of single injection of cross-linked sodium hyaluronate vs. three injections of high molecular weight sodium hyaluronate for osteoarthritis of the knee: A double-blind, randomized, multi-center, non-inferiority study. BMC Musculoskelet. Disord. 2017, 18, 223. [Google Scholar] [CrossRef] [PubMed]
- Bolognese, J.A.; Schnitzer, T.J.; Ehrich, E.W. Response relationship of VAS and Likert scales in osteoarthritis efficacy measurement. Osteoarthr. Cartil. 2003, 11, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, N.; Campbell, J.; Robinson, V.; Gee, T.; Bourne, R.; Wells, G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst. Rev. 2006, 2006, CD005328. [Google Scholar] [CrossRef] [PubMed]
- Newberry, S.J.; FitzGerald, J.; SooHoo, N.F.; Booth, M.; Marks, J.; Motala, A.; Apaydin, E.; Chen, C.; Raaen, L.; Shanman, R.; et al. Treatment of Osteoarthritis of the Knee: An Update Review; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2017. [Google Scholar] [CrossRef] [PubMed]
- Leighton, R.; Akermark, C.; Therrien, R.; Richardson, J.B.; Andersson, M.; Todman, M.G.; Arden, N.K. NASHA hyaluronic acid vs. methylprednisolone for knee osteoarthritis: A prospective, multi-centre, randomized, non-inferiority trial. Osteoarthr. Cartil. 2014, 22, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Maheu, E.; Zaim, M.; Appelboom, T.; Jeka, S.; Trc, T.; Berenbaum, F.; Maasalu, K.; Berenbaum, F. Comparative efficacy and safety of two different molecular weight (MW) hyaluronans F60027 and Hylan G-F20 in symptomatic osteoarthritis of the knee (KOA). Results of a non inferiority, prospective, randomized, controlled trial. Clin. Exp. Rheumatol. 2011, 29, 527–535. [Google Scholar] [PubMed]
- Park, Y.G.; Ha, C.W.; Yoo, J.H.; Lee, W.S.; Lee, H.J.; In, Y.; Bae, K.C.; Shon, O.J.; Kim, Y.M.; Seon, J.K.; et al. Intra-Articular Injection of a Novel DVS Cross-Linked Hyaluronic Acid Manufactured by Biological Fermentation (YYD302) in Patients With Knee Osteoarthritis: A Double-Blind, Randomized, Multicenter, Noninferiority Study. Clin. Ther. 2021, 43, 1843–1860. [Google Scholar] [CrossRef] [PubMed]
- Strand, V.; Baraf, H.S.B.; Lavin, P.T.; Lim, S.; Hosokawa, H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthr. Cartil. 2012, 20, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Blicharski, T.; Łukasik, P.; Plebanski, R.; Żęgota, Z.; Szuścik, M.; Moster, E.; Pavelka, K.; Jeon, S.; Park, S.L. Efficacy and Safety of Intra-Articular Cross-Linked Sodium Hyaluronate for the Treatment of Knee Osteoarthritis: A Prospective, Active-Controlled, Randomized, Parallel-Group, Double-Blind, Multicenter Study. J. Clin. Med. 2023, 12, 2982. [Google Scholar] [CrossRef]
- Vincent, P. Intra-Articular Hyaluronic Acid in the Symptomatic Treatment of Knee Osteoarthritis: A Meta-Analysis of Single-Injection Products. Curr. Ther. Res. Clin. Exp. 2019, 90, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Maheu, E.; Rannou, F.; Reginster, J.Y. Efficacy and safety of hyaluronic acid in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin. Arthritis Rheum. 2016, 45, S28–S33. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.V.; Juni, P.; Saadat, P.; Xing, D.; Yao, L.; Bobos, P.; Agarwal, A.; Hincapie, C.A.; da Costa, B.R. Viscosupplementation for knee osteoarthritis: Systematic review and meta-analysis. BMJ 2022, 378, e069722. [Google Scholar] [CrossRef] [PubMed]
- Previtali, D.; Merli, G.; Di Laura Frattura, G.; Candrian, C.; Zaffagnini, S.; Filardo, G. The Long-Lasting Effects of “Placebo Injections” in Knee Osteoarthritis: A Meta-Analysis. Cartilage 2021, 13, 185s–196s. [Google Scholar] [CrossRef] [PubMed]
- Brophy, R.H.; Fillingham, Y.A. AAOS Clinical Practice Guideline Summary: Management of Osteoarthritis of the Knee (Nonarthroplasty), Third Edition. J. Am. Acad. Orthop. Surg. 2022, 30, e721–e729. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, M.S.; McIntyre, L.; Huang, Y.; Chevalier, X. Intra-articular placebo effect in the treatment of knee osteoarthritis: A survey of the current clinical evidence. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720x211066689. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Devji, T.; Bhandari, M.; Fierlinger, A.; Niazi, F.; Christensen, R. Clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: A systematic review and meta-analysis of randomized trials. Semin. Arthritis Rheum. 2016, 46, 151–159. [Google Scholar] [CrossRef] [PubMed]

| Variables | HMDA-HA (N = 107) | BDDE-HA (N = 113) | p Value [1] |
|---|---|---|---|
| Mean age (SD), years | 63.00 (7.53) | 63.93 (7.57) | 0.3626 (t) |
| Female, n (%) | 81 (75.70) | 86 (76.11) | 0.9440 (c) |
| Mean body mass index (SD), kg/m2 | 25.18 (2.80) | 24.75 (2.93) | 0.2685 (t) |
| Kellgren & Lawrence grade, n (%) | |||
| Grade I | 10 (9.35) | 17 (15.04) | 0.4245 (c) |
| Grade II | 58 (54.21) | 59 (52.21) | |
| Grade III | 39 (36.45) | 37 (32.74) | |
| Mean weight-bearing pain (SD), mm † | 55.71 (11.18) | 55.77 (12.46) | 0.6416 (w) |
| Mean investigator global assessment (SD), mm † | 51.29 (15.80) | 52.20 (16.58) | 0.7091 (t) |
| Mean WOMAC total score (SD) ‡ | 44.67 (16.49) | 47.29 (15.83) | 0.3567 (w) |
| Outcome Measures | HMDA-HA (N = 83) | BDDE-HA (N = 95) | LS Mean Difference [95% CI] | p Value [1] |
|---|---|---|---|---|
| Primary outcome * | ||||
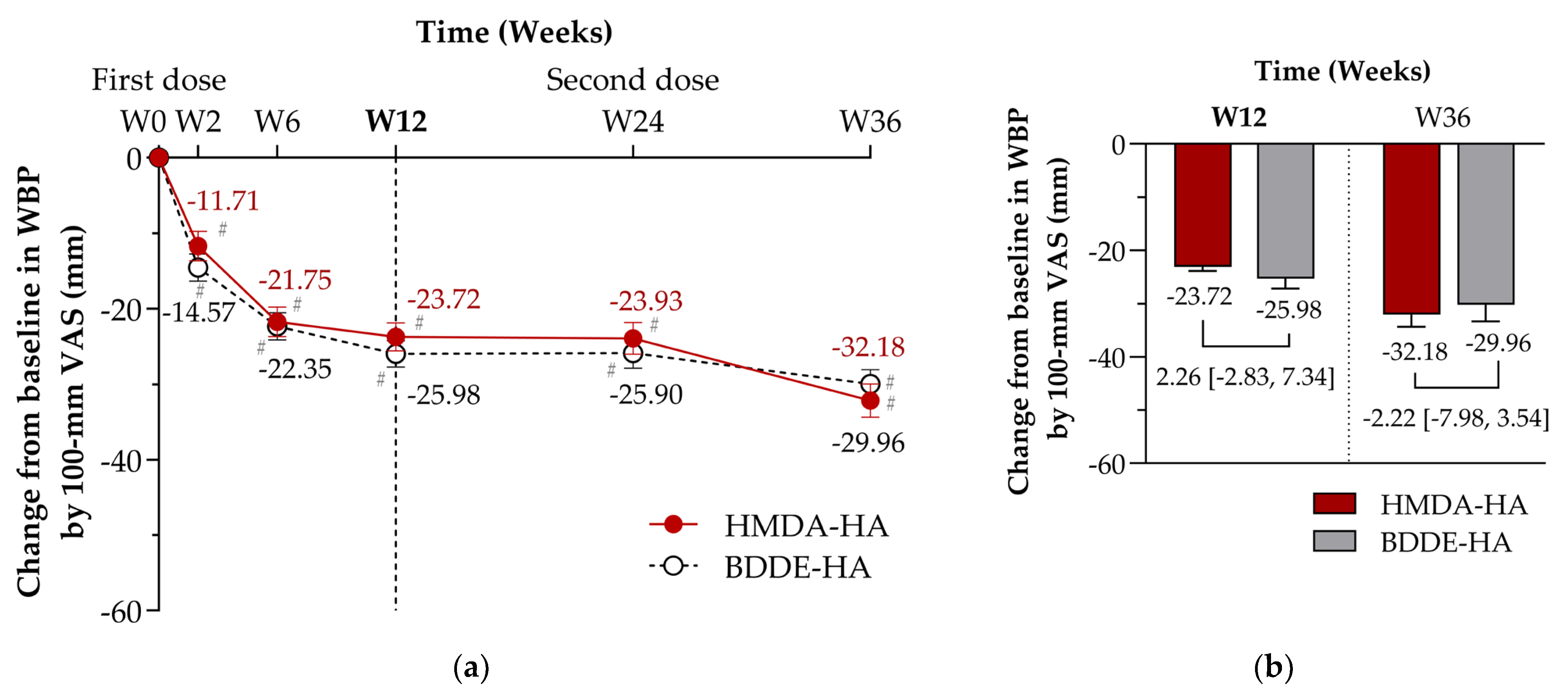
| Changes from baseline in WBP at Week 12—100 mm VAS, LS mean (SE) mm | −23.72 (1.88) | −25.98 (1.76) | 2.26 [−2.83, 7.34] | 0.3825 |
| Secondary outcomes ** | ||||
| Changes from baseline in WBP—100 mm VAS, LS mean (SE) mm | ||||
| At Week 2 | −11.71 (1.93) | −14.57 (1.80) | 2.86 [−2.35, 8.08] | 0.2795 |
| At Week 6 | −21.75 (1.94) | −22.35 (1.83) | 0.60 [−4.66, 5.86] | 0.8213 |
| At Week 24 | −23.93 (2.08) | −25.90 (2.00) | 1.97 [−3.73, 7.67] | 0.4957 |
| At Week 36 | −32.18 (2.21) | −29.96 (1.90) | −2.22 [−7.98, 3.54] | 0.4957 |
| Changes from baseline at Week 12—100 mm VAS, LS mean (SE) mm | ||||
| Rest pain | −17.06 (1.85) | −19.32 (1.73) | 2.26 [−2.73, 7.26] | 0.3717 |
| Night pain | −16.61 (1.99) | −18.43 (1.86) | 1.82 [−3.57, 7.21] | 0.5054 |
| Motion pain | −22.67 (2.10) | −24.87 (1.96) | 2.20 [−3.48, 7.88] | 0.4457 |
| Patient global assessment | −23.37 (2.00) | −24.98 (1.87) | 1.60 [−3.81, 7.01] | 0.5593 |
| Investigator global assessment | −22.54 (1.56) | −25.38 (1.46) | 2.84 [−1.39, 7.06] | 0.1874 |
| Changes from baseline in WOMAC index at Week 12, LS mean (SE) | ||||
| Total score | −15.82 (1.58) | −17.01 (1.48) | 1.19 [−3.10, 5.47] | 0.5856 |
| Pain subscore | −3.14 (0.34) | −3.58 (0.32) | 0.44 [−0.47, 1.35] | 0.3429 |
| Function subscore | −11.25 (1.18) | −11.90 (1.10) | 0.65 [−2.54, 3.83] | 0.6896 |
| Stiffness subscore | −1.47 (0.14) | −1.51 (0.13) | 0.04 [−0.35, 0.43] | 0.8467 |
| Changes from baseline in physical assessments at Week 12, LS mean (SE) | ||||
| Swelling † | −0.20 (0.04) | −0.32 (0.04) | 0.12 [0.01, 0.23] | 0.0367 |
| Joint-line tenderness on pressure ‡ | −0.67 (0.06) | −0.50 (0.06) | −0.17 [−0.34, 0.00] | 0.0470 |
| Range of motion [extension], degree | −0.24 (0.14) | −0.19 (0.13) | −0.06 [−0.44, 0.32] | 0.7672 |
| Range of motion [flexion], degree | −0.55 (0.55) | 0.23 (0.52) | −0.78 [−2.27, 0.71] | 0.3037 |
| Consumed dose of rescue medication at Week 12, mean (SD) g | 5.98 (9.48) | 6.07 (10.20) | −0.10 [−3.02, 2.83] | 0.4634 (w) |
| HMDA-HA (N = 107) | BDDE-HA (N = 114) | p Value [1] | |
|---|---|---|---|
| AEs †, n (%) [event] | 44 (41.12) [84] | 32 (28.07) [52] | 0.0412 (c) |
| Mild | 32 (29.91) [57] | 24 (21.05) [37] | |
| Moderate | 20 (18.69) [26] | 11 (9.65) [15] | |
| Severe | 1 (0.93) [1] | 0 | |
| SAEs, n (%) [event] | 4 (3.74) [4] | 3 (2.63) [3] | 0.7146 (f) |
| AEs leading to discontinuation of study intervention, n (%) [event] | 1 (0.93) [1] | 1 (0.88) [1] | 1.0000 (f) |
| AEs leading to death | 0 | 0 | NC |
| ADRs | 0 | 0 | NC |
| SADRs | 0 | 0 | NC |
| Solicitated local AEs at injection site, n (%) [event] | 97 (90.65) [337] | 95 (83.33) [307] | 0.1072 (c) |
| Pain | 96 (89.72) [161] | 95 (83.33) [163] | |
| Swelling | 18 (16.82) [25] | 17 (14.91) [20] | |
| Oedema | 38 (35.51) [57] | 34 (29.82) [42] | |
| Erythema | 25 (23.36) [32] | 21 (18.42) [23] | |
| Warmth | 45 (42.06) [62] | 44 (38.60) [59] |
| HMDA-HA (N = 107) | BDDE-HA (N = 114) | |
|---|---|---|
| AEs Occurring in ≥1% of Participants †, n (%) [event] | ||
| Infections and Infestations | 15 (14.02) [18] | 12 (10.53) [14] |
| COVID-19 | 10 (9.35) [10] | 6 (5.26) [6] |
| Urinary tract infection | 2 (1.87) [3] | 2 (1.75) [2] |
| Cystitis | 2 (1.87) [2] | 0 |
| Nasopharyngitis | 0 | 2 (1.75) [2] |
| Musculoskeletal and Connective Tissue Disorders | 16 (14.95) [20] | 5 (4.39) [7] |
| Arthralgia | 11 (10.28) [11] | 2 (1.75) [2] |
| Back pain | 3 (2.80) [3] | 1 (0.88) [1] |
| Pain in extremity | 2 (1.87) [2] | 0 (0.00) |
| Gastrointestinal Disorders | 6 (5.61) [8] | 4 (3.51) [5] |
| Investigations | 6 (5.61) [10] | 2 (1.75) [3] |
| ALT increased | 3 (2.80) [3] | 1 (0.88) [1] |
| AST increased | 2 (1.87) [2] | 1 (0.88) [1] |
| Blood glucose increased | 2 (1.87) [2] | 1 (0.88) [1] |
| Metabolism and Nutrition Disorders | 4 (3.74) [4] | 2 (1.75) [2] |
| Dyslipidaemia | 2 (1.87) [2] | 0 |
| Eye Disorders | 5 (4.67) [6] | 0 |
| Conjunctivitis allergic | 4 (3.74) [4] | 0 |
| Injury, Poisoning and Procedural Complications | 2 (1.87) [2] | 3 (2.63) [3] |
| Neoplasms, Benign, Malignant and Unspecified (including Cysts and Polyps) | 2 (1.87) [2] | 2 (1.75) [2] |
| Skin and Subcutaneous Tissue Disorders | 3 (2.80) [3] | 1 (0.88) [1] |
| Vascular Disorders | 2 (1.87) [2] | 2 (1.75) [2] |
| Ear and Labyrinth Disorders | 1 (0.93) [1] | 2 (1.75) [2] |
| Vertigo positional | 0 | 2 (1.75) [2] |
| Renal and Urinary Disorders | 1 (0.93) [1] | 2 (1.75) [2] |
| Respiratory, Thoracic and Mediastinal Disorders | 0 | 2 (1.75) [3] |
| Cardiac Disorders | 2 (1.87) [2] | 0 |
| Endocrine Disorders | 0 | 2 (1.75) [2] |
| Thyroid mass | 0 | 2 (1.75) [2] |
| Nervous System Disorders | 2 (1.87) [2] | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-I.; In, Y.; Choi, H.-S.; Lee, J.-H.; Sim, J.-A.; Lee, H.-J.; Moon, Y.-W.; Shon, O.-J.; Seon, J.-K.; Kim, Y.-M.; et al. A Multicentre, Double-Blind, Randomised, Non-Inferiority Trial of a Novel Single-Injection Intra-Articular HMDA-Cross-Linked Hyaluronate Gel for Knee Osteoarthritis. J. Clin. Med. 2025, 14, 4384. https://doi.org/10.3390/jcm14124384
Kim K-I, In Y, Choi H-S, Lee J-H, Sim J-A, Lee H-J, Moon Y-W, Shon O-J, Seon J-K, Kim Y-M, et al. A Multicentre, Double-Blind, Randomised, Non-Inferiority Trial of a Novel Single-Injection Intra-Articular HMDA-Cross-Linked Hyaluronate Gel for Knee Osteoarthritis. Journal of Clinical Medicine. 2025; 14(12):4384. https://doi.org/10.3390/jcm14124384
Chicago/Turabian StyleKim, Kang-Il, Yong In, Hyung-Suk Choi, Ju-Hong Lee, Jae-Ang Sim, Han-Jun Lee, Young-Wan Moon, Oog-Jin Shon, Jong-Keun Seon, Young-Mo Kim, and et al. 2025. "A Multicentre, Double-Blind, Randomised, Non-Inferiority Trial of a Novel Single-Injection Intra-Articular HMDA-Cross-Linked Hyaluronate Gel for Knee Osteoarthritis" Journal of Clinical Medicine 14, no. 12: 4384. https://doi.org/10.3390/jcm14124384
APA StyleKim, K.-I., In, Y., Choi, H.-S., Lee, J.-H., Sim, J.-A., Lee, H.-J., Moon, Y.-W., Shon, O.-J., Seon, J.-K., Kim, Y.-M., Song, S.-J., Chang, C.-B., & Han, H.-S. (2025). A Multicentre, Double-Blind, Randomised, Non-Inferiority Trial of a Novel Single-Injection Intra-Articular HMDA-Cross-Linked Hyaluronate Gel for Knee Osteoarthritis. Journal of Clinical Medicine, 14(12), 4384. https://doi.org/10.3390/jcm14124384







