Effectiveness of Endovascular Treatment in Native Hemodialysis Fistula Dysfunction: Long-Term Outcomes
Abstract
1. Introduction
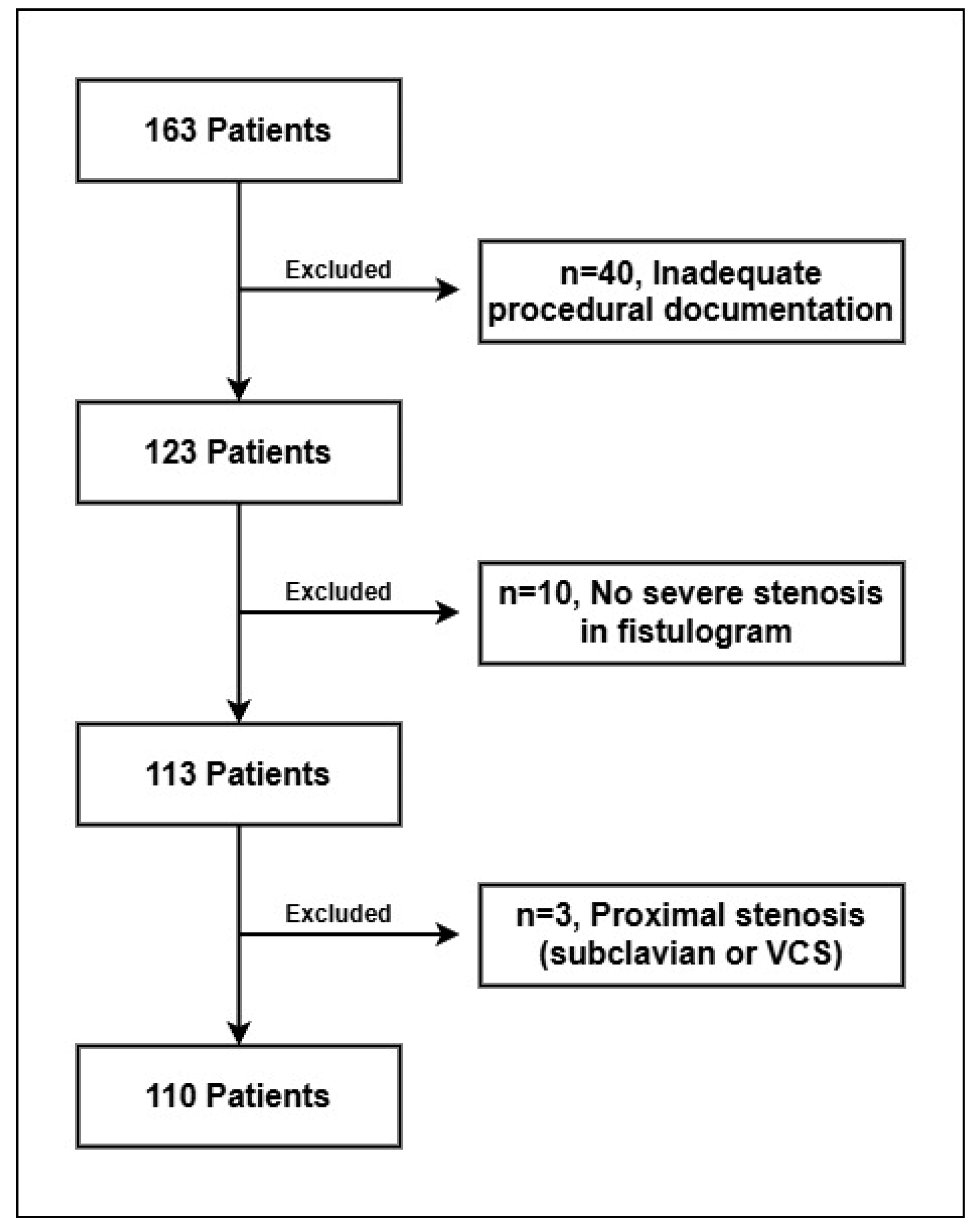
2. Materials and Methods
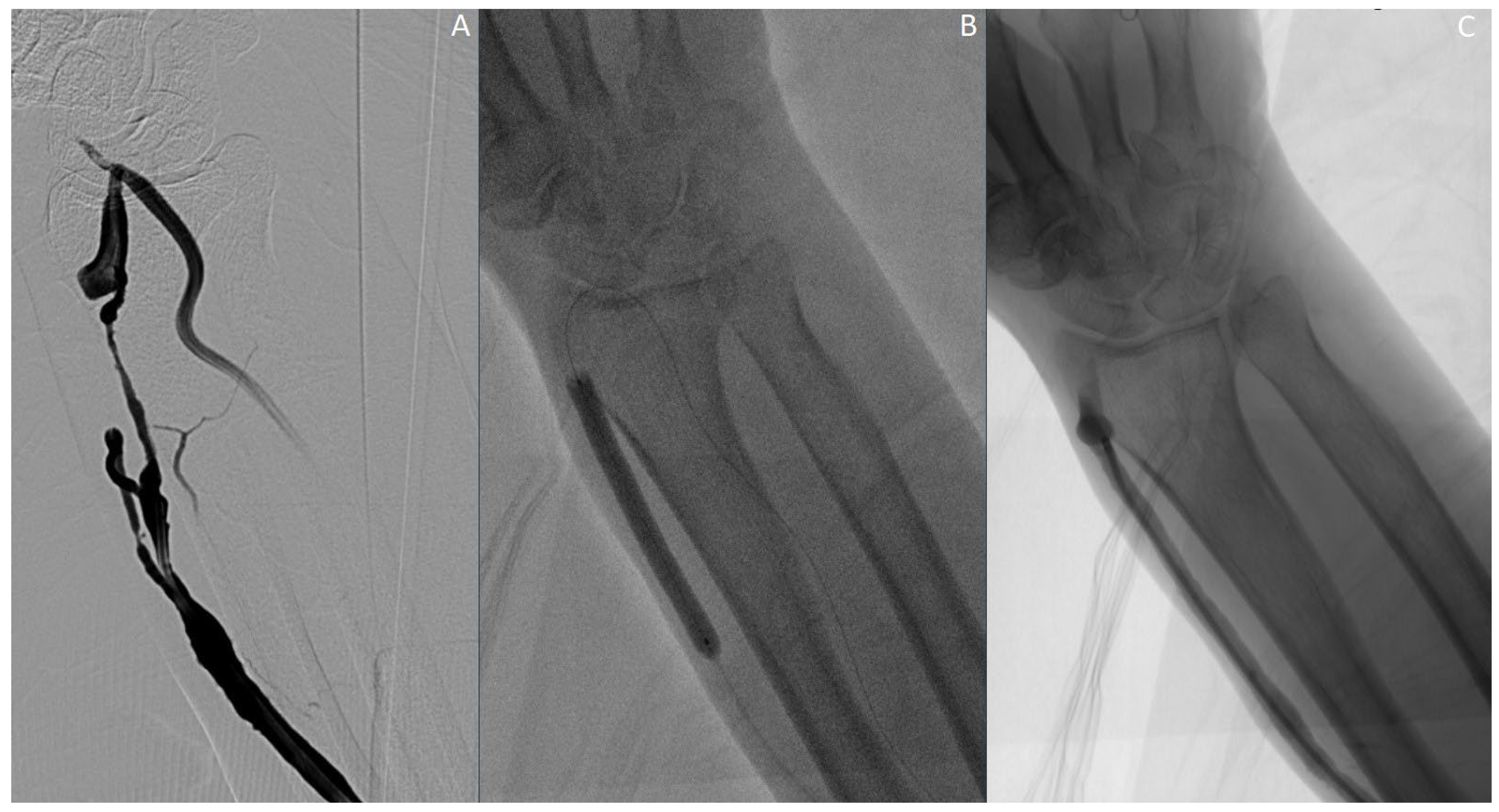
2.1. Procedure Details
2.2. Procedure Outcome and Follow-Up
2.3. Statistical Analysis
3. Results
3.1. Primary Patency Outcomes
3.2. 3-Month and 6-Month Follow-Up
3.3. Comparative Analysis Between Treatment Groups
3.4. Long-Term Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.E.; Huber, T.S.; Lee, T.; Shenoy, S.; Yevzlin, A.S.; Abreo, K.; Allon, M.; Asif, A.; Astor, B.C.; Glickman, M.H.; et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am. J. Kidney Dis. 2020, 75 (Suppl. S2), S1–S164. [Google Scholar] [CrossRef] [PubMed]
- Steinke, T.; Rieck, J.; Nuth, L. Endovascular arteriovenous fistula for hemodialysis access. Gefässchirurgie 2019, 24 (Suppl. S1), 25–31. [Google Scholar] [CrossRef]
- Quencer, K.B.; Arici, M. Arteriovenous fistulas and their characteristic sites of stenosis. Am. J. Roentgenol. 2015, 205, 726–734. [Google Scholar] [CrossRef]
- Sidawy, A.; Gray, R.; Henry, M.; Ascher, E.; Silva, M.; Miller, A.; Scher, L.; Trerotola, S.; Gregory, R.; Rutherford, R.; et al. Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J. Vasc. Surg. 2002, 35, 603–610. [Google Scholar] [CrossRef]
- Vazquez-Padron, R.I.; Duque, J.C.; Tabbara, M.; Salman, L.H.; Martinez, L. Intimal Hyperplasia and Arteriovenous Fistula Failure: Looking Beyond Size Differences. Kidney360 2021, 2, 1360–1372. [Google Scholar] [CrossRef]
- Heerwagen, S.T.; Hansen, M.A.; Schroeder, T.V.; Ladefoged, S.D.; Lönn, L. Endovascular treatment of hemodialysis arteriovenous fistulas: Is immediate post-interventional blood flow a predictor of patency. J. Vasc. Access. 2012, 13, 315–320. [Google Scholar] [CrossRef]
- Duque, J.C.; Tabbara, M.; Martinez, L.; Cardona, J.; Vazquez-Padron, R.I.; Salman, L.H. Dialysis Arteriovenous Fistula Failure and Angioplasty: Intimal Hyperplasia and Other Causes of Access Failure. Am. J. Kidney Dis. 2017, 69, 147–151. [Google Scholar] [CrossRef]
- Quencer, K.B.; Oklu, R. Hemodialysis access thrombosis. Cardiovasc. Diagn. Ther. 2017, 7 (Suppl. S3), S299–S308. [Google Scholar] [CrossRef]
- MacRae, J.M.; Dipchand, C.; Oliver, M.; Moist, L.; Lok, C.; Clark, E.; Hiremath, S.; Kappel, J.; Kiaii, M.; Luscombe, R.; et al. Arteriovenous Access Failure, Stenosis, and Thrombosis. Can. J. Kidney Health Dis. 2016, 3, 2054358116669126. [Google Scholar] [CrossRef]
- So, Y.H.; Choi, Y.H.; Oh, S.; Jung, I.M.; Chung, J.K.; Lucatelli, P. Thrombosed native hemodialysis fistulas: Technical and clinical outcomes of endovascular recanalization and factors influencing patency. J. Vasc. Access. 2019, 20, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Horoz, M.; Yoğurtçu, M.; Hüvez, A.; Balli, Ö.; Gür, S. Plain versus drug-eluting balloon angioplasty in the treatment of non-thrombotic hemodialysis arteriovenous fistula stenosis: Results from a single center comparative retrospective analysis. Acta Chir Belg. 2025. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, S.; Gu, J.; Lou, W.; He, X.; Chen, L.; Chen, G.; Zee, C.; Chen, B.T. Transcatheter Thrombolysis with Percutaneous Transluminal Angioplasty Using a Trans-Brachial Approach to Treat Thrombosed Arteriovenous Fistulas. Med. Sci. Monit. 2019, 25, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Santoro, D.; Benedetto, F.; Mondello, P.; Spinelli, F.; Ricciardi, C.A.; Cernaro, V.; Buemi, M.; Pipito, N.; Barilla, D. Vascular access for hemodialysis: Current perspectives. Int. J. Nephrol. Renov. Dis. 2014, 7, 281–294. [Google Scholar] [CrossRef]
- Yildiz, I. The efficacy of percutaneous transluminal angioplasty for the endovascular management of arteriovenous fistula dysfunction: A retrospective analysis in patients with end-stage renal disease. Int. Angiol. 2020, 39, 341–348. [Google Scholar] [CrossRef]
- Yang, C.C.; Yang, C.W.; Wen, S.C.; Wu, C.C. Comparisons of clinical outcomes for thrombectomy devices with different mechanisms in hemodialysis arteriovenous fistulas. Catheter. Cardiovasc. Interv. 2012, 80, 1035–1041. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, S.B.; Kim, Y.H.; Chung, H.H.; Lee, S.H.; Sung, D.J. Transjugular percutaneous endovascular treatment of dysfunctional hemodialysis access. J. Vasc. Access. 2019, 20, 488–494. [Google Scholar] [CrossRef]
- Zirek, S.; Özyurt, G.M.; Özen, A.; Olmaz, R.; Esen, K. Thrombus stiffness as an independent predictor of endovascular treatment success in hemodialysis fistulas: A study using ultrasound elastography. Ultrasonography 2025, 44, 153–159. [Google Scholar] [CrossRef]
- Granata, A.; Maccarrone, R.; Di Lullo, L.; Morale, W.; Battaglia, G.G.; Di Nicolò, P.; Bellasi, A.; Pesce, F.; Khater, E.; Gesualdo, L.; et al. Feasibility of routine ultrasound-guided percutaneous transluminal angioplasty in the treatment of native arteriovenous fistula dysfunction. J. Vasc. Access. 2021, 22, 739–743. [Google Scholar] [CrossRef]
- Thakker, V.; Sarda, P.; Ruhela, V.; Arora, M.; Sharma, R.; Azad, R.K. Role of Endovascular Treatment in Dysfunctional Hemodialysis Fistulae: A Single Center Experience. Indian. J. Nephrol. 2022, 32, 452–459. [Google Scholar]
- El Hendawy, K.A.; Lotfi, U.A.; Hussein, M.M.; Eldesouky, M.; Nagi, M.M. Endovascular treatment of failing arterio-venous fistula for hemodialysis. Syst. Rev. Pharm. 2021, 12, 259–264. [Google Scholar]
- Vignesh, S.; Mukuntharajan, T.; Sampathkumar, K. Outcomes of Endovascular Treatment for Salvaging Failed Hemodialysis Arteriovenous Fistula—Role of Balloon Angioplasty as Initial Therapy. Indian J. Nephrol. 2024, 34, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, B.; Mao, J.; Zhang, J.; Guo, H.; Wang, D.; Zhang, Y.; He, J.; Zhao, N.; Zhang, H.; et al. Hemodialysis Arteriovenous Fistula Dysfunction: Retrospective Comparison of Post-thrombotic Percutaneous Endovascular Interventions with Pre-emptive Angioplasty. Ann. Vasc. Surg. 2022, 84, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Lookstein, R.A.; Haruguchi, H.; Ouriel, K.; Weinberg, I.; Lei, L.; Cihlar, S.; Holden, A. Drug-Coated Balloons for Dysfunctional Dialysis Arteriovenous Fistulas. N. Engl. J. Med. 2020, 383, 733–742. [Google Scholar] [CrossRef]

| Variables | Cathegories | Patency (n = 100) | Non-Patency (n = 10) | p-Value |
|---|---|---|---|---|
| Age | 61.93 | 60 | 0.9875 | |
| Left-right arm | Left | 84 | 6 | 0.1481 |
| Rigt | 16 | 4 | ||
| Fistula Locations | Brachiobasilic | 4 | 0 | 0.5969 |
| Brachiocephalic | 36 | 5 | ||
| Radiocephalic | 60 | 5 | ||
| Stenosis Locations | Juxta-anastomotic | 54 | 5 | 1.0 |
| Vein | 46 | 5 | ||
| Thrombus | Yes | 58 | 9 | 0.1 |
| No | 42 | 1 |
| Primary Patency (n = 110) | p-Value | |||
|---|---|---|---|---|
| Yes (n = 100) | No (n = 10) | |||
| tPA | Yes | 53 47 | 5 5 | 1.0 |
| No | ||||
| Thrombectomy | Yes | 33 67 | 4 6 | 0.9237 |
| No | ||||
| Balloon angioplasty | Yes | 82 18 | 4 6 | 0.0077 |
| No | ||||
| Drug-coated balloon angioplasty | Yes | 20 80 | 0 10 | 0.2570 |
| No | ||||
| 3-Month Patency (n = 88) | p-Value | OR | 6-Month Patency (n = 88) | p-Value | OR | ||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 73) | No (n = 15) | Yes (n = 64) | No (n = 24) | ||||||
| tPA | Yes | 36 37 | 11 4 | 0.1539 | 0.35 | 32 32 | 15 9 | 0.3434 | 0.60 |
| No | |||||||||
| Thrombectomy | Yes | 24 49 | 8 7 | 0.1539 | 0.43 | 21 43 | 11 13 | 0.3216 | 0.58 |
| No | |||||||||
| Balloon angioplasty | Yes | 61 12 | 10 5 | 0.1556 | 2.54 | 55 9 | 16 8 | 0.0662 | 3.06 |
| No | |||||||||
| Drug-coated balloon angioplasty | Yes | 17 56 | 1 14 | 0.2889 | 4.25 | 15 49 | 3 21 | 0.3761 | 2.14 |
| No | |||||||||
| Treatment Cohorts | p-Value |
|---|---|
| Balloon angioplasty vs. non-balloon angioplasty | 0.124 |
| Balloon angioplasty vs. combined treatment | 0.512 |
| Non-balloon angioplasty vs. combined treatment | 0.335 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyazal, M.; Kaba, E. Effectiveness of Endovascular Treatment in Native Hemodialysis Fistula Dysfunction: Long-Term Outcomes. J. Clin. Med. 2025, 14, 4382. https://doi.org/10.3390/jcm14124382
Beyazal M, Kaba E. Effectiveness of Endovascular Treatment in Native Hemodialysis Fistula Dysfunction: Long-Term Outcomes. Journal of Clinical Medicine. 2025; 14(12):4382. https://doi.org/10.3390/jcm14124382
Chicago/Turabian StyleBeyazal, Mehmet, and Esat Kaba. 2025. "Effectiveness of Endovascular Treatment in Native Hemodialysis Fistula Dysfunction: Long-Term Outcomes" Journal of Clinical Medicine 14, no. 12: 4382. https://doi.org/10.3390/jcm14124382
APA StyleBeyazal, M., & Kaba, E. (2025). Effectiveness of Endovascular Treatment in Native Hemodialysis Fistula Dysfunction: Long-Term Outcomes. Journal of Clinical Medicine, 14(12), 4382. https://doi.org/10.3390/jcm14124382







