Axis I of DC/TMD in Diagnosis of Temporomandibular Disorders in People with Multiple Sclerosis—Preliminary Reports
Abstract
1. Introduction
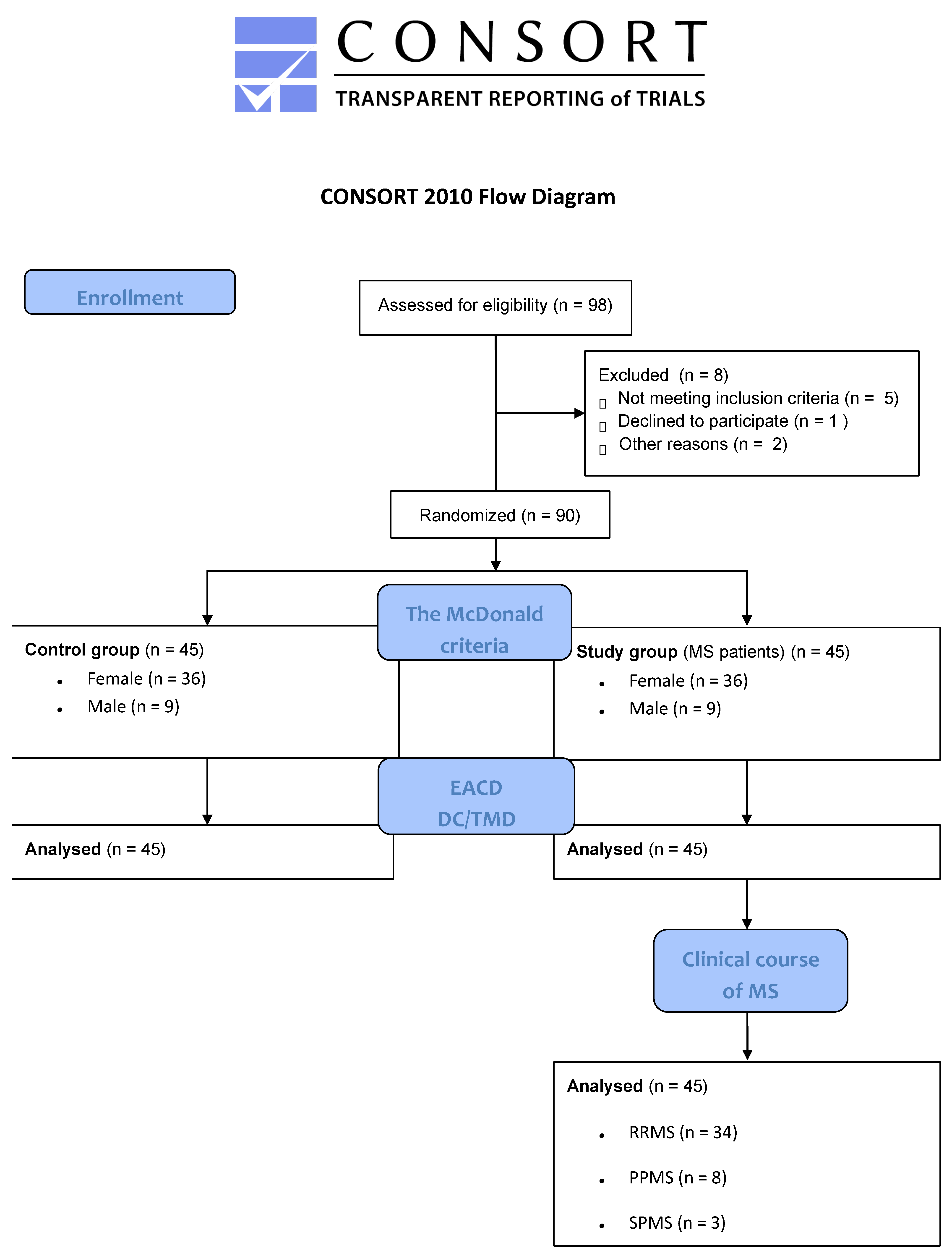
2. Materials and Methods
2.1. Study Methodology and Ethics Approval Statement
2.2. Inclusion and Exclusion Criteria
2.3. Study Population
2.4. Research Instruments
- Do you experience pain when opening your mouth wide or while chewing, occurring at least once a week or more frequently?
- Do you experience pain in the temple, facial region, temporomandibular joint, or jaw, occurring at least once a week or more frequently?
- Do you feel that your jaw is restricted or have difficulty opening it fully?
- Group I: Masticatory muscle disorders.
- Group II: Joint disorders related to disk derangements.
- Group III: Arthralgia, arthritis, and arthrosis.
2.5. Procedure
2.6. Data Processing and Statistical Evaluation
3. Research Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jakimovski, D.; Bittner, S.; Zivadinov, R.; Morrow, S.A.; Benedict, R.H.; Zipp, F.; Weinstock-Guttman, B. Multiple sclerosis. Lancet 2024, 403, 183–202. [Google Scholar] [CrossRef] [PubMed]
- McGinley, M.P.; Goldschmidt, C.H.; Rea-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Kamińska, J.; Koper, O.M.; Piechal, K.; Kemona, H. Multiple sclerosis—Etiology and diagnostic potential. Postep. Hig. Med. Dosw. 2017, 71, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Haki, M.; Al-Biati, H.E.; Al-Tameemi, Z.S.; Sami Ali, I.; Al-Hussaniy, H.A. Review of multiple sclerosis: Epidemiology, etiology, pathophysiology, and treatment. Medicine 2024, 103, e37297. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Liou, Y.-J.; Bai, Y.-M.; Tsai, S.-J.; Chen, T.-J.; Chen, M.-H.; Lo, W.-L. Bidirectional Associations of Temporomandibular Joint Disorders with Major Depressive and Anxiety Disorders. J. Evid.-Based Dent. Pract. 2023, 23, 101860. [Google Scholar] [CrossRef] [PubMed]
- Garstka, A.A.; Kozowska, L.; Kijak, K.; Brzózka, M.; Gronwald, H.; Skomro, P.; Lietz-Kijak, D. Accurate Diagnosis and Treatment of Painful Temporomandibular Disorders: A Literature Review Supplemented by Own Clinical Experience. Pain Res. Manag. 2023, 2023, 1002235. [Google Scholar] [CrossRef]
- Zieliński, G.; Pająk-Zielińska, B.; Ginszt, M. A Meta-Analysis of the Global Prevalence of Temporomandibular Disorders. J. Clin. Med. 2024, 13, 1365. [Google Scholar] [CrossRef]
- Maini, K.; Dua, A. Temporomandibular Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Velly, A.M.; Anderson, G.C.; Look, J.O.; Riley, J.L.; Rindal, D.B.; Johnson, K.J.; Wang, Q.; Fricton, J.; Huff, K.; Ohrbach, R.; et al. Management of painful temporomandibular disorders: Methods and overview of The National Dental Practice-Based Research Network prospective cohort study. J. Am. Dent. Assoc. 2022, 153, 144–157. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- De Boever, J.A.; Nilner, M.; Orthlieb, J.-D.; Steenks, M.H. Recommendations by the EACD for examination, diagnosis, and management of patients with temporomandibular disorders and orofacial pain by the general dental practitioner. J. Orofac. Pain. 2008, 22, 268–278. [Google Scholar]
- Odzimek, M.; Brola, W. Occurrence of Cervical Spine Pain and Its Intensity in Young People with Temporomandibular Disorders. J. Clin. Med. 2024, 13, 1941. [Google Scholar] [CrossRef]
- Osiewicz, M.; Ciapała, B.; Bolt, K.; Kołodziej, P.; Więckiewicz, M.; Ohrbach, R. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD): Polish assessment instruments. Dent. Med. Probl. 2024, 61, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Alquataibi, A.Y.; Alhammadi, M.S.; Hamadallah, H.H.; Altarjami, A.A.; Malosh, O.T.; Aloufi, A.M.; Alkahtani, L.M.; Alharbi, F.S.; Halboub, E.; Almashraqi, A.A. Global prevalence of temporomandibular disorders: A systematic review and meta-analysis. J. Oral. Facial Pain Headache 2025, 39, 48–65. [Google Scholar] [CrossRef]
- Zakrzewska, J.M. Temporomandibular disorders, headaches and chronic pain. J. Pain Palliat. Care Pharmacother. 2015, 29, 61–63. [Google Scholar] [CrossRef]
- Li, D.T.S.; Leung, Y.Y. Temporomandibular Disorders: Current Concepts and Controversies in Diagnosis and Management. Diagnostics 2021, 11, 459. [Google Scholar] [CrossRef]
- Greene, C.S.; Manfredini, D. Treating Temporomandibular Disorders in the 21st Century: Can We Finally Eliminate the “Third Pathway”? J. Oral Facial Pain Headache 2020, 34, 206–216. [Google Scholar] [CrossRef]
- Jussila, P.; Knuutila, J.; Salmela, S.; Näpänkangas, R.; Päkkilä, J.; Pirttinirmi, P.; Raustia, A. Association of risk factors with temporomandibular disorders in the Northern Finland Birth Cohort 1966. Acta Odontol. Scand. 2018, 76, 525–529. [Google Scholar] [CrossRef]
- Kapos, F.P.; Exposto, F.G.; Oyarzo, J.F.; Durham, J. Temporomandibular disorders: A review of current concepts in aetiology, diagnosis and management. Oral Surg. 2020, 13, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Loster, J.E.; Osiewicz, M.A.; Groch, M.; Ryniewicz, W.; Wieczorek, A. The Prevalence of TMD in Polish Young Adults. J. Prosthodont. 2017, 26, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Guarda-Nardini, L.; Winocur, E.; Piccotti, F.; Ahlberh, J.; Lobbezzo, F. Research diagnostic criteria for temporomandibular disorders: A systematic review of axis I epidemiologic findings. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Walczyńska-Dragon, K.; Baron, S.; Nitecka-Buchta, A.; Tkacz, E. Correlation between TMD and cervical spine pain and mobility: Is the whole body balance TMJ related? Biomed. Res. Int. 2014, 2014, 582414. [Google Scholar] [CrossRef]
- Torres, F.; Campos, L.G.; Fillipini, H.F.; Weigert, K.L.; Vecchia, G.F.D. Efeitos dos tratamentos fisioterapêutico e odontológico em pacientes com disfunção temporomandibular. Fisioter. Mov. 2012, 25, 117–125. [Google Scholar] [CrossRef]
- Danesh-Sani, S.A.; Rahimdoost, A.; Soltani, M.; Ghiyasi, M.; Haghdoost, N.; Sabzali-Zanjankhah, S. Clinical assessment of orofacial manifestations in 500 patients with multiple sclerosis. J. Oral Maxillofac. Surg. 2013, 71, 290–294. [Google Scholar] [CrossRef]
- Kovac, Z.; Uhac, I.; Buković, D.; Cabov, T.; Kovacević, D.; Grzić, R. Oral health status and temporomandibular disorders in multiple sclerosis patients. Coll. Antropol. 2005, 29, 441–444. [Google Scholar]
- Carvalho, L.S.C.; Nascimento, O.J.M.; Rodrigues, L.L.F.R.; Matta, A.P.D.C. Relationship between Expanded Disability Status Scale scores and the presence of temporomandibular disorders in patients with multiple sclerosis. Eur. J. Dent. 2018, 12, 144–148. [Google Scholar] [CrossRef]
- Minervini, G.; Mariani, P.; Fiorillo, L.; Cervino, G.; Cicciu, M.; Laino, L. Prevalence of temporomandibular disorders in people with multiple sclerosis: A systematic review and meta-analysis. Cranio 2025, 43, 312–320. [Google Scholar] [CrossRef]
- Symons, A.L.; Bortolanza, M.; Godden, S.; Seymour, G. A preliminary study into the dental health status of multiple sclerosis patients. Spec. Care Dentist. 1993, 13, 96–101. [Google Scholar] [CrossRef]
- Lasemi, E.; Ali Sahraian, M.; Motamedi, M.H.K.; Valayi, N.; Moradi, N.; Lasemi, R. Prevalence of oral and maxillofacial manifestations in patients with multiple sclerosis. J. Res. Dent. Sci. 2011, 8, 20–26. [Google Scholar]
- Carvalho, L.S.C.; Matta, A.P.C.; Nascimento, O.J.M.; Guimarães, A.S.; Rodrigues, L.R. Prevalence of temporomandibular disorders symptoms in patients with multiple sclerosis. Arq. Neuropsiquiatr. 2014, 72, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Clauw, D.J.; Braley, T.J.; Arewasikporn, A.; Kratz, A.L. Characterizing Temporomandibular Disorders in Multiple Sclerosis. J. Pain 2024, 25 (Suppl. S4), 43–44. [Google Scholar] [CrossRef]
- Kapel-Reguła, A.; Chojdak-Łukasiewicz, J.; Rybińska, A.; Duś-Ilnicka, I.; Radwan-Oczko, M. Oral State and Salivary Cortisol in Multiple Sclerosis Patients. Biomedicines 2024, 12, 2277. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, Z.; Xu, J.; Wang, Q.; Zhao, Z.; Jiang, Q. Causal effects of autoimmune diseases on temporomandibular disorders and the mediating pathways: A Mendelian randomization study. Front. Immunol. 2024, 15, 1390516. [Google Scholar] [CrossRef]
| Characteristics | Experimental Group (MS Patients) n = 45, (%) | Comparison Group (Without MS) n = 45, (%) | Sum, n = 90, (%) |
|---|---|---|---|
| Sex, n (%) | |||
| Males | 9 (20.0) | 9 (20.0) | 18 (20.0) |
| Females | 36 (80.0) | 36 (80.0) | 72 (80.0) |
| Age Range (years) | |||
| 20 to 30 years | 23 (51.1) | 23 (51.1) | 46 (51.1) |
| 31 to 40 years | 14 (31.1) | 14 (31.1) | 18 (20.0) |
| 41 to 50 years | 8 (17.8) | 8 (17.8) | 16 (17.8) |
| Residential Area, n (%) | |||
| Town | 23 (51.1) | 23 (51.1) | 46 (51.1) |
| Large City | 10 (22.2) | 10 (22.2) | 20 (22.2) |
| Countryside | 12 (26.7) | 12 (26.7) | 24 (26.7) |
| Features of the Study Group | Females, n = 36, (%) | Males, n = 9, (%) | Sum, n = 45, (%) | p-Value * |
|---|---|---|---|---|
| Subtypes of disease course, n (%) | ||||
| Relapsing-remitting | 28 (77.8) | 6 (66.7) | 34 (75.5) | 0.74 |
| Primary progressive | 6 (16.7) | 2 (22.2) | 8 (17.8) | |
| Secondary progressive | 2 (5.5) | 1 (11.1) | 3 (6.7) | |
| EDSS value (mean ± SD) | 2.1 (1.3) | 2.9 (1.6) | 2.5 (1.5) | - |
| Disease progression time (years, mean ± SD) | 3.5 (2.2) | 3.2 (2.6) | 3.3 (2.4) | - |
| Characteristics of TMDs Based on EACD | Experimental Group (MS Patients) n = 45, (%) | Comparison Group (Without MS) n = 45, (%) | Sum, n = 90 | p-Value * | ES Cohen’s d |
|---|---|---|---|---|---|
| Do you experience pain when opening your mouth wide or while chewing, occurring at least once a week or more frequently? | |||||
| Yes | 39 (86.7) | 12 (26.7) | 51 (56.7) | <0.001 | 0.52 |
| No | 6 (13.3) | 33 (73.3) | 39 (43.3) | ||
| Do you experience pain in the temple, facial region, temporomandibular joint, or jaw, occurring at least once a week or more frequently? | |||||
| Yes | 26 (57.8) | 10 (22.2) | 36 (40.0) | <0.001 | 0.77 |
| No | 19 (42.2) | 35 (77.8) | 54 (60.0) | ||
| Do you feel that your jaw is restricted or have difficulty opening it fully? | |||||
| Yes | 13 (28.9) | 4 (8.9) | 17 (18.9) | <0.05 | 0.53 |
| No | 32 (71.1) | 41 (91.1) | 73 (81.1) | ||
| Do you suffer from headaches occurring more than once a week? | |||||
| Yes | 33 (73.3) | 17 (37.8) | 50 (55.6) | <0.001 | 0.86 |
| No | 12 (26.7) | 28 (62.2) | 40 (44.4) |
| TMDs Based on DC/TMD | Experimental Group (MS Patients) n = 45, (%) | Comparison Group (Without MS) n = 45, (%) | Sum, n = 90, (%) | p-Value * |
|---|---|---|---|---|
| Yes | 18 (40.0) | 5 (11.1) | 23 (25.6) | <0.05 |
| No | 27 (60.0) | 40 (88.9) | 67 (74.4) |
| Characteristics of TMDs Based on DC/TMD | Experimental Group (MS Patients) n = 45, (%) | Comparison Group (Without MS) n = 45, (%) | Sum, n = 90, (%) | p-Value * | ES Cohen’s d |
|---|---|---|---|---|---|
| Group I | |||||
| Myalgia/Myofascial pain | 14 (31.1) | 5 (11.1) | 19 (21.1) | <0.05 | 0.61 |
| Myofascial pain with referral | 2 (4.4) | 0 (0.0) | 2 (2.2) | 0.55 | NS |
| Group II | |||||
| Disk displacement with reduction | 5 (11.1) | 1 (2.2) | 4 (4.4) | <0.05 | 0.53 |
| Disk displacement without reduction, with limited opening | 1 (2.2) | 0 (0.0) | 1 (1.1) | 0.67 | NS |
| Disk displacement without reduction, without limited opening | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | NS |
| Group III | |||||
| Arthralgia | 4 (8.9) | 0 (0.0) | 4 (4.4) | 0.16 | NS |
| Arthritis | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | NS |
| Arthrosis | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | NS |
| Type of MS | Group with TMDs, n = 18, % | Group Without TMDs, n = 27, % | Sum, n = 45, % | p-Value * |
|---|---|---|---|---|
| RRMS | n = 11 (61.1) | 23 (85.9) | 34 (75.6) | <0.05 |
| Group I—11 (100.0) | ||||
| Group II—4 (36.4) | ||||
| Group III—2 (18.9) | ||||
| PPMS | n = 4 (22.2) | 4 (14.1) | 8 (17.8) | NS |
| Group I—4 (100.0) | ||||
| Group II—1 (25.0) | ||||
| Group III—1 (25.0) | ||||
| SPMS | n = 3 (16.7) | 0 (0.0) | 3 (6.6) | NS |
| Group I—1 (33.3) | ||||
| Group II—1 (33.3) | ||||
| Group III—1 (33.3) |
| EDSS | Group with TMDs, n = 18, % | Group Without TMDs, n = 27, % | Total, n = 45, % | p-Value * |
|---|---|---|---|---|
| 1.0–4.5 | n = 13 (72.2) | 23 (85.9) | 36 (80.0) | 0.92 |
| Group I—13 (100.0) | ||||
| Group II—5 (38.5) | ||||
| Group III—3 (23.1) | ||||
| 5.0–10.0 | n = 5 (27.8) | 4 (14.1) | 9 (80.0) | 0.89 |
| Group I—3 (60.0) | ||||
| Group II—1 (20.0) | ||||
| Group III—1 (20.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odzimek, M.; Lipiński, H.; Dubiński, P.; Żak, M.; Brola, W. Axis I of DC/TMD in Diagnosis of Temporomandibular Disorders in People with Multiple Sclerosis—Preliminary Reports. J. Clin. Med. 2025, 14, 4338. https://doi.org/10.3390/jcm14124338
Odzimek M, Lipiński H, Dubiński P, Żak M, Brola W. Axis I of DC/TMD in Diagnosis of Temporomandibular Disorders in People with Multiple Sclerosis—Preliminary Reports. Journal of Clinical Medicine. 2025; 14(12):4338. https://doi.org/10.3390/jcm14124338
Chicago/Turabian StyleOdzimek, Martyna, Hubert Lipiński, Piotr Dubiński, Marek Żak, and Waldemar Brola. 2025. "Axis I of DC/TMD in Diagnosis of Temporomandibular Disorders in People with Multiple Sclerosis—Preliminary Reports" Journal of Clinical Medicine 14, no. 12: 4338. https://doi.org/10.3390/jcm14124338
APA StyleOdzimek, M., Lipiński, H., Dubiński, P., Żak, M., & Brola, W. (2025). Axis I of DC/TMD in Diagnosis of Temporomandibular Disorders in People with Multiple Sclerosis—Preliminary Reports. Journal of Clinical Medicine, 14(12), 4338. https://doi.org/10.3390/jcm14124338








