Advances in Clinical Outcomes of Endoscopic Lumbar Sympathectomy: Analysis of 494 Consecutive Patients at a Single Institution
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Patients Selection
2.3. Study Definitions and Outcome Assessment
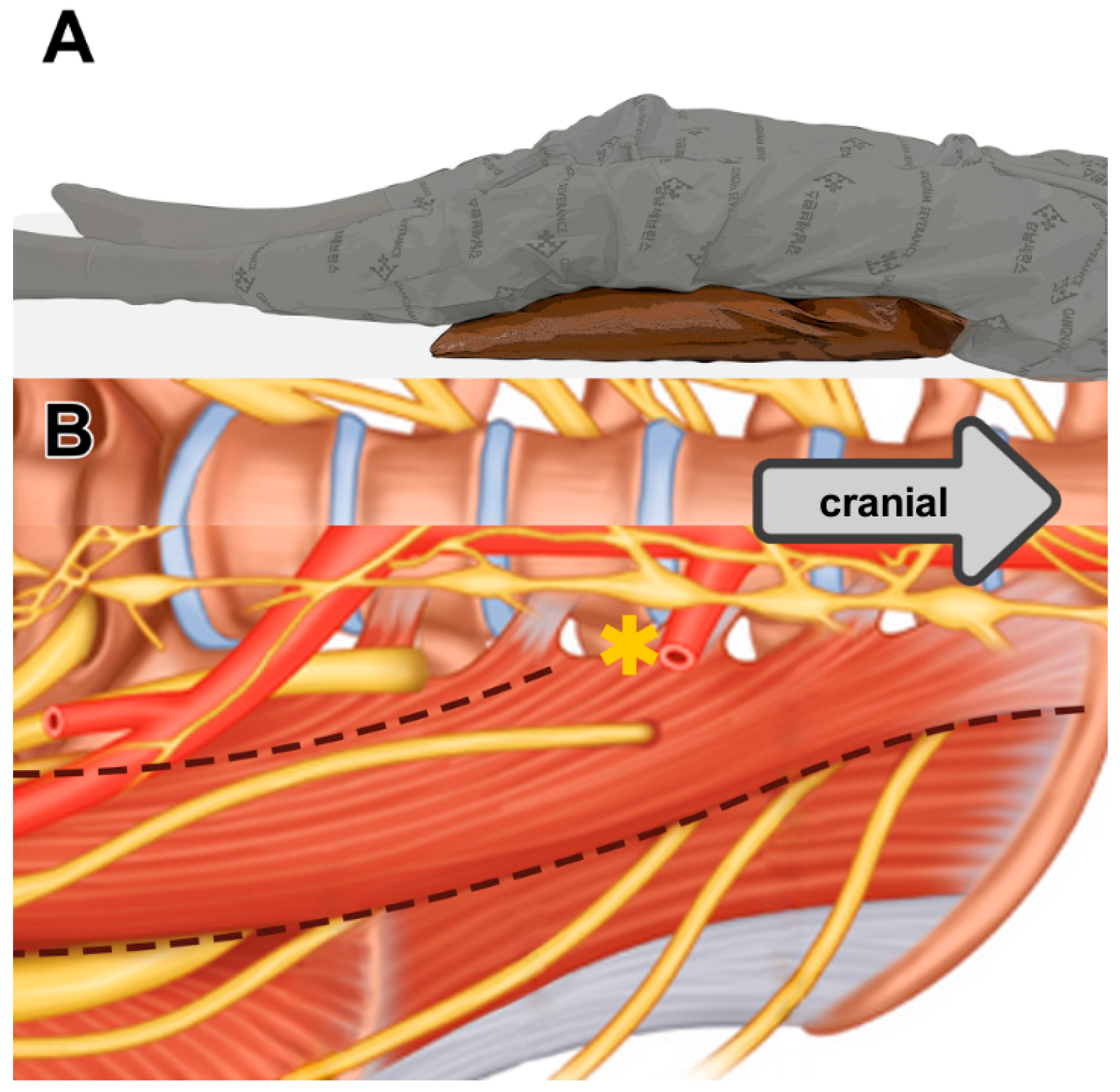
2.4. Surgical Technique
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics and Surgical Outcomes
3.2. Reappearance of Plantar Sweating After ELS and Risk Factor Analysis
3.3. Compensatory Hyperhidrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chick, J.F.B.; Srinivasa, R.N. Regarding Retroperitoneoscopic lumbar sympathectomy for plantar hyperhidrosis. J. Vasc. Surg. 2018, 68, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kaur, S.; Wilson, P. Early experience with endoscopic lumbar sympathectomy for plantar hyperhidrosis. Asian J. Endosc. Surg. 2016, 9, 128–134. [Google Scholar] [CrossRef] [PubMed]
- de Paula Loureiro, M.; de Campos, J.R.M.; Kauffman, P.; Jatene, F.B.; Weigmann, S.; Fontana, A. Endoscopic lumbar sympathectomy for women: Effect on compensatory sweat. Clinics 2008, 63, 189–196. [Google Scholar] [CrossRef]
- Rieger, R. Management of plantar hyperhidrosis with endoscopic lumbar sympathectomy. Thorac. Surg. Clin. 2016, 26, 465–469. [Google Scholar] [CrossRef]
- Rieger, R.; Pedevilla, S.; Lausecker, J. Quality of life after endoscopic lumbar sympathectomy for primary plantar hyperhidrosis. World J. Surg. 2015, 39, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Kathouda, N.; Wattanasirichaigoon, S.; Tang, E.; Yassini, P.; Ngaorungsri, U. Laparoscopic lumbar sympathectomy. Surg. Endosc. 1997, 11, 257–260. [Google Scholar] [CrossRef]
- Housset, M.; Dessertenne, G.; Marchand, E.; Daligault, M.; Maillard, H. A Multicentre Retrospective Study of Lumbar Sympathectomy for Plantar Hyperhidrosis: Satisfaction and Postoperative Complications. Clin. Exp. Dermatol. 2024, 49, 241–246. [Google Scholar] [CrossRef]
- Hur, K.J.; Moon, H.W.; Park, Y.H.; Bae, W.J.; Cho, H.J.; Ha, U.-S.; Lee, J.Y.; Kim, S.W.; Hong, S.-H. Retroperitoneoscopic Lumbar Sympathectomy for the Treatment of Primary Plantar Hyperhidrosis. BMC Surg. 2021, 21, 397. [Google Scholar] [CrossRef]
- Lima, S.O.; Santos, R.S.; Moura, A.M.M.; de O. Neto, E.G.; de Andrade, R.L.B.; Valido, A.D.; Dos Santos, V.F.; Mendonça, A.K.R.H. A Systematic Review and Meta-Analysis to Evaluate the Efficacy of Lumbar Sympathectomy for Plantar Hyperhidrosis. Int. J. Dermatol. 2019, 58, 982–986. [Google Scholar] [CrossRef]
- Yun, S.W.; Kim, Y.S.; Lee, Y.; Lim, H.J.; Park, S.I.; Jung, J.P.; Park, C.R. Outcome of Limited Video-Assisted Lumbar Sympathetic Block for Plantar Hyperhidrosis Using Clipping Method. J. Laparoendosc. Adv. Surg. Tech. A 2017, 27, 36–42. [Google Scholar] [CrossRef]
- Reisfeld, R.; Pasternack, G.A.; Daniels, P.D.; Basseri, E.; Nishi, G.K.; Berliner, K.I. Severe Plantar Hyperhidrosis: An Effective Surgical Solution. Am. Surg. 2013, 79, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Reisfeld, R. Endoscopic Lumbar Sympathectomy for Focal Plantar Hyperhidrosis Using the Clamping Method. Surg. Laparosc. Endosc. Percutan. Tech. 2010, 20, 231–236. [Google Scholar] [CrossRef]
- Vlahovic, T.C. Plantar Hyperhidrosis: An Overview. Clin. Podiatr. Med. Surg. 2016, 33, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kaur, S.; Wilson, P. Plantar Hyperhidrosis: A Review of Current Management. J. Dermatolog. Treat. 2016, 27, 556–561. [Google Scholar] [CrossRef]
- Maguire, S.C.; Fleming, C.A.; O’Brien, G.; McGreal, G. Lumbar Sympathectomy Can Improve Symptoms Associated with Ischaemia, Vasculitis, Diabetic Neuropathy and Hyperhidrosis Affecting the Lower Extremities-a Single-Centre Experience. Ir. J. Med. Sci. 2018, 187, 1045–1049. [Google Scholar] [CrossRef]
- Zheng, Z.-F.; Liu, Y.-S.; Min, X.; Tang, J.-B.; Liu, H.-W.; Cheng, B. Recovery of Sympathetic Nerve Function after Lumbar Sympathectomy Is Slower in the Hind Limbs than in the Torso. Neural Regen. Res. 2017, 12, 1177–1185. [Google Scholar] [CrossRef]
- de H. Name, B.; Silva, C.N.; Amoroso, M.H.R.; Pedrosa, E.M.; Sousa, D.A. Epidemiological Insights into Thoracic and Lumbar Sympathectomies in Brazil: A Comparative Analysis of Open versus Video-Assisted Procedures. Acta Cir. Bras. 2024, 39, e397124. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; You, Y.K.; Lee, S.; Moon, D.H.; Lee, J.-W. Evaluation of blood perfusion using laser doppler flowmetry during endoscopic lumbar sympathectomy in patients with plantar hyperhidrosis: A retrospective observational study. Sci. Rep. 2022, 12, 11456. [Google Scholar] [CrossRef]
- Lima, S.O.; de Santana, V.R.; Valido, D.P.; de Andrade, R.L.B.; Fontes, L.M.; Leite, V.H.O.; Neto, J.M.; Santos, J.M.; Varjão, L.L.; Reis, F.P. Retroperitoneoscopic lumbar sympathectomy for plantar hyperhidrosis. J. Vasc. Surg. 2017, 66, 1806–1813. [Google Scholar] [CrossRef]
- Loureiro, M.; Lemos Junior ANde Salvalaggio, P.R.O.; Alwazzan, M. Minilaparoscopic lumbar sympathectomy with 3 mm instruments for plantar hyperhidrosis. J. Vasc. Bras. 2020, 19, e20190072. [Google Scholar] [CrossRef]
- Jani, K. Retroperitoneoscopic lumbar sympathectomy for plantar hyperhidrosis. J. Am. Coll. Surg. 2009, 209, e12–e15. [Google Scholar] [CrossRef] [PubMed]
- Rieger, R.; de Paula Loureiro, M.; Pedevilla, S.; de Oliveira, R.A. Endoscopic lumbar sympathectomy following thoracic sympathectomy in patients with palmoplantar hyperhidrosis. World J. Surg. 2011, 35, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Rieger, R.; Pedevilla, S. Retroperitoneoscopic lumbar sympathectomy for the treatment of plantar hyperhidrosis: Technique and preliminary findings. Surg. Endosc. 2007, 21, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Vulpio, C.; Borzone, A.; Iannace, C.; Agnes, S.; Mascaro, A.; De Santis, M.; Mingrone, G.; Flore, R.; Pola, P.; Castagneto, M.; et al. Lumbar chemical sympathectomy in end stage of arterial disease: Early and late results. Angiology 1989, 40, 948–952. [Google Scholar] [CrossRef]

| Factor | Group | Total | Period A (Initial Period) | Period B (with LDF) | Period C (with LDF + PMR) | p-Value |
|---|---|---|---|---|---|---|
| N = 474 | n = 28 | n = 198 | n = 248 | |||
| Sex | 0.512 | |||||
| F | 277 (58.4) | 18 (64.3) | 110 (55.6) | 149 (60.1) | ||
| M | 197 (41.6) | 10 (35.7) | 88 (44.4) | 99 (39.9) | ||
| BMI | 22.1 [20.0, 24.1] | 20.7 [18.9, 22.6] | 22.8 [20.6, 24.6] | 22.0 [20.0, 24.0] | 0.004 | |
| Obesity (BMI over 25) | 86 (18.1) | 1 (3.6) | 40 (20.2) | 45 (18.1) | 0.102 | |
| Weight | 60.8 [53.0, 70.0] | 56.5 [50.4, 66.8] | 62.7 [53.9, 72.0] | 60.0 [52.1, 69.5] | 0.022 | |
| Height | 165.6 [160.0, 173.0] | 163.7 [159.3, 172.5] | 165.5 [160.6, 173.3] | 166.1 [160.0, 173.0] | 0.614 | |
| previous ETS | 79 (16.7) | 13 (46.4) | 33 (16.7) | 33 (13.3) | <0.001 | |
| Previous LSGB | 8 (1.7) | 2 (7.1) | 2 (1.0) | 4 (1.6) | 0.061 | |
| Operating time | 46.0 [40.0, 52.0] | 76.5 [61.7, 96.5] | 49.0 [43.2, 55.0] | 42.0 [39.0, 49.0] | <0.001 | |
| Concomitant ETS | 390 (82.3) | 13 (46.4) | 163 (82.3) | 214 (86.3) | <0.001 | |
| Unidentifiable lumbar chain | 8 (1.7) | 1 (3.6) | 3 (1.5) | 4 (1.6) | 0.725 | |
| Peritoneal injury | 38 (8.0) | 4 (14.3) | 25 (12.6) | 9 (3.6) | 0.001 | |
| Reappearance of plantar sweating | 21 (4.4) | 5 (17.9) | 6 (3.0) | 10 (4.0) | 0.002 | |
| Compensatory Hyperhidrosis | 0.005 | |||||
| N | 256 (54.0) | 23 (82.1) | 98 (49.5) | 135 (54.4) | ||
| Y | 218 (46.0) | 5 (17.9) | 100 (50.5) | 113 (45.6) | ||
| Compensation degree | HDSS0 | 256 (54.0) | 23 (82.1) | 98 (49.5) | 135 (54.4) | 0.027 |
| HDSS1 | 210 (44.3) | 5 (17.9) | 97 (49.0) | 108 (43.5) | ||
| HDSS2+ | 8 (1.7) | 0 (0.0) | 3 (1.5) | 5 (2.0) |
| Factor | Group | No Reappearance | Reappearance | p-Value |
|---|---|---|---|---|
| N = 453 | N = 21 | |||
| Sex | 0.503 | |||
| F | 263 (58.1) | 14 (66.7) | ||
| M | 190 (41.9) | 7 (33.3) | ||
| BMI | 22.1 [20.1, 24.2] | 20.0 [18.7, 23.0] | 0.003 | |
| Weight | 61.2 [53.5, 70.1] | 53.0 [50.1, 60.8] | 0.012 | |
| Height | 165.5 [160.0, 173.0] | 165.9 [161.8, 170.0] | 0.838 | |
| Previous ETS | 73 (16.1) | 6 (28.6) | 0.138 | |
| Previous LSGB | 1 (0.2) | 7 (33.3) | <0.001 | |
| Concomitant ETS | 379 (83.7) | 11 (52.4) | 0.001 | |
| Operation Time | 46.0 [40.0, 52.0] | 52.0 [42.0, 66.0] | 0.009 | |
| Compensatory hyperhidrosis | N | 236 (52.1) | 20 (95.2) | <0.001 |
| Y | 217 (47.9) | 1 (4.8) | ||
| Peritoneal injury | 34 (7.5) | 4 (19.0) | 0.078 | |
| Unidentifiable lumbar chain | 0 (0.0) | 8 (38.1) | <0.001 | |
| Study period | 0.008 | |||
| A | 23 (5.1) | 5 (23.8) | ||
| B | 192 (42.4) | 6 (28.6) | ||
| C | 238 (52.5) | 10 (47.6) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Factor | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Age over 35 | 3.67 (1.47–9.21) | 0.006 | 4.57 (1.56–13.40) | 0.006 |
| BMI over 25 | 0.46 (0.11–2.02) | 0.314 | ||
| Sex (Ref. Female) | 0.69 (0.27–1.75) | 0.442 | ||
| Previous ETS | 2.08 (0.78–5.54) | 0.143 | ||
| Previous LSGB | 226 (26.0–1960) | <0.001 | 269 (29.3–2460) | <0.001 |
| Intraoperative peritoneal injury | 2.90 (0.92–9.10) | 0.068 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, D.H.; Woo, W.; Lee, J.; Lee, S. Advances in Clinical Outcomes of Endoscopic Lumbar Sympathectomy: Analysis of 494 Consecutive Patients at a Single Institution. J. Clin. Med. 2025, 14, 4311. https://doi.org/10.3390/jcm14124311
Moon DH, Woo W, Lee J, Lee S. Advances in Clinical Outcomes of Endoscopic Lumbar Sympathectomy: Analysis of 494 Consecutive Patients at a Single Institution. Journal of Clinical Medicine. 2025; 14(12):4311. https://doi.org/10.3390/jcm14124311
Chicago/Turabian StyleMoon, Duk Hwan, Wongi Woo, Jimin Lee, and Sungsoo Lee. 2025. "Advances in Clinical Outcomes of Endoscopic Lumbar Sympathectomy: Analysis of 494 Consecutive Patients at a Single Institution" Journal of Clinical Medicine 14, no. 12: 4311. https://doi.org/10.3390/jcm14124311
APA StyleMoon, D. H., Woo, W., Lee, J., & Lee, S. (2025). Advances in Clinical Outcomes of Endoscopic Lumbar Sympathectomy: Analysis of 494 Consecutive Patients at a Single Institution. Journal of Clinical Medicine, 14(12), 4311. https://doi.org/10.3390/jcm14124311






