High Disease Burden and Oral Corticosteroid Use in Patients with Hypereosinophilic Syndrome and Eosinophilic Granulomatosis with Polyangiitis: Country-Level Insights into Real-World Management in Europe
Abstract
1. Introduction
2. Materials and Methods
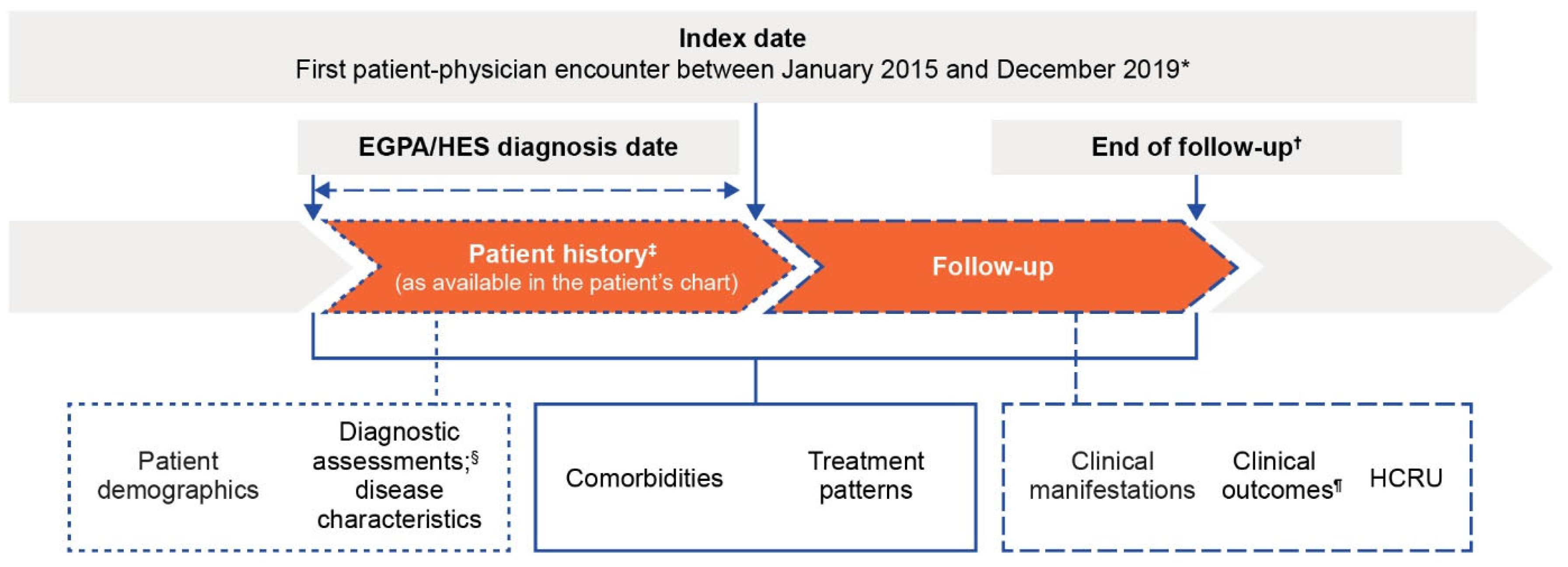
2.1. Study Design
2.2. Patient and Physician Eligibility Criteria
2.3. Data Source and Collection
2.4. Outcomes
2.5. Sample Size and Statistical Analysis
2.6. Ethics
3. Results
3.1. Physician Characteristics
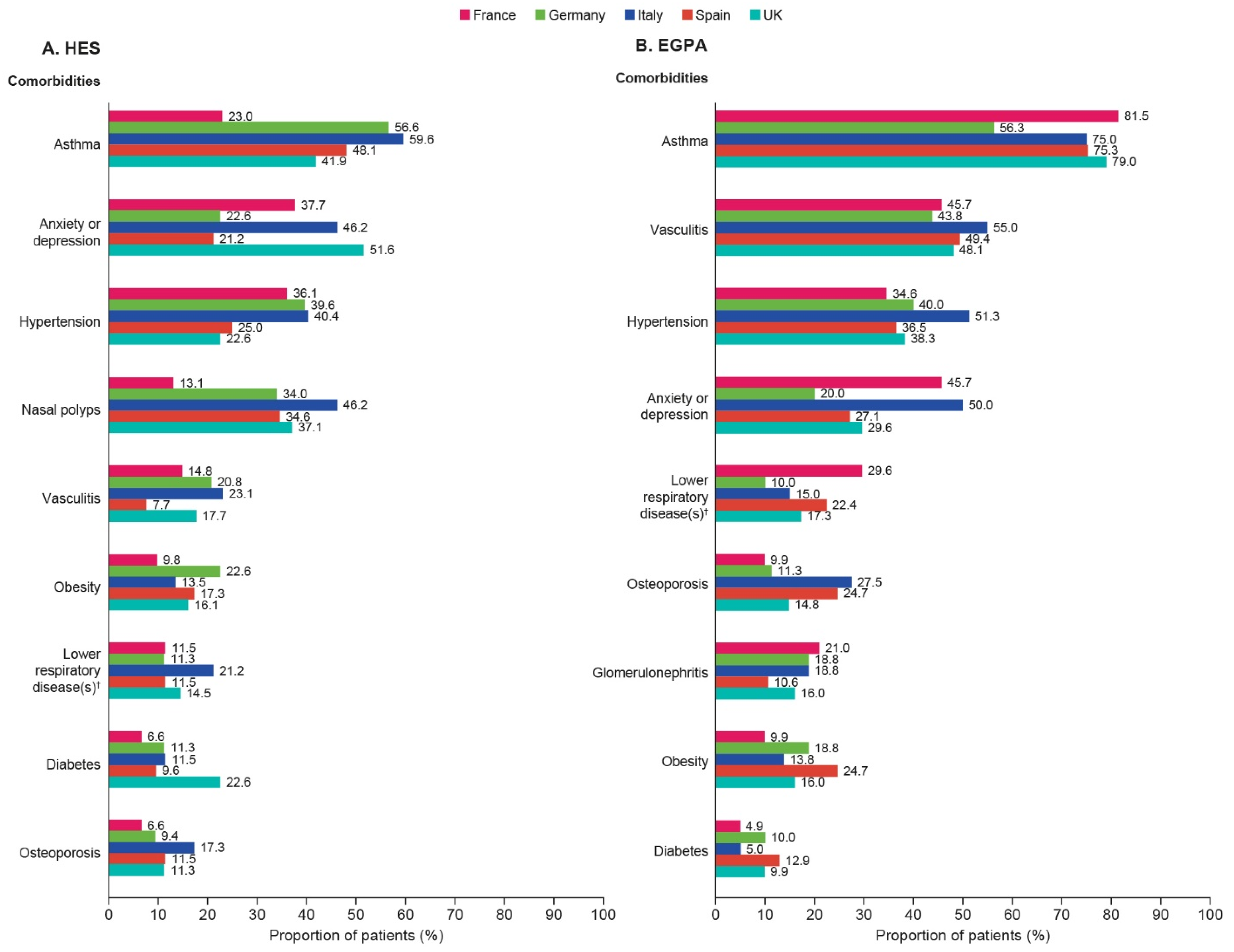
3.2. Patient Demographics, Clinical Characteristics, and Comorbidities
3.3. Diagnostic Assessments and Monitoring
3.4. Treatment Patterns
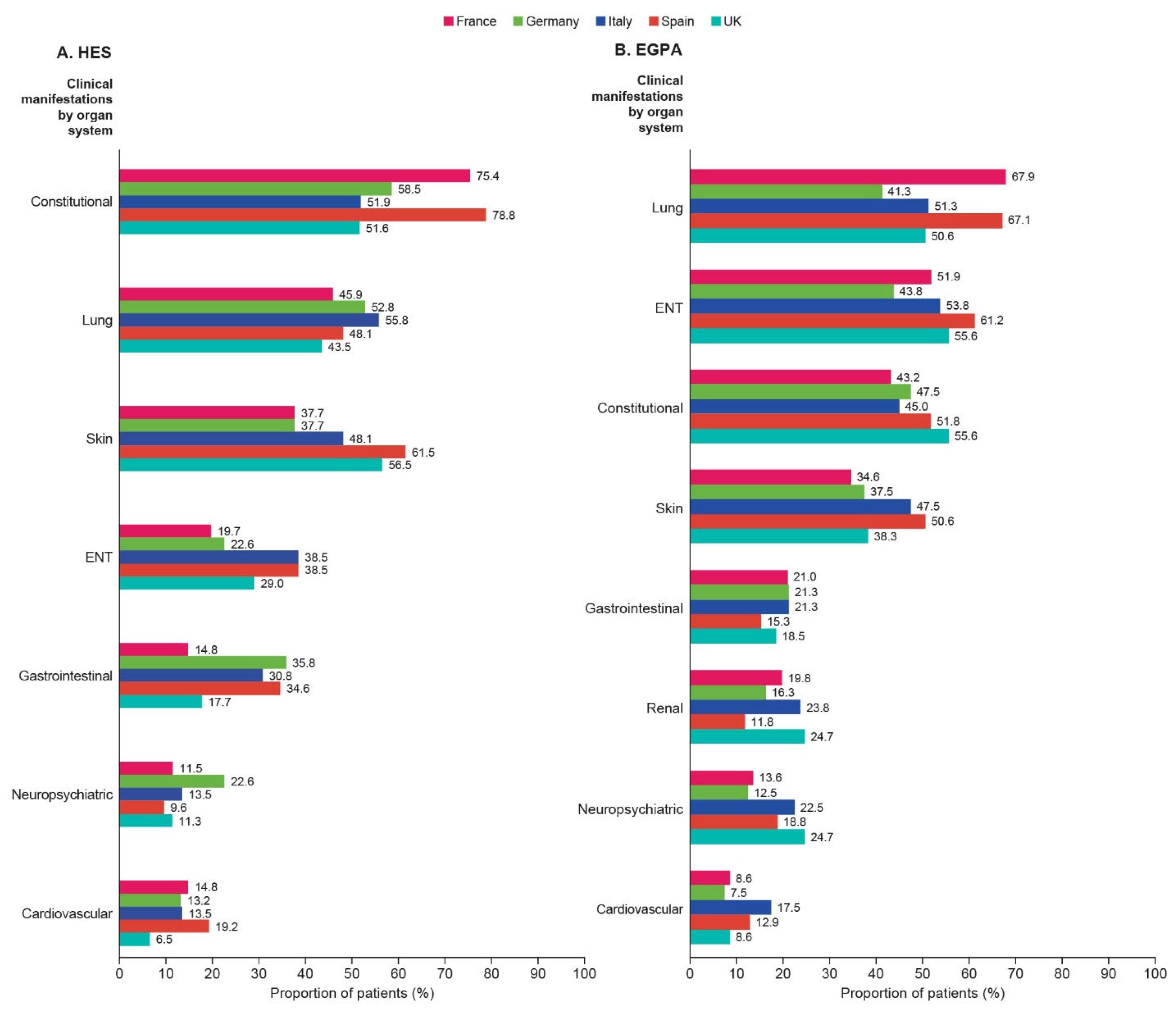
3.5. Clinical Manifestations
3.6. Clinical Outcomes
3.7. HES and EGPA-Related HCRU
3.8. Post Hoc Pooled Analysis of HES and EGPA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khoury, P.; Akuthota, P.; Kwon, N.; Steinfeld, J.; Roufosse, F. HES and EGPA: Two sides of the same coin. Mayo Clin. Proc. 2023, 98, 1054–1070. [Google Scholar] [CrossRef] [PubMed]
- Emmi, G.; Bettiol, A.; Gelain, E.; Bajema, I.M.; Berti, A.; Burns, S.; Cid, M.C.; Cohen Tervaert, J.W.; Cottin, V.; Durante, E.; et al. Evidence-based guideline for the diagnosis and management of eosinophilic granulomatosis with polyangiitis. Nat. Rev. Rheumatol. 2023, 19, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Shomali, W.; Gotlib, J. World Health Organization and International Consensus Classification of eosinophilic disorders: 2024 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2024, 99, 946–968. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Klion, A.D.; Roufosse, F.; Simon, D.; Metzgeroth, G.; Leiferman, K.M.; Schwaab, J.; Butterfield, J.H.; Sperr, W.R.; Sotlar, K.; et al. Proposed refined diagnostic criteria and classification of eosinophil disorders and related syndromes. Allergy 2023, 78, 47–59. [Google Scholar] [CrossRef]
- Grayson, P.C.; Ponte, C.; Suppiah, R.; Robson, J.C.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Luqmani, R.A.; Watts, R.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Ann. Rheum. Dis. 2022, 81, 309–314. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Comarmond, C.; Pagnoux, C.; Khellaf, M.; Cordier, J.F.; Hamidou, M.; Viallard, J.F.; Maurier, F.; Jouneau, S.; Bienvenu, B.; Puéchal, X.; et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): Clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 2013, 65, 270–281. [Google Scholar] [CrossRef]
- Cottin, V.; Bel, E.; Bottero, P.; Dalhoff, K.; Humbert, M.; Lazor, R.; Sinico, R.A.; Sivasothy, P.; Wechsler, M.E.; Groh, M.; et al. Respiratory manifestations of eosinophilic granulomatosis with polyangiitis (Churg–Strauss). Eur. Respir. J. 2016, 48, 1429. [Google Scholar] [CrossRef]
- Doubelt, I.; Cuthbertson, D.; Carette, S.; Chung, S.A.; Forbess, L.J.; Khalidi, N.A.; Koening, C.L.; Langford, C.; McAlear, C.A.; Moreland, L.W.; et al. Clinical manifestations and long-term outcomes of eosinophilic granulomatosis with polyangiitis in North America. ACR Open Rheumatol. 2021, 3, 404–412. [Google Scholar] [CrossRef]
- Ogbogu, P.U.; Bochner, B.S.; Butterfield, J.H.; Gleich, G.J.; Huss-Marp, J.; Kahn, J.E.; Leiferman, K.M.; Nutman, T.B.; Pfab, F.; Ring, J.; et al. Hypereosinophilic syndrome: A multicenter, retrospective analysis of clinical characteristics and response to therapy. J. Allergy Clin. Immunol. 2009, 124, 1319–1325.e3. [Google Scholar] [CrossRef]
- Hellmich, B.; Sanchez-Alamo, B.; Schirmer, J.H.; Berti, A.; Blockmans, D.; Cid, M.C.; Holle, J.U.; Hollinger, N.; Karadag, O.; Kronbichler, A.; et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann. Rheum. Dis. 2024, 83, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Dispenza, M.C.; Bochner, B.S. Diagnosis and novel approaches to the treatment of hypereosinophilic syndromes. Curr. Hematol. Malig. Rep. 2018, 13, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Klion, A.D. Approach to the patient with suspected hypereosinophilic syndrome. Hematology 2022, 2022, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Robson, J.; Doll, H.; Suppiah, R.; Flossmann, O.; Harper, L.; Hoglund, P.; Jayne, D.; Mahr, A.; Westman, K.; Luqmani, R. Glucocorticoid treatment and damage in the anti-neutrophil cytoplasm antibody-associated vasculitides: Long-term data from the European Vasculitis Study Group trials. Rheumatology 2015, 54, 471–481. [Google Scholar] [CrossRef]
- Volmer, T.; Effenberger, T.; Trautner, C.; Buhl, R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: A focused review of the impact data in the literature. Eur. Respir. J. 2018, 52, 1800703. [Google Scholar] [CrossRef]
- Strehl, C.; Bijlsma, J.W.; de Wit, M.; Boers, M.; Caeyers, N.; Cutolo, M.; Dasgupta, B.; Dixon, W.G.; Geenen, R.; Huizinga, T.W.; et al. Defining conditions where long-term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: Viewpoints from an EULAR task force. Ann. Rheum. Dis. 2016, 75, 952–957. [Google Scholar] [CrossRef]
- Hwee, J.; Huynh, L.; Du, S.; Kwon, N.; Jakes, R.W.; Alfonso-Cristancho, R.; Baylis, L.; Requena, G.; Khanal, A.; Rothenberg, M.E.; et al. Hypereosinophilic syndrome in Europe: Retrospective study of treatment patterns, clinical manifestations, and healthcare resource utilization. Ann. Allergy Asthma Immunol. 2023, 130, 768–775. [Google Scholar] [CrossRef]
- Jakes, R.W.; Kwon, N.; Nordstrom, B.; Goulding, R.; Fahrbach, K.; Tarpey, J.; Van Dyke, M.K. Burden of illness associated with eosinophilic granulomatosis with polyangiitis: A systematic literature review and meta-analysis. Clin. Rheumatol. 2021, 40, 4829–4836. [Google Scholar] [CrossRef]
- Bell, C.F.; Ajmera, M.; Meyers, J. Retrospective analysis of the burden of illness of eosinophilic granulomatosis with polyangiitis (EGPA) versus asthma in commercially insured US patients. Cureus 2023, 15, e42241. [Google Scholar] [CrossRef]
- Hwee, J.; Harper, L.; Fu, Q.; Nirantharakumar, K.; Mu, G.; Jakes, R.W. Prevalence, incidence and healthcare burden of eosinophilic granulomatosis with polyangiitis in the UK. ERJ Open Res. 2024, 10, 00430–02023. [Google Scholar] [CrossRef]
- Jakes, R.W.; Kwon, N.; Huynh, L.; Hwee, J.; Baylis, L.; Alfonso-Cristancho, R.; Du, S.; Khanal, A.; Duh, M.S.; Terrier, B. Burden of eosinophilic granulomatosis with polyangiitis in Europe. ERJ Open Res. 2024, 10, 00912–2023. [Google Scholar] [CrossRef] [PubMed]
- Strobel, M.J.; Alves, D.; Roufosse, F.; Antoun, Z.; Kwon, N.; Baylis, L.; Wechsler, M.E. Insights from social media on the patient experience of living with rare eosinophil-driven diseases. J. Patient Exp. 2022, 9, 23743735221143953. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.J.; Akuthota, P.; Andradas, R.; Bredenoord, A.J.; Cordell, A.; Gray, S.; Kullman, J.; Mathur, S.K.; Pavord, I.; Roufosse, F.; et al. Improving care in eosinophil-associated diseases: A charter. Adv. Ther. 2022, 39, 2323–2341. [Google Scholar] [CrossRef]
- Mundell, L.; Lindemann, R.; Douglas, J. Monitoring long-term oral corticosteroids. BMJ Open Qual. 2017, 6, e000209. [Google Scholar] [CrossRef]
- Price, D.B.; Trudo, F.; Voorham, J.; Xu, X.; Kerkhof, M.; Jie, L.J.Z.; Tran, T.N. Adverse outcomes from initiation of systemic corticosteroids for asthma: Long-term observational study. J. Asthma Allergy 2018, 11, 193–204. [Google Scholar] [CrossRef]
- Dalal, A.A.; Duh, M.S.; Gozalo, L.; Robitaille, M.N.; Albers, F.; Yancey, S.; Ortega, H.; Forshag, M.; Lin, X.; Lefebvre, P. Dose-response relationship between long-term systemic corticosteroid use and related complications in patients with severe asthma. J. Manag. Care Spec. Pharm. 2016, 22, 833–847. [Google Scholar] [CrossRef]
- Samson, M.; Puéchal, X.; Devilliers, H.; Ribi, C.; Cohen, P.; Stern, M.; Pagnoux, C.; Mouthon, L.; Guillevin, L. Long-term outcomes of 118 patients with eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome) enrolled in two prospective trials. J. Autoimmun. 2013, 43, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Canzian, A.; Venhoff, N.; Urban, M.L.; Sartorelli, S.; Ruppert, A.M.; Groh, M.; Girszyn, N.; Taillé, C.; Maurier, F.; Cottin, V.; et al. Use of Biologics to Treat Relapsing and/or Refractory Eosinophilic Granulomatosis with Polyangiitis: Data From a European Collaborative Study. Arthritis Rheumatol. 2021, 73, 498–503. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Hellmich, B.; Cid, M.C.; Jayne, D.; Tian, X.; Baylis, L.; Roufosse, F. Unmet needs and evidence gaps in hypereosinophilic syndrome and eosinophilic granulomatosis with polyangiitis. J. Allergy Clin. Immunol. 2023, 151, 1415–1428. [Google Scholar] [CrossRef]
- Caminati, M.; Maule, M.; Bello, F.; Emmi, G. Biologics for eosinophilic granulomatosis with polyangiitis. Curr. Opin. Allergy Clin. Immunol. 2023, 23, 36–43. [Google Scholar] [CrossRef]
- Kuang, F.L.; Khoury, P.; Weller, P.F.; Wechsler, M.E.; Klion, A.D. Biologics and hypereosinophilic syndromes: Knowledge gaps and controversies. J. Allergy Clin. Immunol. Pract. 2023, 11, 2666–2671. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Mepolizumab (Nucala) Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/nucala-epar-product-information_en.pdf (accessed on 15 May 2025).
- European Medicines Agency (EMA). Benralizumab (Fasnera) Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/fasenra-epar-product-information_en.pdf (accessed on 15 May 2025).
- Chen, M.M.; Roufosse, F.; Wang, S.A.; Verstovsek, S.; Durrani, S.R.; Rothenberg, M.E.; Pongdee, T.; Butterfield, J.; Lax, T.; Wechsler, M.E.; et al. An international, retrospective study of off-label biologic use in the treatment of hypereosinophilic syndromes. J. Allergy Clin. Immunol. Pract. 2022, 10, 1217–1228.e3. [Google Scholar] [CrossRef] [PubMed]
- Roufosse, F.; Butterfield, J.; Steinfeld, J.; Bentley, J.H.; von Maltzahn, R.; Kwon, N.; Nelsen, L. Mepolizumab therapy improves the most bothersome symptoms in patients with hypereosinophilic syndrome. Front. Med. 2023, 10, 1035250. [Google Scholar] [CrossRef] [PubMed]
- Roufosse, F.; Kahn, J.E.; Rothenberg, M.E.; Wardlaw, A.J.; Klion, A.D.; Kirby, S.Y.; Gilson, M.J.; Bentley, J.H.; Bradford, E.S.; Yancey, S.W.; et al. Efficacy and safety of mepolizumab in hypereosinophilic syndrome: A phase III, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2020, 146, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Akuthota, P.; Jayne, D.; Khoury, P.; Klion, A.; Langford, C.A.; Merkel, P.A.; Moosig, F.; Specks, U.; Cid, M.C.; et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N. Engl. J. Med. 2017, 376, 1921–1932. [Google Scholar] [CrossRef]
- Ishii, T.; Kunishige, H.; Kobayashi, T.; Hayashi, E.; Komatsubara, M.; Alfonso-Cristancho, R.; Tamaoki, J.; Howarth, P. Real-world safety and effectiveness of mepolizumab for patients with eosinophilic granulomatosis with polyangiitis in Japan: Long-term observation of the MARS study. Mod. Rheumatol. 2024, 35, 505–515. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Silver, J.; Wolff, G.; Price, R.G.; Verghis, R.; Weller, P.F.; Merkel, P.A.; Khoury, P.; EGPA Mepolizumab Open-Label Extension study group. Long-Term Safety and Efficacy of Mepolizumab in Eosinophilic Granulomatosis with Polyangiitis. Arthritis Rheumatol. 2025. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Nair, P.; Terrier, B.; Walz, B.; Bourdin, A.; Jayne, D.R.W.; Jackson, D.J.; Roufosse, F.; Börjesson Sjö, L.; Fan, Y.; et al. Benralizumab versus mepolizumab for eosinophilic granulomatosis with polyangiitis. N. Engl. J. Med. 2024, 390, 911–921. [Google Scholar] [CrossRef]
- Mathur, S.K.; Silver, J.; MacKnight, S.D.; Urosevic, A.; Martinez, C.; Zhang, K.; Laliberté, F.; Deb, A. Real-world mepolizumab treatment in eosinophilic granulomatosis with polyangiitis reduces disease burden in the United States. Ann. Allergy Asthma Immunol. 2025, 134, 341–350.e2. [Google Scholar] [CrossRef]
- Caminati, M.; Maule, M.; Benoni, R.; Micheletto, C.; Tecchio, C.; Vaia, R.; De Franceschi, L.; Guarnieri, G.; Vianello, A.; Senna, G. Low-dose anti-IL 5 treatment in idiopathic hypereosinophilic syndrome: Towards a precision medicine approach for remission maintenance. Orphanet J. Rare Dis. 2023, 18, 302. [Google Scholar] [CrossRef]
- Kuang, F.L.; Fay, M.P.; Ware, J.; Wetzler, L.; Holland-Thomas, N.; Brown, T.; Ortega, H.; Steinfeld, J.; Khoury, P.; Klion, A.D. Long-Term Clinical Outcomes of High-Dose Mepolizumab Treatment for Hypereosinophilic Syndrome. J. Allergy Clin. Immunol. Pract. 2018, 6, 1518–1527.e5. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Jayne, D.R.W.; Hellmich, B.; Bentley, J.H.; Steinfeld, J.; Yancey, S.W.; Kwon, N.; Akuthota, P.; Khoury, P.; Baylis, L.; et al. Clinical Benefit of Mepolizumab in Eosinophilic Granulomatosis with Polyangiitis for Patients With and Without a Vasculitic Phenotype. ACR Open Rheumatol. 2023, 5, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Jayne, D.R.W.; Hellmich, B.; Merkel, P.A.; Agmon-Levin, N.; Nair, P.; Fan, Y.; Maho, E.; Necander, S.; Shavit, A. POS0862 the Efficacy of Eosinophil-Targeting Therapies According to Anca Status in Patients with Eosinophilic Granulomatosis with Polyangiitis: A Post-Hoc Analysis of the Phase 3 Mandara Study. Ann. Rheum. Dis. 2024, 83, 1118–1120. [Google Scholar] [CrossRef]
- Rothenberg, M.E.; Roufosse, F.; Faguer, S.; Gleich, G.J.; Steinfeld, J.; Yancey, S.W.; Mavropoulou, E.; Kwon, N. MepolizumabReduces Hypereosinophilic Syndrome Flares Irrespective of Blood Eosinophil Count and Interleukin-5. J. Allergy Clin. Immunol. Pract. 2022, 10, 2367–2374.e3. [Google Scholar] [CrossRef]
- Lyons, P.A.; Peters, J.E.; Alberici, F.; Liley, J.; Coulson, R.M.R.; Astle, W.; Baldini, C.; Bonatti, F.; Cid, M.C.; Elding, H.; et al. Genome-wide association study of eosinophilic granulomatosis with polyangiitis reveals genomic loci stratified by ANCA status. Nat. Commun. 2019, 10, 5120. [Google Scholar] [CrossRef]
- Martorana, D.; Bonatti, F.; Alberici, F.; Gioffredi, A.; Reina, M.; Urban, M.L.; Maritati, F.; Adorni, A.; Radice, A.; Pizzolato, S.; et al. Fcgamma-receptor 3B (FCGR3B) copy number variations in patients with eosinophilic granulomatosis with polyangiitis. J. Allergy Clin. Immunol. 2016, 137, 1597–1599.e8. [Google Scholar] [CrossRef]
- Vaglio, A.; Martorana, D.; Maggiore, U.; Grasselli, C.; Zanetti, A.; Pesci, A.; Garini, G.; Manganelli, P.; Bottero, P.; Tumiati, B.; et al. HLA-DRB4 as a genetic risk factor for Churg-Strauss syndrome. Arthritis Rheum. 2007, 56, 3159–3166. [Google Scholar] [CrossRef]
- Maritati, F.; Peyronel, F.; Fenaroli, P.; Pegoraro, F.; Lastrucci, V.; Benigno, G.D.; Palmisano, A.; Rossi, G.M.; Urban, M.L.; Alberici, F.; et al. Occupational exposures and smoking in eosinophilic granulomatosis with polyangiitis: A case-control study. Arthritis Rheumatol. 2021, 73, 1694–1702. [Google Scholar] [CrossRef]
- Fagni, F.; Bello, F.; Emmi, G. Eosinophilic granulomatosis with polyangiitis: Dissecting the pathophysiology. Front. Med. 2021, 8, 627776. [Google Scholar] [CrossRef]
- Detiček, A.; Locatelli, I.; Kos, M. Patient access to medicines for rare diseases in European countries. Value Health 2018, 21, 553–560. [Google Scholar] [CrossRef]
- Akehurst, R.L.; Abadie, E.; Renaudin, N.; Sarkozy, F. Variation in health technology assessment and reimbursement processes in Europe. Value Health 2017, 20, 67–76. [Google Scholar] [CrossRef]
| Patients with HES (N = 280) | France n = 61 | Germany n = 53 | Italy n = 52 | Spain n = 52 | UK n = 62 |
|---|---|---|---|---|---|
| Length of follow-up, years, mean (SD) | 3.1 (1.4) | 2.3 (1.1) | 2.8 (1.5) | 3.0 (1.4) | 2.5 (1.3) |
| Male, n (%) | 46 (75.4) | 28 (52.8) | 30 (57.7) | 38 (73.1) | 40 (64.5) |
| Age on index date, years, mean (SD) | 48.5 (14.5) | 42.3 (17.7) | 42.3 (15.1) | 43.6 (12.1) | 41.3 (18.1) |
| Age at HES diagnosis, years, mean (SD) | 47.5 (15.3) | 42.0 (17.9) | 40.2 (14.8) | 42.3 (12.6) | 39.7 (18.6) |
| Disease duration, years, from diagnosis date to EOF | |||||
| Mean (SD) | 4.1 (4.4) | 2.6 (1.6) | 4.9 (5.9) | 4.3 (4.2) | 4.1 (4.9) |
| Median (IQR) | 3.0 (2.1, 4.4) | 2.3 (1.8, 2.9) | 3.0 (1.8, 5.1) | 2.9 (1.7, 4.5) | 2.4 (1.7, 4.1) |
| HES diagnosis before study (≤2014), n (%) | 7 (11.5) | 1 (1.9) | 9 (17.3) | 7 (13.5) | 8 (12.9) |
| HES diagnosis during the study (2015–2019), n (%) | 54 (88.5) | 52 (98.1) | 43 (82.7) | 45 (86.5) | 54 (87.1) |
| Disease subtype, † n (%) | |||||
| Idiopathic | 33 (54.1) | 30 (56.6) | 32 (61.5) | 27 (51.9) | 33 (53.2) |
| Myeloid variant | 16 (26.2) | 10 (18.9) | 9 (17.3) | 18 (34.6) | 13 (21.0) |
| Lymphocytic variant | 11 (18.0) | 8 (15.1) | 4 (7.7) | 5 (9.6) | 14 (22.6) |
| Other †,‡ | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) | 1 (1.6) |
| Unknown | 1 (1.6) | 5 (9.4) | 6 (11.5) | 2 (3.8) | 1 (1.6) |
| Blood eosinophil count data available, § n (%) | 56 (91.8) | 44 (83.0) | 45 (86.5) | 45 (86.5) | 51 (82.3) |
| Blood eosinophil count, § cells/µL | |||||
| Mean (SD) | 3641.3 (2837.0) | 2242.6 (1730.1) | 1412.1 (1573.3) | 2920.6 (2233.7) | 2303.4 (2694.1) |
| Median (IQR) | 2900.0 (1050.0, 6075.0) | 1650.0 (1000.0, 3100.0) | 700.0 (150.0, 2000.0) | 2479.0 (1600.0, 3200.0) | 1600.0 (89.0, 3500.0) |
| Patients with EGPA (N = 407) | France n = 81 | Germany n = 80 | Italy n = 80 | Spain n = 85 | UK n = 81 |
| Length of follow-up, years, mean (SD) | 2.4 (1.3) | 2.6 (1.3) | 3.2 (1.6) | 2.9 (1.5) | 2.6 (1.4) |
| Male, n (%) | 51 (63.0) | 44 (55.0) | 47 (58.8) | 42 (49.4) | 47 (58.0) |
| Age on index date, years, mean (SD) | 45.8 (13.9) | 42.6 (15.8) | 41.4 (16.1) | 44.2 (14.8) | 46.1 (14.0) |
| Age at EGPA diagnosis, years, mean (SD) | 45.5 (14.0) | 42.3 (15.6) | 39.9 (15.4) | 42.8 (14.8) | 45.4 (14.3) |
| Disease duration, years, from diagnosis date to EOF | |||||
| Mean (SD) | 2.7 (1.7) | 3.0 (2.1) | 4.6 (3.9) | 4.2 (4.2) | 3.3 (3.1) |
| Median (IQR) | 2.1 (1.8, 2.9) | 2.2 (1.7, 3.1) | 3.7 (2.0, 5.9) | 2.8 (1.9, 4.4) | 2.3 (1.7, 3.5) |
| EGPA diagnosis before study (≤2014), n (%) | 4 (4.9) | 4 (5.0) | 19 (23.8) | 12 (14.1) | 9 (11.1) |
| EGPA diagnosis during the study (2015–2019), n (%) | 77 (95.1) | 76 (95.0) | 61 (76.3) | 73 (85.9) | 72 (88.9) |
| Proportion of patients with asthma, n (%) | 66 (81.5) | 45 (56.3) | 60 (75.0) | 64 (75.3) | 64 (79.0) |
| Proportion of patients with an asthma diagnosis date prior to EGPA diagnosis, ¶ n (%) | 39 (78.0) | 24 (100.0) | 36 (80.0) | 33 (64.7) | 28 (80.0) |
| Time from asthma diagnosis to EGPA diagnosis, years | |||||
| Mean (SD) | 2.3 (3.2) | 8.2 (11.3) | 1.9 (3.9) | 5.6 (6.2) | 5.5 (5.6) |
| Median (IQR) | 1.2 (0.3, 3.2) | 4.4 (2.2, 7.3) | 0.1 (0.0, 2.0) | 2.9 (0.5, 8.1) | 3.6 (0.9, 9.2) |
| Disease phase, † n (%) | |||||
| Eosinophilic | 47 (58.0) | 40 (50.0) | 44 (55.0) | 48 (56.5) | 41 (50.6) |
| Vasculitic | 21 (25.9) | 33 (41.3) | 28 (35.0) | 20 (23.5) | 23 (28.4) |
| Prodromal | 9 (11.1) | 4 (5.0) | 4 (5.0) | 9 (10.6) | 10 (12.3) |
| Unknown | 4 (4.9) | 3 (3.8) | 4 (5.0) | 8 (9.4) | 7 (8.6) |
| Blood eosinophil count data available, § n (%) | 77 (95.1) | 73 (91.3) | 66 (82.5) | 75 (88.2) | 73 (90.1) |
| Blood eosinophil count, § cells/µL | |||||
| Mean (SD) | 2125.6 (2035.0) | 3098.1 (2266.2) | 2345.6 (2123.2) | 2345.7 (2399.2) | 1948.1 (2542.1) |
| Median (IQR) | 1500.0 (875.0, 2800.0) | 2800.0 (1200.0, 4500.0) | 1800.0 (900.0, 3000.0) | 1400.0 (600.0, 4000.0) | 800.0 (45.0, 3200.0) |
| Patients with HES † (N = 280) | France n = 61 | Germany n = 53 | Italy n = 52 | Spain n = 52 | UK n = 62 |
|---|---|---|---|---|---|
| Number of distinct HES therapies used, ‡ median (IQR) | 2.0 (2.0, 4.0) | 2.0 (2.0, 3.0) | 2.0 (1.0, 3.0) | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) |
| Number of distinct HES therapies used, ‡ categorical, n (%) | |||||
| 0 | 2 (3.3) | 0 (0.0) | 1 (1.9) | 0 (0.0) | 1 (1.6) |
| 1 | 8 (13.1) | 13 (24.5) | 15 (28.8) | 12 (23.1) | 12 (19.4) |
| 2 | 22 (36.1) | 20 (37.7) | 14 (26.9) | 22 (42.3) | 29 (46.8) |
| 3 | 13 (21.3) | 11 (20.8) | 13 (25.0) | 14 (26.9) | 14 (22.6) |
| ≥4 | 16 (26.2) | 9 (17.0) | 9 (17.3) | 4 (7.7) | 6 (9.7) |
| HES therapies by treatment category § | |||||
| OCS, n (%) | 49 (80.3) | 53 (100) | 45 (86.5) | 50 (96.2) | 53 (85.5) |
| Maximum daily dose across all OCS, mg, ¶ mean (SD) | 31.6 (20.2) | 28.0 (18.1) | 24.8 (16.8) | 42.4 (19.6) | 32.0 (17.7) |
| Total duration across all oral corticosteroids, months | |||||
| Mean (SD) | 17.5 (19.0) | 22.3 (18.7) | 43.5 (54.3) | 22.0 (18.3) | 17.9 (12.0) |
| Median (IQR) | 8.5 (5.7, 24.1) | 21.3 (10.2, 28.7) | 23.1 (8.6, 59.0) | 18.4 (5.8, 30.8) | 15.9 (10.4, 23.6) |
| Prednisone or prednisolone, n (%) | 39 (63.9) | 46 (86.8) | 30 (57.7) | 43 (82.7) | 42 (67.7) |
| Methylprednisolone, n (%) | 4 (6.6) | 4 (7.5) | 18 (34.6) | 8 (15.4) | 12 (19.4) |
| Cortisone, n (%) | 7 (11.5) | 3 (5.7) | 1 (1.9) | 0 (0) | 3 (4.8) |
| Immunosuppressants or cytotoxic agents, used by ≥5% of patients in any country, ** n (%) | 44 (72.1) | 33 (62.3) | 34 (65.4) | 33 (63.5) | 34 (54.8) |
| Azathioprine | 8 (13.1) | 6 (11.3) | 13 (25) | 5 (9.6) | 8 (12.9) |
| Cyclophosphamide | 3 (4.9) | 6 (11.3) | 3 (5.8) | 2 (3.8) | 3 (4.8) |
| Cyclosporine | 2 (3.3) | 2 (3.8) | 4 (7.7) | 2 (3.8) | 4 (6.5) |
| Hydroxyurea | 6 (9.8) | 6 (11.3) | 4 (7.7) | 4 (7.7) | 6 (9.7) |
| Imatinib mesylate | 16 (26.2) | 12 (22.6) | 10 (19.2) | 11 (21.2) | 8 (12.9) |
| Interferon-alpha | 3 (4.9) | 8 (15.1) | 1 (1.9) | 1 (1.9) | 2 (3.2) |
| Methotrexate | 7 (11.5) | 5 (9.4) | 6 (11.5) | 10 (19.2) | 1 (1.6) |
| Tofacitinib | 2 (3.3) | 1 (1.9) | 3 (5.8) | 0 (0.0) | 1 (1.6) |
| Mycophenolate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (5.8) | 2 (3.2) |
| Biologics, n (%) | 30 (49.2) | 17 (32.1) | 25 (48.1) | 20 (38.5) | 31 (50.0) |
| Mepolizumab | 9 (14.8) | 4 (7.5) | 16 (30.8) | 9 (17.3) | 5 (8.1) |
| Alemtuzumab | 1 (1.6) | 4 (7.5) | 2 (3.8) | 2 (3.8) | 13 (21) |
| Benralizumab | 9 (14.8) | 9 (17) | 6 (11.5) | 3 (5.8) | 7 (11.3) |
| Dupilumab | 5 (8.2) | 5 (9.4) | 6 (11.5) | 0 (0) | 6 (9.7) |
| Omalizumab | 1 (1.6) | 2 (3.8) | 5 (9.6) | 5 (9.6) | 0 (0) |
| Reslizumab | 7 (11.5) | 1 (1.9) | 0 (0) | 2 (3.8) | 3 (4.8) |
| Rituximab | 11 (18) | 5 (9.4) | 4 (7.7) | 9 (17.3) | 5 (8.1) |
| Patients with EGPA †† (N = 407) | France n = 81 | Germany n = 80 | Italy n = 80 | Spain n = 85 | UK n = 81 |
| Number of distinct EGPA therapies used, ‡ median (IQR) | 4.0 (2.0, 5.0) | 3.0 (2.0, 4.0) | 4.0 (3.0, 5.0) | 4.0 (3.0, 5.0) | 4.0 (3.0, 5.0) |
| Number of distinct EGPA therapies used, categorical, ‡ n (%) | |||||
| 1–2 | 26 (32.1) | 33 (41.3) | 9 (11.3) | 14 (16.5) | 20 (24.7) |
| 3–4 | 31 (38.3) | 30 (37.5) | 36 (45.0) | 34 (40.0) | 35 (43.2) |
| 5–7 | 19 (23.5) | 16 (20.0) | 31 (38.8) | 36 (42.4) | 24 (29.6) |
| ≥8 | 5 (6.2) | 1 (1.3) | 4 (5.0) | 1 (1.2) | 2 (2.5) |
| Time from diagnosis to initiation of EGPA therapy, years | |||||
| Mean (SD) | 0.3 (1.2) | 0.2 (0.6) | 0.7 (1.7) | 0.9 (2.7) | 0.0 (0.1) |
| Median (IQR) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.2) | 0.0 (0.0, 0.9) | 0.0 (0.0, 0.0) |
| Time from diagnosis to initiation of biologics, years, median (IQR) | 1.0 (0.1, 1.5) | 0.7 (0.1, 1.6) | 2.8 (1.2, 4.6) | 1.9 (1.2, 3.0) | 0.5 (0.1, 1.8) |
| EGPA therapies by treatment category ‡‡ | |||||
| OCS, n (%) | 79 (97.5) | 80 (100.0) | 79 (98.8) | 83 (97.6) | 81 (100.0) |
| Maximum daily OCS dose (mg) ¶ | 35.8 (18.9) | 28.8 (19.9) | 19.9 (16.2) | 34.0 (19.8) | 31.6 (20.4) |
| Duration of OCS use, months | |||||
| Mean (SD) | 22.3 (15.0) | 23.1 (16.8) | 38.3 (38.1) | 31.6 (39.3) | 28.3 (26.3) |
| Median (IQR) | 19.1 (12.3, 27.0) | 21.4 (8.9, 29.9) | 27.9 (19.2, 47.0) | 19.2 (8.2, 38.2) | 22.5 (12.7, 39.7) |
| Prednisone or prednisolone, n (%) | 72 (88.9) | 75 (93.8) | 56 (70.0) | 71 (83.5) | 75 (92.6) |
| Methylprednisolone, n (%) | 26 (32.1) | 7 (8.8) | 22 (27.5) | 13 (15.3) | 30 (37.0) |
| Cortisone, n (%) | 2 (2.5) | 1 (1.3) | 7 (8.8) | 4 (4.7) | 5 (6.2) |
| Immunosuppressive agents and other therapies, used by ≥5% of patients in any country, §§ n (%) | 37 (45.7) | 54 (67.5) | 56 (70.0) | 51 (60.0) | 62 (76.5) |
| Azathioprine | 14 (17.3) | 29 (36.3) | 22 (27.5) | 16 (18.8) | 29 (35.8) |
| Cyclophosphamide | 6 (7.4) | 23 (28.8) | 21 (26.3) | 13 (15.3) | 15 (18.5) |
| Cyclosporine | 3 (3.7) | 2 (2.5) | 2 (2.5) | 5 (5.9) | 3 (3.7) |
| Immunoglobulin (intravenous) | 5 (6.2) | 1 (1.3) | 1 (1.3) | 2 (2.4) | 0 (0.0) |
| Methotrexate | 13 (16.0) | 12 (15.0) | 17 (21.3) | 19 (22.4) | 16 (19.8) |
| Mycophenolate | 1 (1.2) | 1 (1.3) | 3 (3.8) | 8 (9.4) | 19 (23.5) |
| Biologics, used by ≥5% of patients in any country, ¶¶ n (%) | 43 (53.1) | 17 (21.3) | 55 (68.8) | 43 (50.6) | 27 (33.3) |
| Mepolizumab | 18 (22.2) | 5 (6.3) | 31 (38.8) | 12 (14.1) | 8 (9.9) |
| Benralizumab | 3 (3.7) | 3 (3.8) | 7 (8.8) | 9 (10.6) | 4 (4.9) |
| Omalizumab | 1 (1.2) | 4 (5.0) | 4 (5.0) | 6 (7.1) | 3 (3.7) |
| Reslizumab | 4 (4.9) | 0 (0.0) | 3 (3.8) | 8 (9.4) | 1 (1.2) |
| Rituximab | 26 (32.1) | 8 (10.0) | 11 (13.8) | 15 (17.6) | 14 (17.3) |
| Patients with HES (N = 280) | France n = 61 | Germany n = 53 | Italy n = 52 | Spain n = 52 | UK n = 62 |
|---|---|---|---|---|---|
| Flares (from index date to EOF) † | |||||
| Patients who experienced a flare, n (%) | 10 (16.4) | 10 (18.9) | 17 (32.7) | 14 (26.9) | 13 (21.0) |
| Number of flares per year ‡ | |||||
| Mean (SD) | 0.7 (0.6) | 0.6 (0.3) | 0.5 (0.2) | 0.3 (0.2) | 0.6 (0.3) |
| Median (IQR) | 0.4 (0.3, 0.7) | 0.5 (0.4, 1.0) | 0.5 (0.3, 0.6) | 0.3 (0.2, 0.4) | 0.6 (0.3, 0.7) |
| Cumulative duration of flare(s), ‡ months | |||||
| Mean (SD) | 4.8 (6.3) | 2.4 (1.6) | 2.1 (1.8) | 4.2 (2.9) | 1.6 (1.2) |
| Median (IQR) | 2.4 (1.0, 5.5) | 2.2 (1.1, 3.7) | 1.2 (1.0, 2.9) | 4.3 (2.0, 6.9) | 2.0 (0.4, 2.4) |
| Time to first flare from diagnosis, months | |||||
| Mean (SD) | 23.2 (15.6) | 11.4 (5.1) | 44.1 (52.7) | 30.6 (22.6) | 32.7 (34.7) |
| Median (IQR) | 24.0 (6.6, 36.5) | 11.1 (6.3, 15.0) | 22.0 (14.7, 56.8) | 29.8 (15.2, 35.9) | 25.1 (8.6, 41.1) |
| Responses (from diagnosis to EOF) § | |||||
| Patients who experienced a response, n (%) ¶ | 43 (70.5) | 48 (90.6) | 34 (65.4) | 38 (73.1) | 37 (59.7) |
| Duration of response(s), cumulative, ¶ months | |||||
| Mean (SD) | 19.3 (15.0) | 18.9 (17.6) | 14.3 (13.1) | 14.2 (12.1) | 11.7 (10.9) |
| Median (IQR) | 17.3 (6.0, 28.6) | 17.2 (5.5, 25.9) | 10.2 (2.0, 23.0) | 13.7 (5.5, 18.3) | 9.8 (3.6, 15.8) |
| Time to first response from diagnosis, months | |||||
| Mean (SD) | 14.0 (14.1) | 11.2 (9.9) | 33.2 (54.4) | 19.3 (23.4) | 23.6 (32.4) |
| Median (IQR) | 8.5 (4.9, 20.9) | 9.2 (2.4, 19.3) | 15.7 (4.9, 36.1) | 9.9 (4.6, 22.6) | 15.2 (4.1, 26.9) |
| Patients who experienced a complete response, n (%) | 26 (42.6) | 28 (52.8) | 19 (36.5) | 19 (36.5) | 21 (33.9) |
| Patients who experienced a partial response, n (%) | 17 (27.9) | 20 (37.7) | 15 (28.8) | 19 (36.5) | 18 (29.0) |
| Patients with EGPA (N = 407) | France n = 81 | Germany n = 80 | Italy n = 80 | Spain n = 85 | UK n = 81 |
| Remission status (from diagnosis to EOF) | |||||
| Patients who experienced remission, n (%) | 41 (50.6) | 57 (71.3) | 42 (52.5) | 57 (67.1) | 45 (55.6) |
| Duration of remission(s), cumulative, months ¶ | |||||
| Mean (SD) | 16.4 (16.9) | 20.8 (15.6) | 12.3 (12.9) | 14.4 (16.5) | 16.0 (13.4) |
| Median (IQR) | 11.7 (3.9, 20.6) | 15.7 (10.0, 27.8) | 6.9 (2.3, 16.2) | 8.3 (4.0, 20.0) | 12.0 (7.4, 22.2) |
| Time to first remission from diagnosis, months | |||||
| Mean (SD) | 16.4 (12.7) | 13.8 (20.5) | 38.0 (45.1) | 35.9 (46.2) | 19.8 (33.0) |
| Median (IQR) | 14.8 (6.9, 23.8) | 8.1 (3.8, 14.0) | 27.0 (17.9, 36.8) | 19.1 (12.9, 37.0) | 9.7 (6.9, 17.0) |
| Remission criteria used, n/N (%) ** | |||||
| BVAS = 0 | 5/26 (19.2) | 7/31 (22.6) | 4/27 (14.8) | 5/30 (16.7) | 3/32 (9.4) |
| OCS dosage use of ≤4.0 mg/day | 17/26 (65.4) | 23/31 (74.2) | 18/27 (66.7) | 20/30 (66.7) | 21/32 (65.6) |
| Other †† | 3/26 (11.5) | 1/31 (3.2) | 1/27 (3.7) | 4/30 (13.3) | 8/32 (25.0) |
| Missing | 3/26 (11.5) | 4/31 (12.9) | 4/27 (14.8) | 3/30 (10.0) | 5/32 (15.6) |
| Relapse status (from index date to EOF) ‡‡ | |||||
| Patients who experienced relapse, n (%) | 13 (16.0) | 7 (8.8) | 15 (18.8) | 25 (29.4) | 18 (22.2) |
| Number of relapse (among patients with a relapse per person per year) | |||||
| Mean (SD) | 0.6 (0.3) | 0.5 (0.4) | 0.9 (0.4) | 0.9 (0.8) | 1.1 (1.9) |
| Median (IQR) | 0.6 (0.3, 0.6) | 0.4 (0.3, 0.5) | 0.8 (0.6, 1.2) | 0.6 (0.3, 0.9) | 0.5 (0.3, 1.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwee, J.; Huynh, L.; Pongdee, T.; Rothenberg, M.E.; Alfonso-Cristancho, R.; da Costa Junior, W.; Duh, M.S. High Disease Burden and Oral Corticosteroid Use in Patients with Hypereosinophilic Syndrome and Eosinophilic Granulomatosis with Polyangiitis: Country-Level Insights into Real-World Management in Europe. J. Clin. Med. 2025, 14, 4309. https://doi.org/10.3390/jcm14124309
Hwee J, Huynh L, Pongdee T, Rothenberg ME, Alfonso-Cristancho R, da Costa Junior W, Duh MS. High Disease Burden and Oral Corticosteroid Use in Patients with Hypereosinophilic Syndrome and Eosinophilic Granulomatosis with Polyangiitis: Country-Level Insights into Real-World Management in Europe. Journal of Clinical Medicine. 2025; 14(12):4309. https://doi.org/10.3390/jcm14124309
Chicago/Turabian StyleHwee, Jeremiah, Lynn Huynh, Thanai Pongdee, Marc E. Rothenberg, Rafael Alfonso-Cristancho, Wilson da Costa Junior, and Mei Sheng Duh. 2025. "High Disease Burden and Oral Corticosteroid Use in Patients with Hypereosinophilic Syndrome and Eosinophilic Granulomatosis with Polyangiitis: Country-Level Insights into Real-World Management in Europe" Journal of Clinical Medicine 14, no. 12: 4309. https://doi.org/10.3390/jcm14124309
APA StyleHwee, J., Huynh, L., Pongdee, T., Rothenberg, M. E., Alfonso-Cristancho, R., da Costa Junior, W., & Duh, M. S. (2025). High Disease Burden and Oral Corticosteroid Use in Patients with Hypereosinophilic Syndrome and Eosinophilic Granulomatosis with Polyangiitis: Country-Level Insights into Real-World Management in Europe. Journal of Clinical Medicine, 14(12), 4309. https://doi.org/10.3390/jcm14124309





