Estimating Left Atrial Pressure Using Diastolic Cutoff Values After Transcatheter Mitral Valve Edge-to-Edge Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. M-TEER Procedure
2.2. LA Pressure Measurement
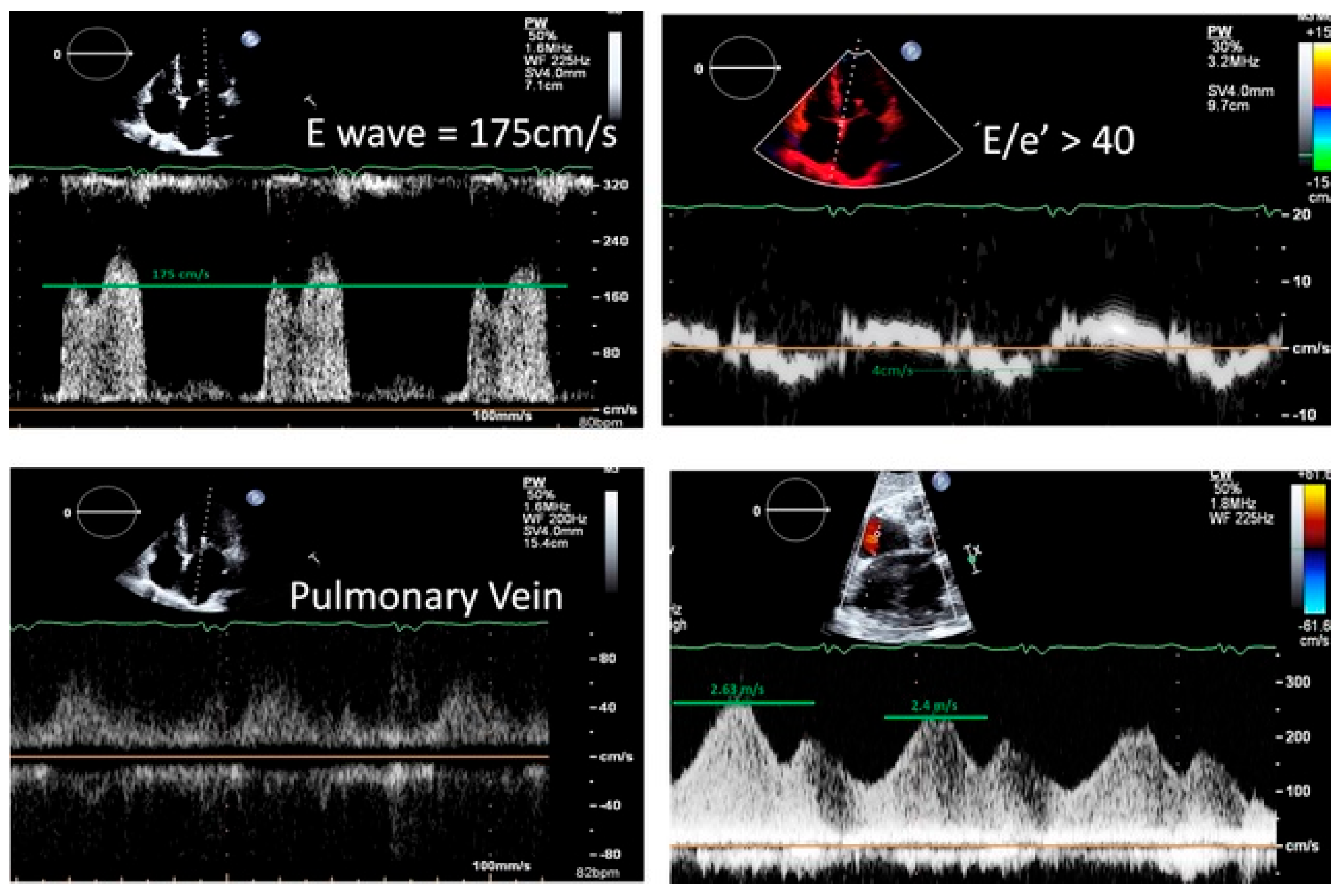
2.3. Echocardiography
2.4. Statistical Analysis
- −
- Pulmonary vein flow pattern: Blunted systolic flow pattern suggests elevated LAP.
- −
- Max flow velocity through residual ASD combined with estimated right atrial pressure: An estimated pressure less than 15 mmHg is considered normal LAP.
3. Results
3.1. Study Population
3.2. Procedural Characteristics
3.3. Effect of M-TEER on Diastolic Parameters Between Baseline and POD30
3.4. Cutoff Predictive for Normalization of Left Atrium Pressure
3.5. Validation of the Suggested Cutoff Values
4. Discussion
- Elevated LAP: Maximum E wave velocity > 163 cm/s
- Normal LAP: Maximum E wave velocity ≤ 85 cm/s
- Elevated LAP: Maximum E wave velocity > 158 cm/s OR E/e′ ratio > 34
- Normal LAP: Maximum E wave velocity ≤ 95 cm/s OR E/e′ ratio ≤ 14
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LVEF | Left ventricle ejection function |
| BSA | Body surface index |
| STS | Society of thoracic surgeons |
| CAD | coronary artery disease |
| CVA | cerebrovascular accident |
| TIA | Transient ischemic attack |
| PVD | peripheral vascular disease |
| COPD | chronic obstructive pulmonary disease |
| PCI | Percutaneous coronary intervention |
| LVEDD | Left ventricle end diastolic diameter |
| LVESD | Left ventricle end systolic diameter |
| EROA | Effective regurgitant orifice area |
| LAP | Left atrial pressure |
| PPV | positive predictive value |
| NPV | negative predictive value |
| PV | Pulmonary vein |
| ASD | Atrial septal defect |
| M-TEER | Transcatheter mitral valve edge-to-edge repair |
| MR | Mitral regurgitation |
| LV | Left ventricle |
| TEE | Transesophageal echocardiography |
| LA | Left atrium |
| LAVI | Left atrial volume index |
| RV | Right ventricle |
| IQR | Interquartile range |
Appendix A
| Abnormal LVEF | Normal LVEF | |||||
|---|---|---|---|---|---|---|
| Normal LAP | High LAP | p | Normal LAP | High LAP | p | |
| LVEF, % | 35 (30–45) | 35 (25–43.8) | 0.263 | 65 (60–66.8) | 60 (55.8–65) | 0.037 |
| LVEDD, cm | 5.5 (5–6.2) | 5.7 (5.4–6.5) | 0.031 | 4.7 (4.4–5.3) | 5 (4.5–5.5) | 0.075 |
| LVESD, cm | 4.4 (3.9–5.4) | 4.5 (3.9–5.6) | 0.430 | 3.2 (2.6–3.7) | 3.3 (3–3.7) | 0.271 |
| LV diastolic volume, mL | 165 (117–201.4) | 192 (144.5–261) | 0.044 | 113.5 (82.1–145.3) | 114.5 (87.8–142.9) | 0.880 |
| LV systolic volume, mL | 99 (61–133) | 123.5 (85.1–188.5) | 0.021 | 41 (29.3–56.3) | 44 (33–64) | 0.440 |
| Mitral regurgitation EROA, cm2 | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.470 | 0.4 (0.3–0.6) | 0.4 (0.3–0.5) | 0.059 |
| Mitral regurgitation regurgitant volume, mL | 61.5 (52–74) | 60 (47–79) | 0.454 | 70 (61–86) | 62 (52–76.5) | 0.028 |
| Left atrium volume index, mL/m2 | 63.7 (45.6–81.8) | 64.3 (48.4–93.9) | 0.620 | 59.3 (50.2–86.6) | 61.5 (44.2–85.4) | 0.589 |
| Max E wave, cm/s | 104.5 (88.4–122.5) | 110 (95–122.3) | 0.287 | 119 (96.8–135.5) | 117 (102–160) | 0.410 |
| Max A wave, cm/s | 64.8 (53.7–91.3) | 69.8 (50.3–98.2) | 0.925 | 80 (62–101.5) | 73.3 (51.5–95.8) | 0.378 |
| E/A ratio | 1.3 (1.1–2.1) | 1.7 (1.3–2) | 0.195 | 1.5 (1.1–1.8) | 1.6 (0.9–2.4) | 0.667 |
| Lateral e′, cm/s | 8 (5.9–11) | 7.1 (6–9) | 0.314 | 8.3 (7–11) | 8 (6.9–9.6) | 0.310 |
| Septal e′, cm/s | 5.1 (4–6.7) | 5 (4–5.9) | 0.562 | 6 (5.2–7.3) | 5.8 (4.4–7.4) | 0.534 |
| E/e′ | 15.4 (11.9–20.9) | 17.9 (13.6–22.5) | 0.184 | 15.1 (11.9–20.6) | 16.8 (13.5–20.7) | 0.302 |
| Tricuspid regurgitation peak velocity, cm/s | 3.1 (2.7–3.3) | 3.3 (2.9–3.5) | 0.188 | 3.3 (3–3.9) | 3.2 (2.8–3.5) | 0.215 |
References
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Feldman, T.; Foster, E.; Glower, D.D.; Glower, D.G.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; et al. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 2011, 364, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Maor, E.; Raphael, C.E.; Panaich, S.S.; Reeder, G.S.; Nishimura, R.A.; Nkomo, V.T.; Rihal, C.S.; Eleid, M.F. Acute Changes in Left Atrial Pressure After MitraClip Are Associated With Improvement in 6-Minute Walk Distance. Circ. Cardiovasc. Interv. 2017, 10, e004856. [Google Scholar] [CrossRef] [PubMed]
- Samimi, S.; Chavez Ponce, A.; Alarouri, H.S.; Shaer, A.E.; Eleid, M.F.; Guerrero, M.E.; Rihal, C.S.; Alkhouli, M. Predictors of hemodynamic response to mitral transcatheter edge-to-edge repair. Catheter. Cardiovasc. Interv. 2023, 101, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Pierre, K.; Adedinsewo, D.A.; Al-Hijji, M.; Miranda, W.R.; Alkhouli, M.; Eleid, M.F.; Guerrero, M.; Pollak, P.M.; Rihal, C.S.; El Sabbagh, A. 30-day patient reported outcomes can be predicted by change in left atrial pressure and not change in transmitral gradient following MitraClip. Catheter. Cardiovasc. Interv. 2021, 97, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Asch, F.M.; Bruce, C.; Gillam, L.D.; Grayburn, P.A.; Hahn, R.T.; Inglessis, I.; Islam, A.M.; Lerakis, S.; Little, S.H.; et al. Guidelines for the Evaluation of Valvular Regurgitation After Percutaneous Valve Repair or Replacement: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2019, 32, 431–475, Erratum in: J. Am. Soc. Echocardiogr. 2019, 32, 914–917. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Dhesi, S.; Miranda, W.R.; Geske, J.B.; Anavekar, N.S.; Morant, K.; Rihal, C.S.; Eleid, M.F.; Oh, J.K. Assessment of left ventricular filling pressure with Doppler velocities across the patent foramen ovale. J. Echocardiogr. 2021, 19, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Gavazzoni, M.; Zuber, M.; Taramasso, M.; Cascella, A.; Voci, D.; Pozzoli, A.; Ferrari, E.; Maisano, F. Transesophageal Echocardiography For The Assessment of Left Atrial Pressure After Trans-Septal Mitral Valve Interventions. Am. J. Cardiol. 2022, 177, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.; Kim, L.K.; Wong, S.C.; Cheung, J.W.; Itagaki, S.; Chikwe, J.; Egorova, N.N. Relation of Hospital Volume With In-Hospital and 90-Day Outcomes After Transcatheter Mitral Valve Repair Using MitraClip. Am. J. Cardiol. 2019, 124, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Kuwata, S.; Taramasso, M.; Czopak, A.; Luciani, M.; Pozzoli, A.; Ho, E.; Ferrero Guadagnoli, A.; Saccocci, M.; Gaemperli, O.; Nietlispach, F.; et al. Continuous Direct Left Atrial Pressure: Intraprocedural Measurement Predicts Clinical Response Following MitraClip Therapy. JACC Cardiovasc. Interv. 2019, 12, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, F.E., 3rd; Chen, J.H.; Maini, A.; Lisko, J.C.; Alvarez, L.; Kamioka, N.; Reginauld, S.; Gleason, P.T.; Condado, J.F.; Wei, J.W.; et al. Pulmonary Venous Waveforms Predict Rehospitalization and Mortality After Percutaneous Mitral Valve Repair. JACC Cardiovasc. Imaging. 2019, 12, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Marwick, T.H. Use and Limitations of E/e′ to Assess Left Ventricular Filling Pressure by Echocardiography. J. Cardiovasc. Ultrasound. 2011, 19, 169–173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jensen, J.L.; Williams, F.E.; Beilby, B.J.; Johnson, B.L.; Miller, L.K.; Ginter, T.L.; Tomaselli-Martin, G.; Appleton, C.P. Feasibility of obtaining pulmonary venous flow velocity in cardiac patients using transthoracic pulsed wave Doppler technique. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 1997, 10, 60–66. [Google Scholar] [CrossRef]
- Lavall, D.; Mehrer, M.; Schirmer, S.H.; Reil, J.-C.; Wagenpfeil, S.; Böhm, M.; Laufs, U. Long-Term Hemodynamic Improvement after Transcatheter Mitral Valve Repair. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2018, 31, 1013–1020. [Google Scholar] [CrossRef]
- Öztürk, C.; Fasell, T.; Sinning, J.; Werner, N.; Nickenig, G.; Hammerstingl, C.; Schueler, R. Left atrial global function in chronic heart failure patients with functional mitral regurgitation after MitraClip. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2020, 96, 678–684. [Google Scholar] [CrossRef]
- Giannini, C.; Petronio, A.S.; De Carlo, M.; Guarracino, F.; Conte, L.; Fiorelli, F.; Pieroni, A.; Di Bello, V. Integrated reverse left and right ventricular remodelling after MitraClip implantation in functional mitral regurgitation: An echocardiographic study. Eur. Heart Journal. Cardiovasc. Imaging 2014, 15, 95–103. [Google Scholar] [CrossRef]
- Biersmith, M.; Orsinelli, D.A.; Harfi, T.T.; Lilly, S.; Boudoulas, K.D. Effect of mitral valve transcatheter edge-to-edge repair on indices of left atrial performance in chronic mitral regurgitation. Echocardiography 2022, 39, 1420–1425. [Google Scholar] [CrossRef]
- Yokoyama, H.; Ruf, T.F.; Geyer, M.; Tamm, A.R.; Da Rocha ESilva, J.G.; Gößler, T.A.M.; Zirbs, J.; Schwidtal, B.; Münzel, T.; von Bardeleben, R.S. Reverse cardiac remodeling in patients undergoing combination therapy of transcatheter mitral valve repair. Front. Cardiovasc. Med. 2023, 10, 1029103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| All (n = 240) | Abnormal LVEF (n = 122) | Normal LVEF (n = 118) | p Value | |
|---|---|---|---|---|
| Age, years * | 82.5 (74.4–87.4) | 80.9 (72.3–85.8) | 84.6 (75.4–89.2) | 0.004 |
| Female, % | 117 (49) | 48 (39) | 69 (58) | 0.003 |
| BSA, m2 | 1.8 ± 0.26 | 1.8 ± 0.25 | 1.7 ± 0.26 | 0.003 |
| STS–Mitral Valve Repair, % * | 6.74% (3.64–10.5) | 7.44% (3.98–11.65) | 5.87% (3.07–9) | 0.006 |
| Baseline Creatinine, mg/dL * | 1.3 (1–1.8) | 1.4 (1.1–2) | 1.1 (0.9–1.5) | <0.001 |
| Frailty, % | 183 (76) | 84 (69) | 99 (84) | 0.006 |
| Scoliosis, % | 13 (5) | 1 (1) | 12 (10) | 0.001 |
| CAD, % | 92 (38) | 62 (51) | 30 (25) | <0.001 |
| Diabetes mellitus, % | 53 (22) | 34 (28) | 19 (16) | 0.028 |
| CVA/TIA, % | 33 (14) | 17 (14) | 16 (14) | 0.933 |
| PVD, % | 23 (10) | 14 (11) | 9 (8) | 0.311 |
| COPD, % | 61 (26) | 29 (24) | 32 (27) | 0.268 |
| Immunocompromised, % | 16 (7) | 8 (7) | 8 (7) | 0.945 |
| Atrial fibrillation, % | 116 (48) | 64 (52) | 52 (44) | 0.193 |
| Dialysis, % | 26 (11) | 21 (17) | 5 (4) | 0.001 |
| Liver disease, % | 7 (3) | 2 (2) | 5 (4) | 0.232 |
| Porcelain Aorta, % | 4 (2) | 3 (2) | 1 (1) | 0.33 |
| Prior PCI, % | 57 (24) | 45 (37) | 12 (10) | <0.001 |
| Cardiac leads, % | 58 (24) | 44 (36) | 14 (12) | <0.001 |
| All (n = 240) | Abnormal LVEF (n = 122) | Normal LVEF (n = 118) | p Value | ||
|---|---|---|---|---|---|
| LVEF, % * | 52.5 (35–61) | 35 (25–45) | 61.5 (58–65) | <0.001 | |
| LVEDD, cm * | 5.2 (4.6–5.9) | 5.6 (5.2–6.4) | 4.9 (4.4–5.3) | <0.001 | |
| LVESD, cm * | 3.7 (3.1–4.5) | 4.5 (3.9–5.5) | 3.2 (2.9–3.7) | <0.001 | |
| LV systolic volume, mL * | 64.5 (40–109.9) | 108 (68–157) | 41 (32–60) | <0.001 | |
| LV diastolic volume, mL * | 138 (96–196) | 176.2 (124–231) | 113.5 (84–143) | <0.001 | |
| Mitral regurgitation EROA, cm2 * | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.062 | |
| Mitral regurgitation Regurgitant volume, mL * | 64 (52–78) | 61 (50–75) | 67.5 (57–81) | 0.013 | |
| Mitral regurgitation etiology | Primary | 96 (40) | 18 (15) | 78 (66) | <0.001 |
| Secondary | 144 (60) | 104 (85) | 40 (34) | <0.001 | |
| Tricuspid regurgitation, % | None/Trace | 23 (10) | 13 (11) | 10 (9) | 0.38 |
| ≤Moderate | 164 (68) | 86 (70) | 78 (65) | ||
| Moderate to Severe | 27 (11) | 12 (10) | 15 (13) | ||
| Severe | 26 (11) | 11 (9) | 15 (13) | ||
| Aortic regurgitation, % | None/Trace | 122 (51) | 67 (55) | 55 (47) | 0.43 |
| ≤Moderate | 116 (48) | 54 (44) | 62 (53) | ||
| >Moderate | 2 (1) | 1 (1) | 1 (1) | ||
| Right ventricle size, % | Normal | 146 (61) | 69 (56) | 77 (65) | 0.57 |
| ≤Moderate | 82 (34) | 46 (38) | 36 (31) | ||
| >Moderate | 12 (5) | 7 (6) | 5 (4) | ||
| Right ventricle function, % | Normal | 137 (57) | 53 (43) | 82 (69) | <0.001 |
| ≤Moderate | 90 (38) | 60 (49) | 32 (27) | ||
| >Moderate | 13 (5) | 9 (7) | 4 (3) | ||
| All (n = 240) | Abnormal LVEF (n = 122) | Normal LVEF (n = 118) | p Value | ||
|---|---|---|---|---|---|
| Left atrium pressure (baseline), mmHg * | 20 (15–25) | 20 (15–25) | 20 (15–25) | 0.435 | |
| Left atrium V wave (baseline), mmHg * | 35 (25–46) | 35 (25–45) | 40 (30–47) | 0.122 | |
| Clip Location | A1–P1 | 13 (5) | 2 (2) | 11 (9) | 0.009 |
| A2–P2 | 223 (93) | 118 (97) | 105 (89) | 0.019 | |
| A3–P3 | 18 (8) | 5 (4) | 13 (11) | 0.042 | |
| Clip generation (G4) | 70 (29) | 32 (26) | 38 (32) | NS | |
| Left atrium pressure (procedure end), mmHg * | 13 (10–18) | 14 (10–18) | 13 (10–16) | 0.95 | |
| Left atrium pressure change, mmHg * | 6 (4–10) | 6 (5–10) | 7 (4–10) | 0.465 | |
| Left atrium V wave (procedure end), mmHg * | 20 (15–25) | 14 (10–18) | 13 (10–16) | 0.725 | |
| Left atrium V wave pressure change, mmHg * | 15 (10–20) | 13 (9–20) | 15 (10–25) | 0.124 | |
| Normal Left atrium pressure post procedure (<15 mmHg) | 130 (54) | 62 (51) | 68 (58) | 0.29 | |
| Abnormal LVEF | Normal LVEF | |||||
|---|---|---|---|---|---|---|
| Pre | 30 Day Follow Up | p | Pre | 30 Day Follow Up | p | |
| Mitral regurgitation grade | 4 (3.5–4) | 2 (2–2.5) | <0.001 | 4 (3.5–4) | 2 (2–2.5) | <0.001 |
| Max E wave, cm/s * | 100 (83–113) | 111 (77.6–142) | 0.044 | 107 (89.7–120.8) | 125 (91–152) | 0.08 |
| Lateral e′, cm/s * | 7 (5–10) | 5.6 (3.9–7.1) | 0.001 | 8 (6.2–9.5) | 6 (5–7.3) | <0.001 |
| septal e′, cm/s * | 5 (3.9–6) | 4.2 (3–5.2) | 0.061 | 5.9 (4.4–6.6) | 4.2 (3.4–5.1) | 0.001 |
| E/e′ * | 16.8 (10.7–20.5) | 24.3 (18–31.4) | 0.001 | 15.1 (12.2–20.3) | 22.9 (17–29.3) | <0.001 |
| Tricuspid regurgitation peak velocity, cm/s * | 3.1 (2.6–3.3) | 3 (2.8–3.4) | 0.853 | 3.2 (2.8–3.5) | 3 (2.7–3.3) | 0.567 |
| Left atrium volume index, mL/m2 * | 66.4 (46.6–83.1) | 61.4 (44.7–86.6) | 0.379 | 55.1 (44–75.9) | 64.9 (40.1–72) | 0.586 |
| Cut-Off | Normal LAP * | High LAP * | ROC † | p | |
|---|---|---|---|---|---|
| Normal LVEF | |||||
| E max, cm/s | ≤85 | 9 (17) | 1 (2.4) | 0.723 (0.584–0.862) | 0.002 |
| 85–163 | 36 (68) | 22 (54) | |||
| >163 | 8 (15) | 18 (44) | |||
| Abnormal LVEF | |||||
| E Max, cm/s | ≤95 | 19 (37) | 4 (10) | 0.742 (0.57–0.914) | 0.006 |
| 95–158 | 31 (60) | 26 (62) | |||
| >158 | 2 (4) | 12 (29) | |||
| E/e′ ratio | ≤14 | 10 (20) | 1 (2) | 0.861 (0.732–0.991) | <0.001 |
| 14–34 | 37 (74) | 23 (56) | |||
| >34 | 3 (6) | 17 (42) | |||
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|
| Normal LVEF | |||||
| E max ≤ 85 cm/s | 20 | 98 | 90 | 52 | 52 |
| E max < 163 cm/s | 85 | 44 | 66 | 69 | 67 |
| Abnormal LVEF | |||||
| E max ≤ 95 cm/s | 37 | 90 | 83 | 54 | 61 |
| E max < 158 cm/s | 96 | 29 | 63 | 86 | 74 |
| E/e′ ≤ 14 | 20 | 98 | 91 | 50 | 55 |
| E/e′ < 34 | 94 | 41 | 66 | 85 | 70 |
| LAP Based on PV/ASD Flow | |||
|---|---|---|---|
| LAP based on suggested cutoff values | Total | Normal * | Elevated * |
| Normal † | 25 | 24 (96) | 1 (4) |
| Indeterminate | 44 | 22 (50) | 22 (50) |
| Elevated ‡ | 30 | 3 (10) | 27 (90) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niv Granot, Y.; Passaniti, G.; Sharma, S.; Kini, A.; Karlsberg, D.; Khera, S.; Tang, G.H.L.; Lerakis, S.; Safi, L.M. Estimating Left Atrial Pressure Using Diastolic Cutoff Values After Transcatheter Mitral Valve Edge-to-Edge Repair. J. Clin. Med. 2025, 14, 4308. https://doi.org/10.3390/jcm14124308
Niv Granot Y, Passaniti G, Sharma S, Kini A, Karlsberg D, Khera S, Tang GHL, Lerakis S, Safi LM. Estimating Left Atrial Pressure Using Diastolic Cutoff Values After Transcatheter Mitral Valve Edge-to-Edge Repair. Journal of Clinical Medicine. 2025; 14(12):4308. https://doi.org/10.3390/jcm14124308
Chicago/Turabian StyleNiv Granot, Yoav, Giulia Passaniti, Samin Sharma, Annapoorna Kini, Daniel Karlsberg, Sahil Khera, Gilbert H. L. Tang, Stamatios Lerakis, and Lucy M. Safi. 2025. "Estimating Left Atrial Pressure Using Diastolic Cutoff Values After Transcatheter Mitral Valve Edge-to-Edge Repair" Journal of Clinical Medicine 14, no. 12: 4308. https://doi.org/10.3390/jcm14124308
APA StyleNiv Granot, Y., Passaniti, G., Sharma, S., Kini, A., Karlsberg, D., Khera, S., Tang, G. H. L., Lerakis, S., & Safi, L. M. (2025). Estimating Left Atrial Pressure Using Diastolic Cutoff Values After Transcatheter Mitral Valve Edge-to-Edge Repair. Journal of Clinical Medicine, 14(12), 4308. https://doi.org/10.3390/jcm14124308






