Fast-Track Protocol for Carotid Surgery
Abstract
1. Introduction
2. Materials and Methods
Statistical Methods
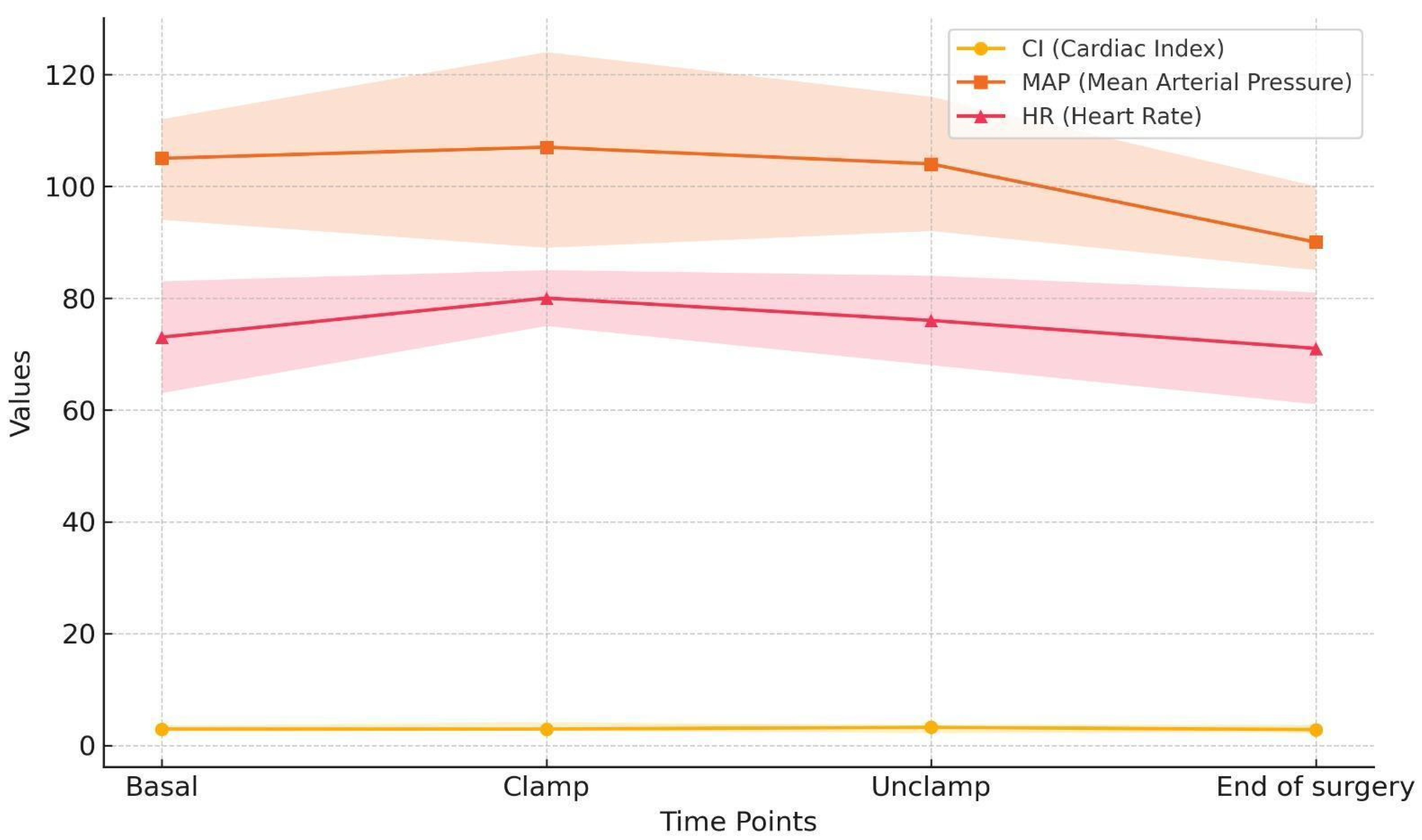
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FT | fast track |
| CEA | carotid endarterectomy |
| NRS | numerical rating scale |
| ICU | intensive care unit |
| CAS | carotid artery stenting |
| SAP | systolic arterial pressure |
| CTA | computed tomography angiography |
| DUS | duplex ultrasound |
| LA | local anesthesia |
| GA | general anesthesia |
| DAPT | dual antiplatelet therapy |
| ASA | American Society of Anesthesiologists |
| ERAS | enhanced recovery after surgery |
| ESVS | European Society of Vascular Surgery |
Appendix A
Humanitas University Fast-Track Protocol for Carotid Surgery
- Surgical indication
- Preoperative assessment
- Hospital admission
- Perioperative blood pressure management
- Surgical timing
- Anesthesia Protocol
- Surgical technique
- Neurological monitoring
- Shunting
- Hemodynamic monitoring
- Anticoagulation strategy
- Intraoperative imaging
- Heparin reversal
- Drainage strategy
- Light wound dressing
- Selective transfer to an intensive care unit
- Postoperative monitoring
- Early postoperative recovery programs
- Discharge
References
- Kehlet, H.; Wilmore, D.W. Evidence-based surgical care and the evolution of fast-track surgery. Ann. Surg. 2008, 248, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.K.; Madsen, C.; Andersen, N.T.; Søballe, K. Efficacy of multimodal optimization of mobilization and nutrition in patients undergoing hip replacement: A randomized clinical trial. Acta Anaesthesiol. Scand. 2006, 50, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Husted, H.; Troelsen, A.; Otte, K.S.; Kristensen, B.B.; Holm, G.; Kehlet, H. Fast-track surgery for bilateral total knee replacement. J. Bone Jt. Surg. Br. 2011, 93, 351–356. [Google Scholar] [CrossRef]
- Holm, B.; Kristensen, M.T.; Myhrmann, L.; Husted, H.; Andersen, L.Ø.; Kristensen, B.; Kehlet, H. The role of pain for early rehabilitation in fast track total knee arthroplasty. Disabil. Rehabil. 2010, 32, 300–306. [Google Scholar] [CrossRef]
- Andersen, L.Ø.; Gaarn-Larsen, L.; Kristensen, B.B.; Husted, H.; Otte, K.S.; Kehlet, H. Subacute pain and function after fast-track hip and knee arthroplasty. Anaesthesia 2009, 64, 508–513. [Google Scholar] [CrossRef]
- Munitiz, V.; Martinez-de-Haro, L.F.; Ortiz, A.; Ruiz-de-Angulo, D.; Pastor, P.; Parrilla, P. Effectiveness of a written clinical pathway for enhanced recovery after transthoracic (Ivor Lewis) oesophagectomy. Br. J. Surg. 2010, 97, 714–718. [Google Scholar] [CrossRef]
- Malik, K.; Poletto, G.; Musto, L.; Giustiniano, E.; Cecconi, M.; Civilini, E. Implementation of a perioperative protocol to enhance open aortic repair. J. Vasc. Surg. 2021, 74, 434–441.e2. [Google Scholar] [CrossRef] [PubMed]
- Muehling, B.; Schelzig, H.; Steffen, P.; Meierhenrich, R.; Sunder-Plassmann, L.; Orend, K.H. A prospective randomized trial comparing traditional and fast-track patient care in elective open infrarenal aneurysm repair. World J. Surg. 2009, 33, 577–585. [Google Scholar] [CrossRef]
- Muehling, B.M.; Halter, G.; Lang, G.; Schelzig, H.; Steffen, P.; Wagner, F.; Meierhenrich, R.; Sunder-Plassmann, L.; Orend, K.-H. Prospective randomized controlled trial to evaluate “fast-track” elective open infrarenal aneurysm repair. Langenbecks Arch. Surg. 2008, 393, 281–287. [Google Scholar] [CrossRef]
- Brustia, P.; Renghi, A.; Aronici, M.; Gramaglia, L.; Porta, C.; Musiani, A.; Martelli, M.; Casella, F.; De Simeis, M.L.; Coppi, G.; et al. Fast-track in abdominal aortic surgery: Experience in over 1000 patients. Ann. Vasc. Surg. 2015, 29, 1151–1159. [Google Scholar] [CrossRef]
- Murphy, M.A.; Richards, T.; Atkinson, C.; Perkins, J.; Hands, L.J. Fast track open aortic surgery: Reduced post operative stay with a goal directed pathway. Eur. J. Vasc. Endovasc. Surg. 2007, 34, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Podore, P.C.; Throop, E.B. Infrarenal aortic surgery with a 3-day hospital stay: A report on success with a clinical pathway. J. Vasc. Surg. 1999, 29, 787–792. [Google Scholar] [CrossRef]
- Tatsuishi, W.; Kohri, T.; Kodera, K.; Asano, R.; Kataoka, G.; Kubota, S.; Nakano, K. Usefulness of an enhanced recovery after surgery protocol for perioperative management following open repair of an abdominal aortic aneurysm. Surg. Today 2012, 42, 1195–1200. [Google Scholar] [CrossRef]
- Debus, E.S.; Ivoghli, A.; Goepfert, M.; Kölbel, T.; Larena-Avellaneda, A. Perioperative management and “Fast-Track” therapy in vascular medicine. Vasa 2011, 40, 281–288. [Google Scholar] [CrossRef]
- Halliday, A.; Bulbulia, R.; Bonati, L.H.; Chester, J.; Cradduck-Bamford, A.; Peto, R.; Pan, H.; Potter, J.; Eckstein, H.H.; Farrell, B.; et al. Second asymptomatic carotid surgery trial (ACST-2): A randomised comparison of carotid artery stenting versus carotid endarterectomy. Lancet 2021, 398, 1065–1073. [Google Scholar] [CrossRef]
- Khan, A.A.; Chaudhry, S.A.; Sivagnanam, K.; Hassan, A.E.; Suri, M.F.K.; Qureshi, A.I. Cost-effectiveness of carotid artery stent placement versus endarterectomy in patients with carotid artery stenosis. J. Neurosurg. 2012, 117, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.; Rantner, B.; Ancetti, S.; de Borst, G.J.; De Carlo, M.; Halliday, A.; Kakkos, S.K.; Markus, H.S.; McCabe, D.J.; Sillesen, H.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 7–111. [Google Scholar] [CrossRef] [PubMed]
- Skydell, J.L.; Machleder, H.I.; Baker, J.D.; Busuttil, R.W.; Moore, W.S. Incidence and Mechanism of Post—Carotid Endarterectomy Hypertension. Arch. Surg. 1987, 122, 1153–1155. [Google Scholar] [CrossRef]
- Demirel, S.; Goossen, K.; Bruijnen, H.; Probst, P.; Böckler, D. Systematic review and meta-analysis of postcarotid endarterectomy hypertension after eversion versus conventional carotid endarterectomy. J. Vasc. Surg. 2017, 65, 868–882. [Google Scholar] [CrossRef]
- Rivolta, N.; Piffaretti, G.; Corazzari, C.; Bush, R.L.; Dorigo, W.; Tozzi, M.; Franchin, M. To drain or not to drain following carotid endarterectomy: A systematic review and meta-analysis. J. Cardiovasc. Surg. 2021, 62, 347–353. [Google Scholar] [CrossRef]
- Kakisis, J.D.; Antonopoulos, C.N.; Mantas, G.; Moulakakis, K.G.; Sfyroeras, G.; Geroulakos, G. Cranial Nerve Injury After Carotid Endarterectomy: Incidence, Risk Factors, and Time Trends. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 320–335. [Google Scholar] [CrossRef] [PubMed]
- Guay, J. Regional or general anesthesia for carotid endarterectomy? Evidence from published prospective and retrospective studies. J. Cardiothorac. Vasc. Anesth. 2007, 21, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Rerkasem, A.; Orrapin, S.; Howard, D.P.J.; Nantakool, S.; Rerkasem, K. Local versus general anaesthesia for carotid endarterectomy. Cochrane Database Syst Rev. 2021, 2021, CD000126. [Google Scholar]
- Gomes, M.; Soares, M.O.; Dumville, J.C.; Lewis, S.C.; Torgerson, D.J.; Bodenham, A.R.; Gough, M.J.; Warlow, C.P. Cost-effectiveness analysis of general anaesthesia versus local anaesthesia for carotid surgery (GALA Trial). Br. J. Surg. 2010, 97, 1218–1225. [Google Scholar] [CrossRef][Green Version]
- Chuatrakoon, B.; Nantakool, S.; Rerkasem, A.; Orrapin, S.; Howard, D.P.J.; Rerkasem, K. Routine or selective carotid artery shunting for carotid endarterectomy (and different methods of monitoring in selective shunting). Cochrane Database Syst Rev. 2022, 2022, CD000190. [Google Scholar]
- Smolock, C.J.; Morrow, K.L.; Kang, J.; Kelso, R.L.; Bena, J.F.; Clair, D.G. Drain placement confers no benefit after carotid endarterectomy in the Vascular Quality Initiative. J. Vasc. Surg. 2020, 72, 204–208.e1. [Google Scholar] [CrossRef]
- Saha, S.P.; Saha, S.; Vyas, K.S. Carotid Endarterectomy: Current Concepts and Practice Patterns. Int. J. Angiol. 2015, 24, 223–235. [Google Scholar] [CrossRef]
- Rismiati, H.; Lee, H.Y. Perioperative Management of Hypertensive Patients. Cardiovasc. Prev. Pharmacother. 2021, 3, 54–63. [Google Scholar] [CrossRef]
- Dalby Kristensen, S.; Knuuti, J.; Saraste, A.; Anker, S.; Erik Bøtker, H.; De Hert, S.; Ford, I.; Gonzalez-Juanatey, J.R.; Gorenek, B.; Heyndrickx, G.R.; et al. ESC/ESA GUIDELINES 2014 ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management The Joint Task Force on non-cardiac surgery: Cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur. Heart J. 2014, 35, 2383–2431. [Google Scholar]
- Tang, T.Y.; Walsh, S.R.; Gillard, J.H.; Varty, K.; Boyle, J.R.; Gaunt, M.E. Carotid sinus nerve blockade to reduce blood pressure instability following carotid endarterectomy: A systematic review and meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2007, 34, 304–311. [Google Scholar] [CrossRef]
- Wind, G.G.; Valentine, R.J. Anatomic Exposures in Vascular Surgery, 3rd ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 41–46. [Google Scholar]
- Becquemin, J.-P.; Marzelle, J. Chirurgie carotidienne: Techniques endovasculaires et stratégie de traitement. EMC—Tech. Chir. —Chir. Vasc. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Litvinova, O.; Bilir, A.; Parvanov, E.D.; Niebauer, J.; Kletecka-Pulker, M.; Kimberger, O.; Atanasov, A.G.; Willschke, H. Patent landscape review of non-invasive medical sensors for continuous monitoring of blood pressure and their validation in critical care practice. Front. Med. 2023, 10, 1138051. [Google Scholar] [CrossRef] [PubMed]
| Preoperative | |
|---|---|
| Surgical intervention indication | Two duplex ultrasound examinations, conducted by two different operators. |
| Hospital admission | The patients are admitted right before surgery. |
| Blood pressure management. | angiotensin receptor blockers, ACE inhibitors, calcium channel blockers |
| Intraoperative | |
| Surgical timing | The CEA procedure is performed during the morning surgical session, ensuring at least 6 h of close postoperative monitoring before night shift. |
| Anesthesia Protocol | Local anesthesia Echo-guided superficial cervical plexus block plus infiltration of local anesthetic along the cutaneous incision line (up to a maximum of ropivacaine 75 mg, lidocaine 200 mg) |
| Neurological monitoring | Clinical examination (allowing selective shunting only for patients showing neurological impairment) Movement check, time/place orientation assessment, basic cognitive task performance |
| Patient coagulation management | Heparin before clamping 60–100 U/kg and a target ACT of 200–250 Protamine to reverse half heparin dose if intraoperative check shows no technical defects. |
| Surgical technique | Eversion |
| Intraoperative control | Completion angiography |
| Postoperative latero-cervical drainage | Selective |
| Dressing | Light wound dressing |
| Postoperative | |
| Transfer to an intensive care unit | Multimorbid patients or severe intraoperative complications |
| Postoperative monitoring | At the end of the surgical procedure: close monitoring in the operating recovery room for one hour At readmission to the ward: nursing staff, along with medical personnel, perform a neurological examination to assess any changes compared to the preoperative state. An ECG is performed, and the patient is monitored using telemetry. On postoperative day 0: Vital signs are measured every 3 h, concurrently assessing the trachea alignment and the potential presence of cervical hematoma. |
| Early postoperative recovery programs | Patients are mobilized 4 h postoperatively, allowed to drink after 2–4 h, and have a light dinner on day 0. |
| Discharge 1st postoperative day | Yes if no pain, no ECG changes, hemodynamic stability, no neck hematoma, no cranial nerve injury, easy access to hospital readmission |
| Age (mean) | 74 |
| Age > 80 | 234 (27) |
| Sex | M 544 (64); F 309 (36) |
| Arterial Hypertension | 738 (86) |
| Dyslipidemia | 698 (82) |
| Diabetes Mellitus | 244 (29) |
| Smoking (active or former) | 182 (21) |
| COPD | 134 (16) |
| CKD | 85 (10) |
| Ischemic heart disease | 297 (35) |
| ASA SCORE (3–4) | 370 (43) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baronetto, N.; Brizzi, S.; Pignataro, A.; Nisi, F.; Giustiniano, E.; Barillà, D.; Civilini, E. Fast-Track Protocol for Carotid Surgery. J. Clin. Med. 2025, 14, 4294. https://doi.org/10.3390/jcm14124294
Baronetto N, Brizzi S, Pignataro A, Nisi F, Giustiniano E, Barillà D, Civilini E. Fast-Track Protocol for Carotid Surgery. Journal of Clinical Medicine. 2025; 14(12):4294. https://doi.org/10.3390/jcm14124294
Chicago/Turabian StyleBaronetto, Noemi, Stefano Brizzi, Arianna Pignataro, Fulvio Nisi, Enrico Giustiniano, David Barillà, and Efrem Civilini. 2025. "Fast-Track Protocol for Carotid Surgery" Journal of Clinical Medicine 14, no. 12: 4294. https://doi.org/10.3390/jcm14124294
APA StyleBaronetto, N., Brizzi, S., Pignataro, A., Nisi, F., Giustiniano, E., Barillà, D., & Civilini, E. (2025). Fast-Track Protocol for Carotid Surgery. Journal of Clinical Medicine, 14(12), 4294. https://doi.org/10.3390/jcm14124294







