Sex Differences in Non-Acute Myocardial Infarction Cardiogenic Shock: Insights from the Northwell-Shock Registry
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Shock Etiologies and Severity of Shock
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Specific Etiologies and Shock Severity
3.3. Management Strategies
3.4. Complications in Advanced Mechanical Circulatory Support
3.5. Hospital Outcomes
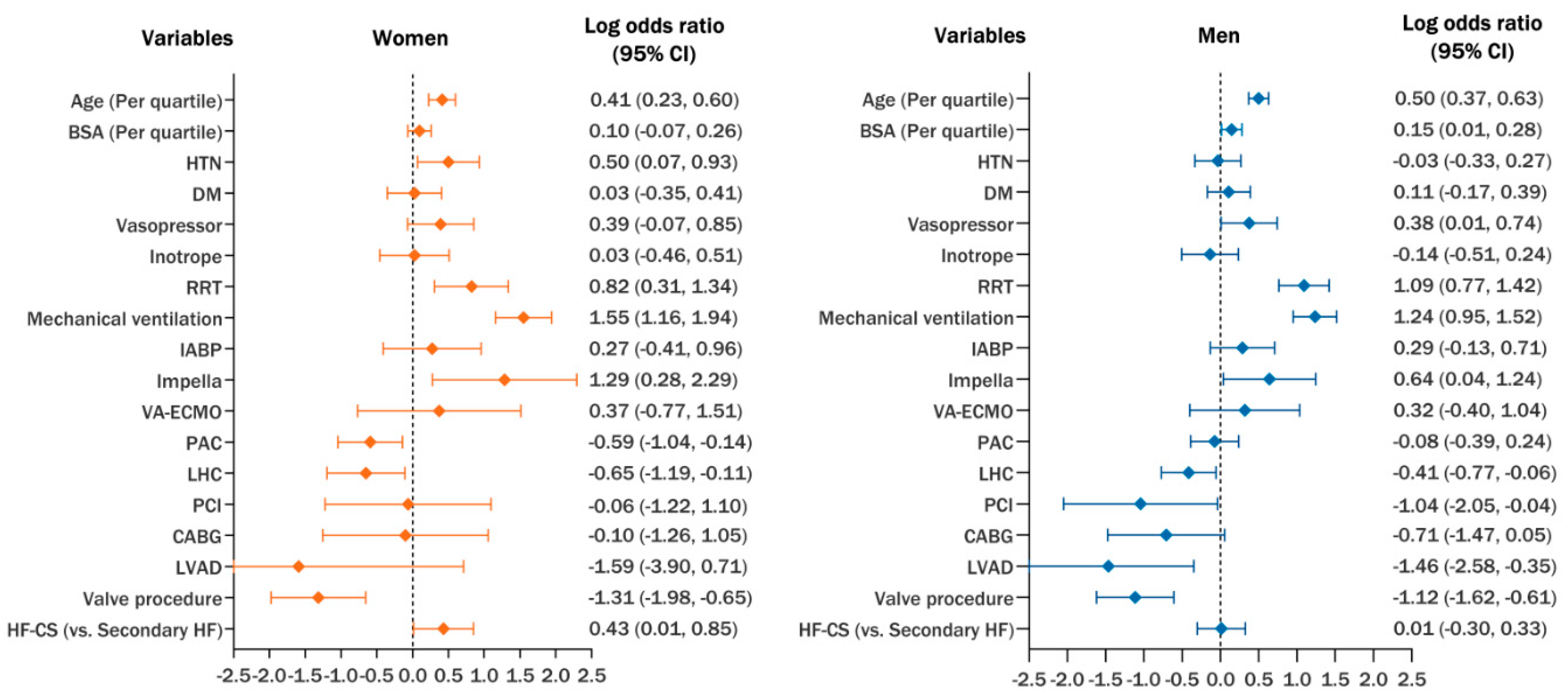
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| CS | Cardiogenic shock |
| CSWG | Cardiogenic shock working group |
| SCAI | Society for Cardiovascular Angiography and Intervention |
| MCS | Mechanical circulatory support |
| AMI-CS | Acute myocardial infarction-related cardiogenic shock |
| nonAMI-CS | non-acute myocardial infarction-related cardiogenic shock |
| ICD | Internation classification of diseases |
| CKD | Chronic kidney disease |
| CAD | Coronary artery disease |
| VA-ECMO | Venoarterial extracorporeal membrane oxygenation |
| mAFP | Microaxial flow pump |
| LVAD | Left ventricular assist device |
| VIS | Vasoactive-inotropic score |
| BARC | Bleeding Academic Research Consortium |
| RRT | Renal replacement therapy |
| SHARC | Shock Academic Research Consortium |
| ACHF | Acute on chronic heart failure |
| VCS | Valvular cardiogenic shock |
| HF-CS | Heart failure-related cardiogenic shock |
| PAC | Pulmonary artery catheter |
| IABP | Intra-aortic balloon pump |
| LHC | Left heart catheterization |
| PCI | Percutaneous coronary intervention |
| ICU | Intensive care unit |
| CABG | Coronary artery bypass graft |
| BSA | Body surface area |
| eGFR | Glomerular filtration rate |
| SNF | Skilled nursing facility |
References
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, 1757–1780. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Choi, K.H.; Ko, Y.G.; Ahn, C.M.; Yu, C.W.; Chun, W.J.; Jang, W.J.; Kim, H.J.; Kim, B.S.; Bae, J.W.; et al. Clinical Characteristics and Predictors of In-Hospital Mortality in Patients With Cardiogenic Shock: Results From the RESCUE Registry. Circ. Heart Fail. 2021, 14, e008141. [Google Scholar] [CrossRef] [PubMed]
- Nordan, T.; Critsinelis, A.C.; Mahrokhian, S.H.; Kapur, N.K.; Vest, A.; DeNofrio, D.; Chen, F.Y.; Couper, G.S.; Kawabori, M. Microaxial Left Ventricular Assist Device Versus Intraaortic Balloon Pump as a Bridge to Transplant. Ann. Thorac. Surg. 2022, 114, 160–166. [Google Scholar] [CrossRef]
- Tehrani, B.N.; Truesdell, A.G.; Psotka, M.A.; Rosner, C.; Singh, R.; Sinha, S.S.; Damluji, A.A.; Batchelor, W.B. A Standardized and Comprehensive Approach to the Management of Cardiogenic Shock. JACC Heart Fail. 2020, 8, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.H.; Perl, L.; Assali, A.; Codner, P.; Greenberg, G.; Samara, A.; Porter, A.; Orvin, K.; Kornowski, R.; Vaknin Assa, H. The Impact of Sex on Cardiogenic Shock Outcomes Following ST Elevation Myocardial Infarction. J. Clin. Med. 2023, 12, 6259. [Google Scholar] [CrossRef]
- Elgendy, I.Y.; Wegermann, Z.K.; Li, S.; Mahtta, D.; Grau-Sepulveda, M.; Smilowitz, N.R.; Gulati, M.; Garratt, K.N.; Wang, T.Y.; Jneid, H. Sex Differences in Management and Outcomes of Acute Myocardial Infarction Patients Presenting With Cardiogenic Shock. JACC Cardiovasc. Interv. 2022, 15, 642–652. [Google Scholar] [CrossRef]
- Ton, V.K.; Kanwar, M.K.; Li, B.; Blumer, V.; Li, S.; Zweck, E.; Sinha, S.S.; Farr, M.; Hall, S.; Kataria, R.; et al. Impact of Female Sex on Cardiogenic Shock Outcomes: A Cardiogenic Shock Working Group Report. JACC Heart Fail. 2023, 11, 1742–1753. [Google Scholar] [CrossRef]
- Daniels, L.B.; Phreaner, N.; Berg, D.D.; Bohula, E.A.; Chaudhry, S.P.; Fordyce, C.B.; Goldfarb, M.J.; Katz, J.N.; Kenigsberg, B.B.; Lawler, P.R.; et al. Sex Differences in Characteristics, Resource Utilization, and Outcomes of Cardiogenic Shock: Data From the Critical Care Cardiology Trials Network (CCCTN) Registry. Circ. Cardiovasc. Qual. Outcomes 2024, 17, e010614. [Google Scholar] [CrossRef]
- Alvarez Villela, M.; Fu, D.; Roslin, K.; Smoller, R.; Asemota, D.; Miklin, D.J.; Kodra, A.; Vullaganti, S.; Roswell, R.O.; Rangasamy, S.; et al. Defining levels of care in cardiogenic shock. Front. Cardiovasc. Med. 2023, 10, 1206570. [Google Scholar] [CrossRef]
- Sundararajan, V.; Henderson, T.; Perry, C.; Muggivan, A.; Quan, H.; Ghali, W.A. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J. Clin. Epidemiol. 2004, 57, 1288–1294. [Google Scholar] [CrossRef]
- Gaies, M.G.; Gurney, J.G.; Yen, A.H.; Napoli, M.L.; Gajarski, R.J.; Ohye, R.G.; Charpie, J.R.; Hirsch, J.C. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr. Crit. Care Med. 2010, 11, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Pahuja, M.; van Diepen, S.; Proudfoot, A.G.; Morrow, D.; Spitzer, E.; Nichol, G.; Weisfeldt, M.L.; Moscucci, M.; Lawler, P.R.; et al. Standardized Definitions for Cardiogenic Shock Research and Mechanical Circulatory Support Devices: Scientific Expert Panel From the Shock Academic Research Consortium (SHARC). Circulation 2023, 148, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K.; Kanwar, M.; Sinha, S.S.; Thayer, K.L.; Garan, A.R.; Hernandez-Montfort, J.; Zhang, Y.; Li, B.; Baca, P.; Dieng, F.; et al. Criteria for Defining Stages of Cardiogenic Shock Severity. J. Am. Coll. Cardiol. 2022, 80, 185–198. [Google Scholar] [CrossRef]
- Naidu, S.S.; Baran, D.A.; Jentzer, J.C.; Hollenberg, S.M.; van Diepen, S.; Basir, M.B.; Grines, C.L.; Diercks, D.B.; Hall, S.; Kapur, N.K.; et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies: This statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100008. [Google Scholar] [CrossRef]
- Bloria, S.D.; Chauhan, R.; Sarna, R.; Gombar, S.; Jindal, S. Comparison of APACHE II and APACHE IV score as predictors of mortality in patients with septic shock in intensive care unit: A prospective observational study. J. Anaesthesiol. Clin. Pharmacol. 2023, 39, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Epps, K.C.; Tehrani, B.N.; Rosner, C.; Bagchi, P.; Cotugno, A.; Damluji, A.A.; deFilippi, C.; Desai, S.; Ibrahim, N.; Psotka, M.; et al. Sex-Related Differences in Patient Characteristics, Hemodynamics, and Outcomes of Cardiogenic Shock: INOVA-SHOCK Registry. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 100978. [Google Scholar] [CrossRef]
- Manzo-Silberman, S.; Martin, A.C.; Boissier, F.; Hauw-Berlemont, C.; Aissaoui, N.; Lamblin, N.; Roubille, F.; Bonnefoy, E.; Bonello, L.; Elbaz, M.; et al. Sex disparities in cardiogenic shock: Insights from the FRENSHOCK registry. J. Crit. Care 2024, 82, 154785. [Google Scholar] [CrossRef]
- DesJardin, J.T.; Chikwe, J.; Hahn, R.T.; Hung, J.W.; Delling, F.N. Sex Differences and Similarities in Valvular Heart Disease. Circ. Res. 2022, 130, 455–473. [Google Scholar] [CrossRef]
- Nair, R.M.; Chawla, S.; Alkhalaileh, F.; Abdelghaffar, B.; Bansal, A.; Higgins, A.; Lee, R.; Rampersad, P.; Khot, U.N.; Jaber, W.A.; et al. Characteristics and Outcomes of Patients With Valvular Cardiogenic Shock. JACC Adv. 2024, 3, 101303. [Google Scholar] [CrossRef]
- Gillis, A.M. Atrial Fibrillation and Ventricular Arrhythmias: Sex Differences in Electrophysiology, Epidemiology, Clinical Presentation, and Clinical Outcomes. Circulation 2017, 135, 593–608. [Google Scholar] [CrossRef]
- Moller, J.E.; Engstrom, T.; Jensen, L.O.; Eiskjaer, H.; Mangner, N.; Polzin, A.; Schulze, P.C.; Skurk, C.; Nordbeck, P.; Clemmensen, P.; et al. Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2024, 390, 1382–1393. [Google Scholar] [CrossRef]
- Miller, P.E.; Bromfield, S.G.; Ma, Q.; Crawford, G.; Whitney, J.; DeVries, A.; Desai, N.R. Clinical Outcomes and Cost Associated With an Intravascular Microaxial Left Ventricular Assist Device vs Intra-aortic Balloon Pump in Patients Presenting With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA Intern. Med. 2022, 182, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Fried, J.; Farr, M.; Kanwar, M.; Uriel, N.; Hernandez-Montfort, J.; Blumer, V.; Li, S.; Sinha, S.S.; Garan, A.R.; Li, B.; et al. Clinical outcomes among cardiogenic shock patients supported with high-capacity Impella axial flow pumps: A report from the Cardiogenic Shock Working Group. J. Heart Lung Transplant. 2024, 43, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Patel, B.; Tripathi, B.; Agarwal, M.; Patnaik, S.; Ram, P.; Patil, S.; Shin, J.; Jorde, U.P. Hospital mortality and thirty day readmission among patients with non-acute myocardial infarction related cardiogenic shock. Int. J. Cardiol. 2018, 270, 60–67. [Google Scholar] [CrossRef]
- Sterling, L.H.; Fernando, S.M.; Talarico, R.; Qureshi, D.; van Diepen, S.; Herridge, M.S.; Price, S.; Brodie, D.; Fan, E.; Di Santo, P.; et al. Long-Term Outcomes of Cardiogenic Shock Complicating Myocardial Infarction. J. Am. Coll. Cardiol. 2023, 82, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, M.D.; Gammelager, H.; Schmidt, M.; Nielsen, H.; Christiansen, C.F. Positive predictive value of International Classification of Diseases, 10th revision, diagnosis codes for cardiogenic, hypovolemic, and septic shock in the Danish National Patient Registry. BMC Med. Res. Methodol. 2015, 15, 23. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Neumann, F.J.; Ferenc, M.; Olbrich, H.G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef]
| Parameter | Women n = 803 | Men n = 1453 | p Value |
|---|---|---|---|
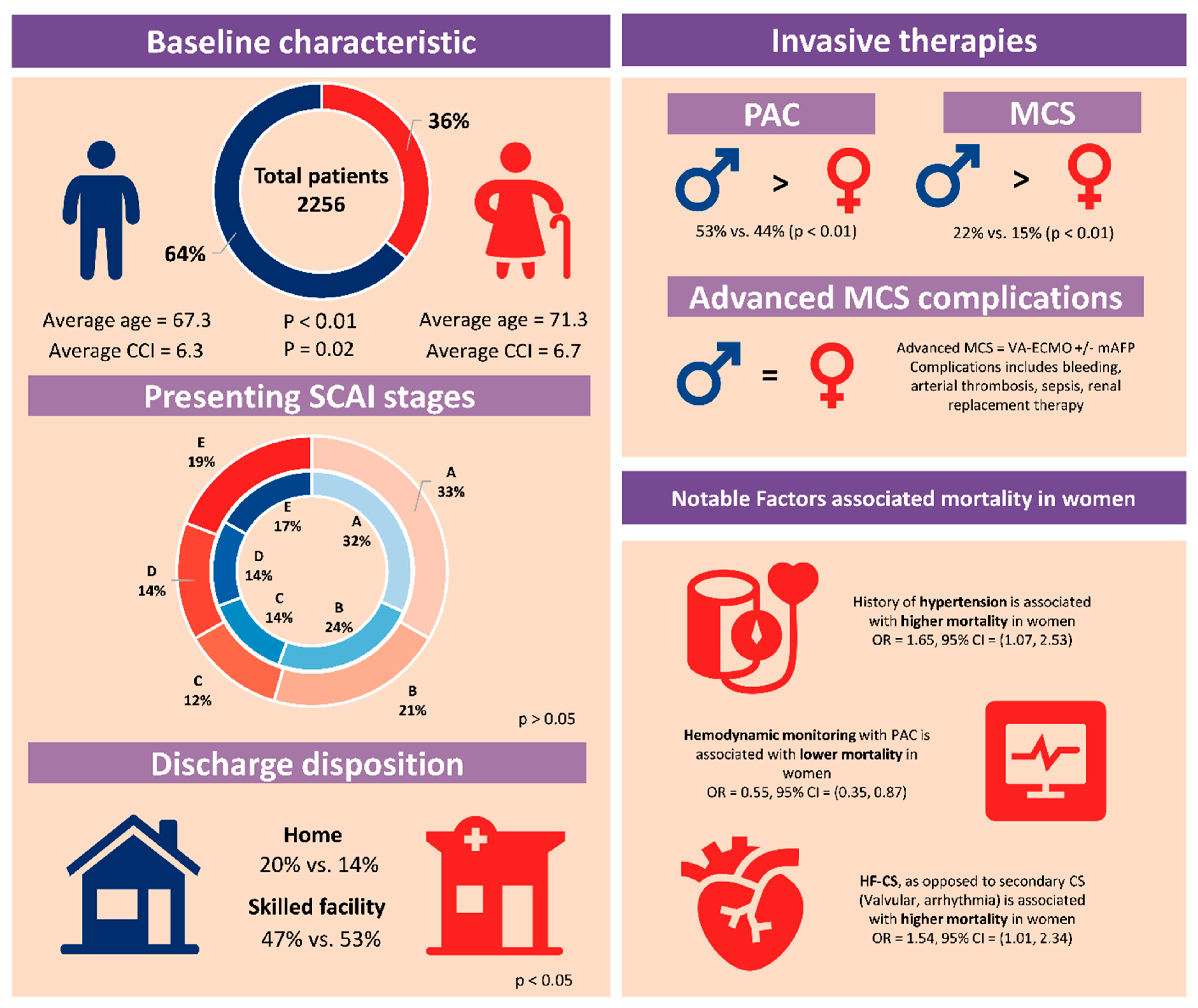
| Age (year) | 71.3 ± 14.6 | 67.7 ± 13.8 | <0.01 |
| BMI (kg/m2) | 30.1 ± 10.1 | 29.6 ± 9.7 | 0.22 |
| BSA (m2) | 1.84 ± 0.33 | 2.06 ± 0.34 | <0.01 |
| Comorbidities (%) | |||
| Diabetes mellitus | 322 (40) | 624 (43) | 0.19 |
| Hypertension | 551 (69) | 976 (67) | 0.48 |
| Chronic kidney disease | 322 (40) | 673 (46) | <0.01 |
| Heart failure | 595 (74) | 1122 (77) | 0.10 |
| Coronary artery disease | 264 (33) | 681 (47) | <0.01 |
| Atrial fibrillation | 403 (50) | 742 (51) | 0.69 |
| CCI | 6.7 ± 3.66 | 6.3 ± 3.66 | 0.02 |
| Cardiac arrest at admission | 18 (2) | 45 (3) | 0.24 |
| Admission APACHE score | 76.1 ± 32.4 | 74.2 ± 32.1 | 0.19 |
| Admission SCAI stage (%) | 0.40 | ||
| A | 256 (32) | 442 (30) | |
| B | 162 (20) | 338 (23) | |
| C | 94 (12) | 190 (13) | |
| D | 111 (14) | 194 (13) | |
| E | 147 (18) | 232 (16) | |
| Not recorded | 33 (4) | 57 (4) | |
| EF (%) (n = 346 vs. 657) | 30 (20, 55) | 23 (15, 35) | <0.01 |
| LVEDD (cm) (n = 348 vs. 705) | 4.8 (4.2, 5.6) | 5.8 (5.0, 6.4) | <0.01 |
| Laboratory | |||
| Lactate (mmol/L) | 2.2 (1.7, 3.8) | 2.2 (1.7, 3.8) | 0.71 |
| Creatinine (mg/dL) | 1.55 (1.02, 2.53) | 1.82 (1.28, 2.87) | <0.01 |
| eGFR (mL/min/1.73 m2) | 41.2 (23.5, 69.0) | 45.2 (27.6, 69.6) | <0.01 |
| ALT (IU/L) | 28 (16, 66) | 33 (19, 87) | <0.01 |
| Bilirubin (mg/dL) | 0.8 (0.5, 1.4) | 1.0 (0.6, 1.8) | <0.01 |
| Sodium (mEq/L) | 137 (133, 140) | 137 (133, 139) | 0.24 |
| Hemoglobin (g/dL) | 11.1 (9.6, 12.5) | 12.1 (10.2, 13.6) | <0.01 |
| WBC (k/uL) | 9.3 (6.4, 13.3) | 8.8 (6.6, 12.3) | 0.16 |
| Platelet (k/uL) | 189 (133, 246) | 177 (126, 234) | <0.01 |
| Albumin (g/dL) | 3.4 (3, 3.8) | 3.4 (3.1, 3.8) | 0.1 |
| Etiology (%) | Women n = 803 | Men n = 1453 | Adjusted p Value |
|---|---|---|---|
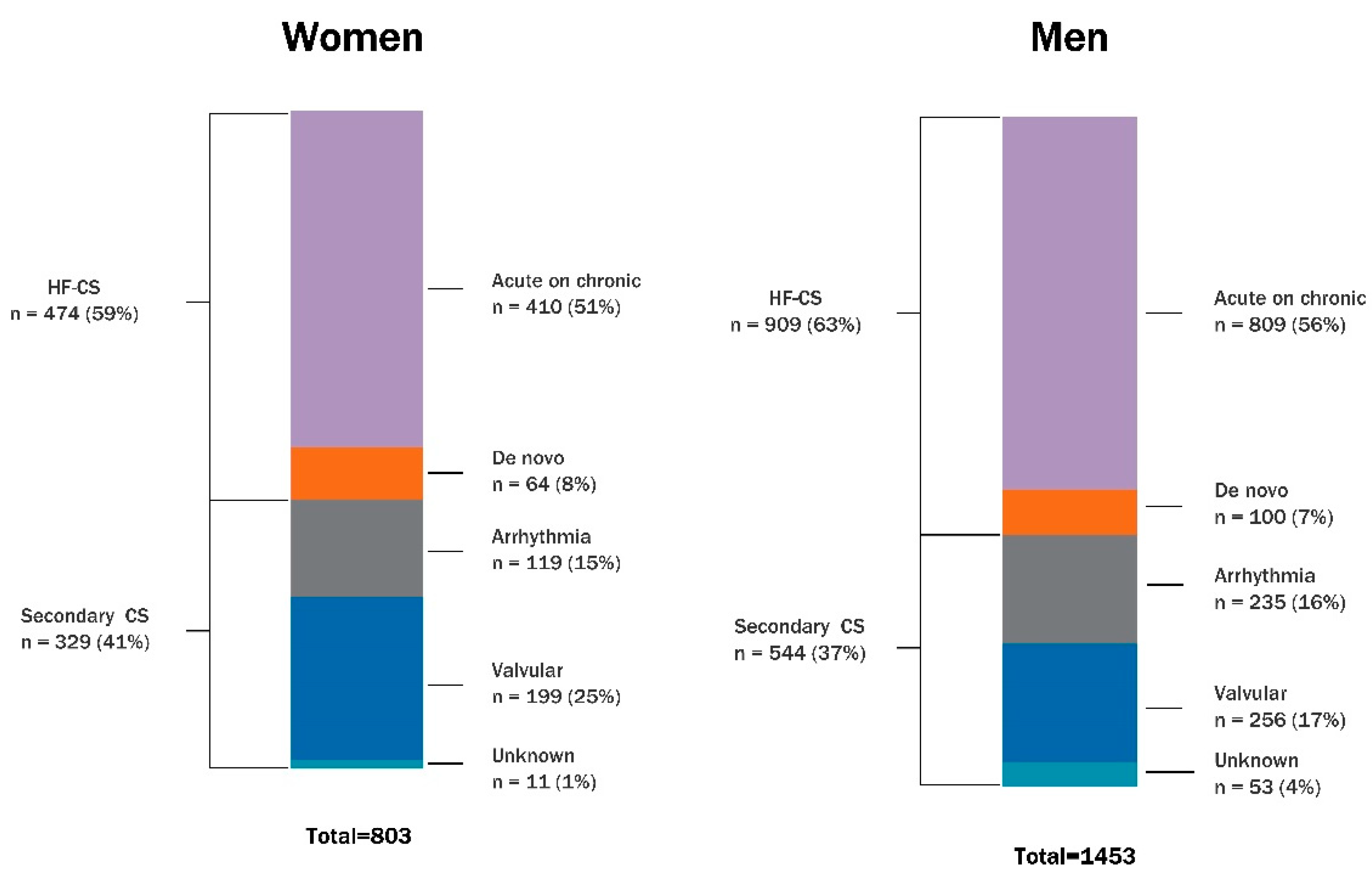
| Heart failure-related CS | 474 (59) | 909 (63) | 0.30 |
| Acute on chronic | 410 (86) | 809 (89) | |
| De novo | 64 (14) | 100 (11) | |
| Arrhythmia-triggered CS | 119 (15) | 235 (16) | 1.0 |
| Atrial | 38 (32) | 63 (27) | 1.0 |
| Ventricular | 29 (24) | 121 (51) | <0.01 |
| Bradyarrhythmia | 50 (42) | 47 (20) | <0.01 |
| Not recorded | 2 (2) | 4 (2) | |
| Valvular CS | 199 (25) | 256 (17) | <0.01 |
| Aortic stenosis | 38 (19) | 65 (25) | 1.0 |
| Aortic regurgitation | 9 (5) | 38 (15) | <0.01 |
| Mitral stenosis | 5 (2) | 2 (1) | 1.0 |
| Mitral regurgitation | 73 (37) | 53 (21) | <0.01 |
| Prosthetic valve | 7 (4) | 24 (9) | 0.24 |
| Right-sided valve | 5 (2) | 5 (2) | 1.0 |
| Multivalvular | 42 (21) | 31 (12) | 0.10 |
| Infectious endocarditis | 18 (9) | 37 (14) | 0.87 |
| Not recorded | 2 (1) | 1 (0.3) | |
| Not recorded | 11 (1) | 53 (4) |
| Intervention (%) | Women n = 803 | Men n = 1453 | Adjusted p Value |
|---|---|---|---|
| Catecholamine | 287 (36) | 515 (35) | 0.89 |
| Any inotrope | 322 (40) | 608 (42) | 0.42 |
| Dobutamine | 234 (29) | 435 (30) | 0.72 |
| Milrinone | 188 (23) | 405 (28) | 0.01 |
| Dopamine | 32 (4) | 35 (2) | 0.02 |
| VIS score | |||
| At 0 h (n = 189 vs. 323) | 10 (5, 25) | 8 (4, 24) | 0.19 |
| At max (n = 366 vs. 623) | 21 (9, 65) | 22 (8, 52) | 0.44 |
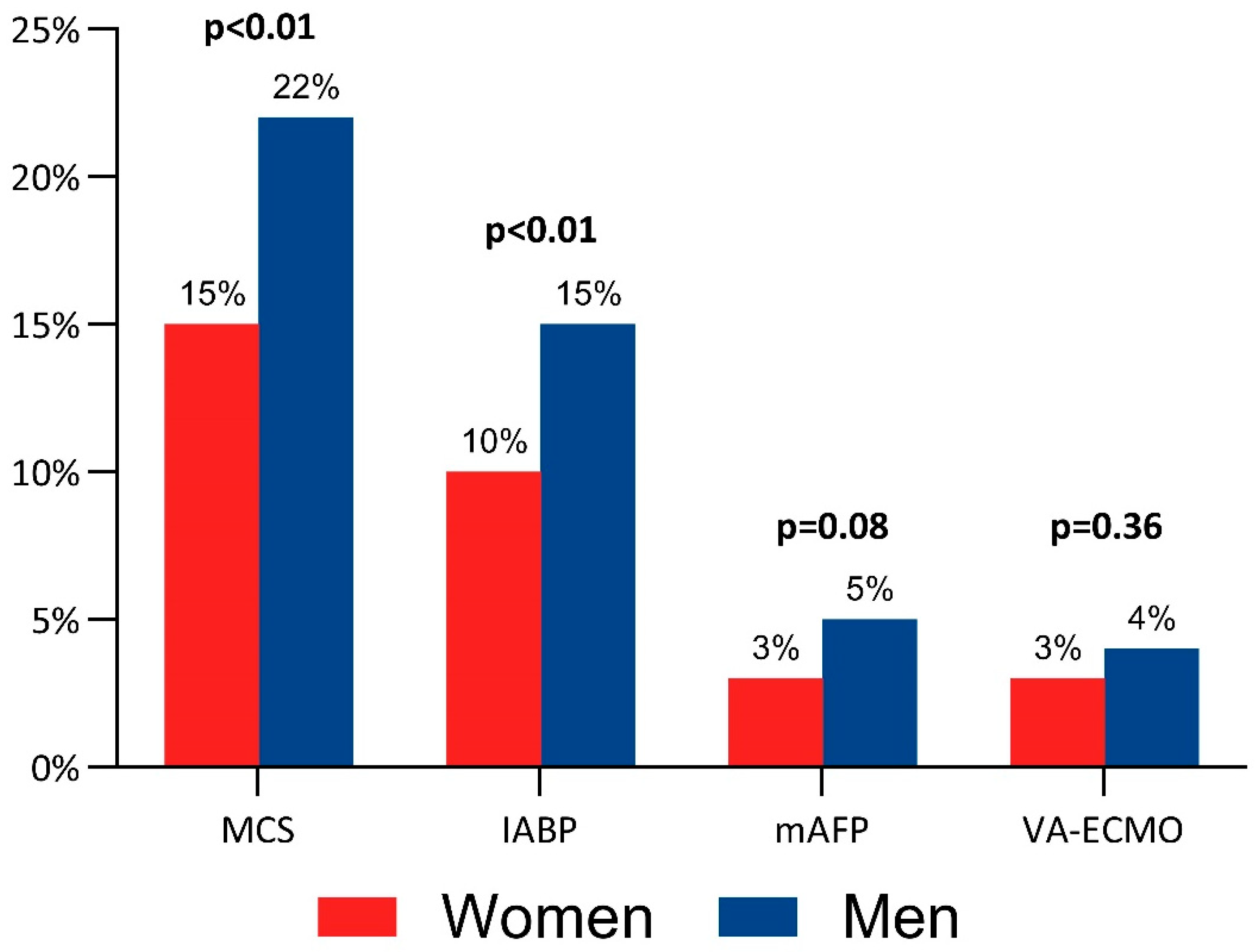
| Any MCS | 117 (15) | 322 (22) | <0.01 |
| IABP | 80 (10) | 215 (15) | <0.01 |
| mAFP | 28 (3) | 74 (5) | 0.08 |
| VA-ECMO | 28 (3) | 62 (4) | 0.36 |
| LVAD | 12 (1) | 54 (4) | <0.01 |
| Transplant | 14 (2) | 22 (2) | 0.68 |
| Pulmonary artery catheter | 353 (44) | 773 (53) | <0.01 |
| Left heart catheterization | 191 (24) | 385 (26) | 0.16 |
| PCI | 23 (3) | 49 (3) | 0.59 |
| CABG | 29 (4) | 93 (6) | <0.01 |
| Valvular procedure | 172 (21) | 258 (18) | 0.03 |
| Surgical | 117 (68) | 155 (60) | |
| Percutaneous | 39 (23) | 42 (16) | |
| Unrecorded | 16 (9) | 61 (24) | |
| Blood transfusion | 277 (34) | 406 (28) | <0.01 |
| Mechanical ventilation | 272 (34) | 499 (34) | 0.82 |
| NIPPV | 180 (22) | 355 (24) | 0.28 |
| RRT | 107 (13) | 240 (17) | 0.04 |
| Advanced MCS Complications (%) | Women n = 46 | Men n = 117 | p Value |
|---|---|---|---|
| Impella only | n = 18 | n = 55 | |
| BARC 3–5 bleeding | 1 (6) | 4 (7) | 1.0 |
| Arterial thrombosis | 1 (6) | 2 (4) | 1.0 |
| Transfusion | 4 (22) | 9 (16) | 0.83 |
| RRT | 7 (56) | 13 (24) | 0.34 |
| Sepsis | 1 (6) | 7 (13) | 0.68 |
| VA-ECMO only | n = 18 | n = 45 | |
| BARC 3–5 bleeding | 0 (0) | 4 (9) | 0.46 |
| Arterial thrombosis | 0 (0) | 0 (0) | NA |
| Transfusion | 1 (6) | 11 (24) | 0.46 |
| RRT | 2 (11) | 6 (13) | 1.0 |
| Sepsis | 1 (6) | 2 (4) | 1.0 |
| Impella and VA-ECMO | n = 10 | n = 17 | |
| BARC 3–5 bleeding | 3 (30) | 3 (18) | 0.79 |
| Arterial thrombosis | 1 (10) | 1 (6) | 1.0 |
| Transfusion | 1 (10) | 6 (35) | 0.32 |
| RRT | 3 (30) | 3 (18) | 0.79 |
| Sepsis | 2 (20) | 2 (12) | 0.98 |
| Outcome | Women n = 803 | Men n = 1453 | Adjusted p Value |
|---|---|---|---|
| Disposition at discharge (%) | <0.01 | ||
| Home | 110 (14) | 290 (20) | |
| Rehab or skilled facility | 379 (47) | 644 (44) | |
| Hospice | 44 (6) | 45 (3) | |
| Unrecorded | 50 (6) | 112 (8) | |
| Length of stay (day) | 12 (6, 20) | 12 (6, 21) | 0.39 |
| In-hospital mortality (%) | 220 (27) | 362 (25) | 0.20 |
| HF-CS | 155 (33) | 231 (25) | 0.02 |
| Arrhythmia-triggered CS | 30 (25) | 69 (29) | 1.0 |
| VCS | 30 (14) | 43 (13) | 1.0 |
| Etiology not recorded | 5 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Adi, A.; Miller, M.; Sakr, F.; Jnani, J.; Rodriguez, E.A.; Tan, S.; Mokhtari, M.B.; Ramsis, R.; Smoller, R.; et al. Sex Differences in Non-Acute Myocardial Infarction Cardiogenic Shock: Insights from the Northwell-Shock Registry. J. Clin. Med. 2025, 14, 4274. https://doi.org/10.3390/jcm14124274
Li S, Adi A, Miller M, Sakr F, Jnani J, Rodriguez EA, Tan S, Mokhtari MB, Ramsis R, Smoller R, et al. Sex Differences in Non-Acute Myocardial Infarction Cardiogenic Shock: Insights from the Northwell-Shock Registry. Journal of Clinical Medicine. 2025; 14(12):4274. https://doi.org/10.3390/jcm14124274
Chicago/Turabian StyleLi, Shuojohn, Abduljabar Adi, Marcy Miller, Fouad Sakr, Jack Jnani, Emily A. Rodriguez, Samuel Tan, Moein Bayat Mokhtari, Ramsis Ramsis, Rebecca Smoller, and et al. 2025. "Sex Differences in Non-Acute Myocardial Infarction Cardiogenic Shock: Insights from the Northwell-Shock Registry" Journal of Clinical Medicine 14, no. 12: 4274. https://doi.org/10.3390/jcm14124274
APA StyleLi, S., Adi, A., Miller, M., Sakr, F., Jnani, J., Rodriguez, E. A., Tan, S., Mokhtari, M. B., Ramsis, R., Smoller, R., Stevens, G. R., Hernandez-Montfort, J., Griffin, M., Pierce, M., & Alvarez Villela, M. (2025). Sex Differences in Non-Acute Myocardial Infarction Cardiogenic Shock: Insights from the Northwell-Shock Registry. Journal of Clinical Medicine, 14(12), 4274. https://doi.org/10.3390/jcm14124274






