Thyroid Cancer in Childhood Leukemia Survivors: A Systematic Review of the Incidence and Survival Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Literature Search
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Quality/Risk-of-Bias Assessment
2.6. Statistical Analysis
3. Results
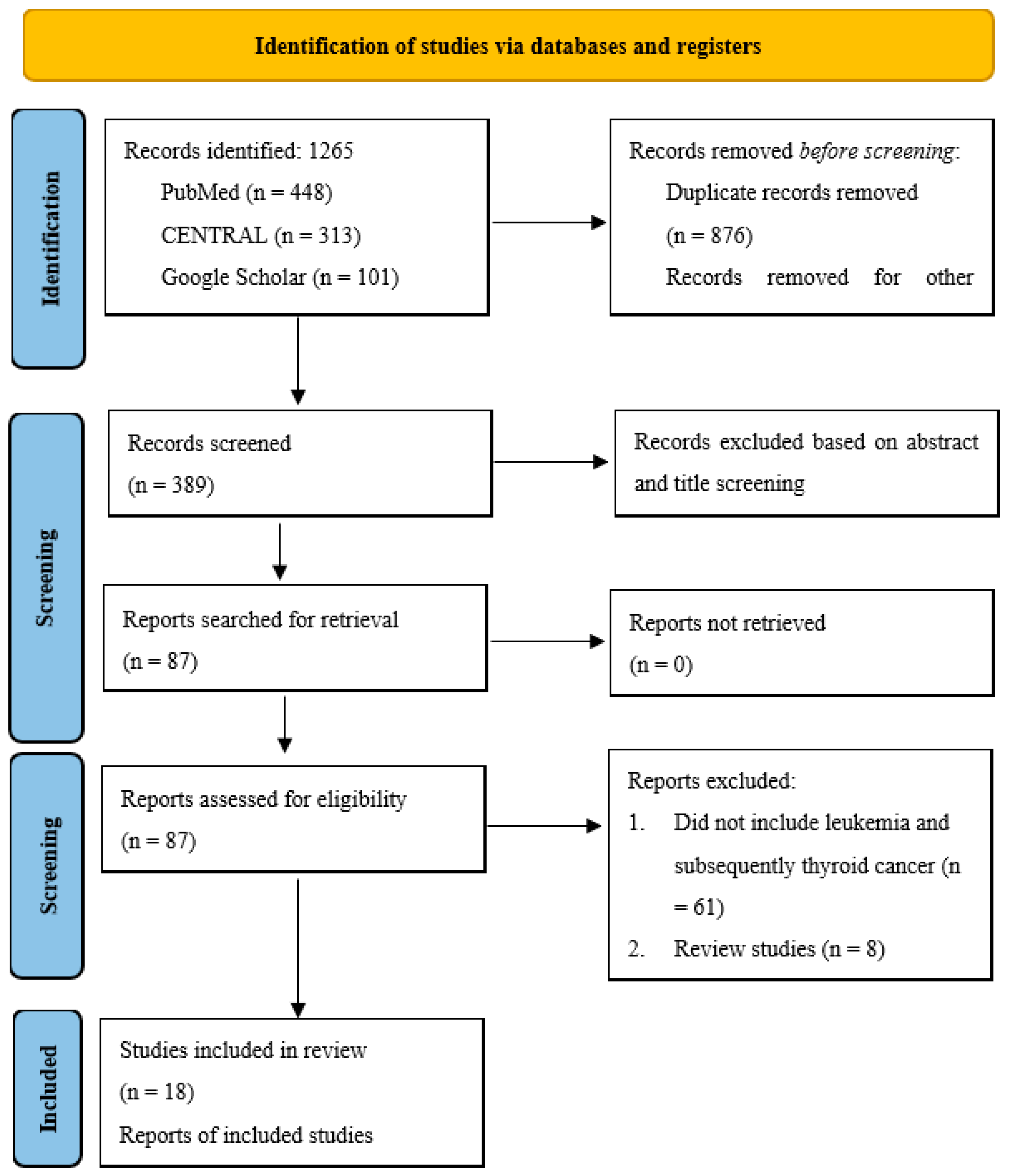
3.1. Search Results
3.2. Characteristics of the Included Studies
3.3. Methodological Quality of the Included Studies
3.4. Incidence of Thyroid Cancer
3.5. Risk Factors for the Development of Thyroid Cancer
3.6. Type and Treatment of Thyroid Cancer
3.7. Outcomes After Second Primary Thyroid Cancer
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALL | acute lymphoblastic leukemia |
| AML | acute myelogenous leukemia |
| CML | chronic myeloid leukemia |
| IRB | Institutional Review Board |
| NOS | Newcastle Ottawa Scale |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RAI | radioactive iodine |
| SEER | Surveillance, Epidemiology, and End Results |
| SPM | second primary malignancies |
References
- Tower, R.L.; Spector, L.G. The Epidemiology of Childhood Leukemia with a Focus on Birth Weight and Diet. Crit. Rev. Clin. Lab. Sci. 2007, 44, 203–242. [Google Scholar] [CrossRef]
- Marcotte, E.L.; Spector, L.G.; Mendes-De-Almeida, D.P.; Nelson, H.H. The prenatal origin of childhood leukemia: Potential applications for epidemiology and newborn screening. Front. Pediatr. 2021, 9, 639479. [Google Scholar] [CrossRef]
- Metayer, C.; Milne, E.; Clavel, J.; Infante-Rivard, C.; Petridou, E.; Taylor, M.; Schüz, J.; Spector, L.G.; Dockerty, J.D.; Magnani, C.; et al. The Childhood Leukemia International Consortium. Cancer Epidemiol. 2013, 37, 336–347. [Google Scholar] [CrossRef]
- Greaves, M.F.; Wiemels, J. Origins of Chromosome Translocations in Childhood Leukaemia. Nat. Rev. Cancer 2003, 3, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Tonorezos, E.S.; Landier, W. Clinical Care for People Who Survive Childhood Cancer: A Review. JAMA 2023, 330, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.L.; Whitton, J.; Leisenring, W.; Mertens, A.C.; Hammond, S.; Stovall, M.; Donaldson, S.S.; Meadows, A.T.; Robison, L.L.; Neglia, J.P. Subsequent Neoplasms in 5-Year Survivors of Childhood Cancer: The Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2010, 102, 1083–1095. [Google Scholar] [CrossRef]
- Demoor-Goldschmidt, C.; De Vathaire, F. Review of Risk Factors of Secondary Cancers among Cancer Survivors. Br. J. Radiol. 2019, 92, 20180390. [Google Scholar] [CrossRef] [PubMed]
- Mirkatouli, N.B.; Hirota, S.; Yoshinaga, S. Thyroid Cancer Risk after Radiation Exposure in Adults-Systematic Review and Meta-Analysis. J. Radiat. Res. 2023, 64, 893–903. [Google Scholar] [CrossRef]
- Jairam, V.; Roberts, K.B.; Yu, J.B. Historical Trends in the Use of Radiation for Pediatric Cancers: 1973–2008. Int. J. Radiat. Oncol. Biol. Phys. 2012, 85, e151. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Landman, A.J.E.M.C.; Don, E.E.; Vissers, G.; Ket, H.C.J.; Oudijk, M.A.; de Groot, C.J.M.; Huirne, J.A.F.; de Boer, M.A. Modified Newcastle Ottawa Quality Assessment Scale and AHRQ Standards. PLoS ONE 2022. [Google Scholar] [CrossRef]
- Acharya, S.; Sarafoglou, K.; LaQuaglia, M.; Lindsley, S.; Gerald, W.; Wollner, N.; Tan, C.; Sklar, C. Thyroid Neoplasms after Therapeutic Radiation for Malignancies during Childhood or Adolescence. Cancer 2003, 97, 2397–2403. [Google Scholar] [CrossRef]
- Bebeshko, V.G.; Bruslova, K.M.; Lyashenko, L.O.; Tsvietkova, N.M.; Galkina, S.G.; Yaroshenko, Z.S.; Gonchar, L.O.; Boyarska, O.Y.; Kuzmenko, V.F.; Trykhlib, I.V.; et al. Thyroid disease in the late observation period upon chemo and radiotherapy in children/survivors of acute lymphoblastic leukemia. Probl. Radiac. Med. Radiobiol. 2021, 26, 309–318. [Google Scholar] [CrossRef]
- Berger, C.; Trombert-Paviot, B.; Casagranda, L.; Mialou, V.; Frappaz, D.; Plantaz, D.; Collardeau-Frachon, S.; Freycon, F. Second Malignant Neoplasms Following Childhood Cancer: A Study of a Recent Cohort (1987–2004) from the Childhood Cancer Registry of the Rhône-Alpes Region (ARCERRA) in France. Pediatr. Hematol. Oncol. 2011, 28, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Sather, H.N.; Pabustan, O.B.; Trigg, M.E.; Gaynon, P.S.; Robison, L.L. Low Incidence of Second Neoplasms among Children Diagnosed with Acute Lymphoblastic Leukemia after 1983. Blood 2002, 99, 4257–4264. [Google Scholar] [CrossRef]
- Borgmann, A.; Zinn, C.; Hartmann, R.; Herold, R.; Kaatsch, P.; Escherich, G.; Möricke, A.; Henze, G.; von Stackelberg, A. Secondary malignant neoplasms after intensive treatment of relapsed acute lymphoblastic leukaemia in childhood. Eur. J. Cancer 2008, 44, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Echecopar, C.; del Val Rey, I.; Galán-Gómez, V.; González-Pérez, C.; Mozo del Castillo, Y.; González Martínez, B.; Pérez-Martínez, A. The Paradigm of Total Body Irradiation in Acute Lymphoblastic Leukaemia: Therapeutic Effectiveness versus the Challenges of Toxicity. An. Pediatr. 2024, 100, 259–267. [Google Scholar] [CrossRef]
- Gow, K.W.; Lensing, S.; Hill, D.A.; Krasin, M.J.; McCarville, M.B.; Rai, S.N.; Zacher, M.; Spunt, S.L.; Strickland, D.K.; Hudson, M.M. Thyroid Carcinoma Presenting in Childhood or after Treatment of Childhood Malignancies: An Institutional Experience and Review of the Literature. J. Pediatr. Surg. 2003, 38, 1574–1580. [Google Scholar] [CrossRef]
- Koh, K.N.; Yoo, K.H.; Im, H.J.; Sung, K.W.; Koo, H.H.; Kim, H.S.; Han, J.W.; Yoon, J.H.; Park, H.J.; Park, B.K.; et al. Characteristics and Outcomes of Second Malignant Neoplasms after Childhood Cancer Treatment: Multi-Center Retrospective Survey. J. Korean Med. Sci. 2016, 31, 1254. [Google Scholar] [CrossRef]
- Martucci, C.; Crocoli, A.; De Pasquale, M.D.; Spinelli, C.; Strambi, S.; Brazzarola, P.; Morelli, E.; Cassiani, J.; Mancera, J.; Luengas, J.P.; et al. Thyroid Cancer in Children: A Multicenter International Study Highlighting Clinical Features and Surgical Outcomes of Primary and Secondary Tumors. Front. Pediatr. 2022, 10, 914942. [Google Scholar] [CrossRef]
- Maule, M.; Scélo, G.; Pastore, G.; Brennan, P.; Hemminki, K.; Tracey, E.; Sankila, R.; Weiderpass, E.; Olsen, J.H.; McBride, M.L.; et al. Risk of Second Malignant Neoplasms after Childhood Leukemia and Lymphoma: An International Study. J. Natl. Cancer Inst. 2007, 99, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Oudin, C.; Auquier, P.; Bertrand, Y.; Chastagner, P.; Kanold, J.; Poirée, M.; Thouvenin, S.; Ducassou, S.; Plantaz, D.; Tabone, M.D.; et al. Late Thyroid Complications in Survivors of Childhood Acute Leukemia. An L.E.A. Study. Haematologica 2016, 101, 747. [Google Scholar] [CrossRef]
- Perkins, S.M.; DeWees, T.; Shinohara, E.T.; Reddy, M.M.; Frangoul, H. Risk of Subsequent Malignancies in Survivors of Childhood Leukemia. J. Cancer Surviv. 2013, 7, 544–550. [Google Scholar] [CrossRef]
- Renard, M.; Suciu, S.; Bertrand, Y.; Uyttebroeck, A.; Ferster, A.; van der Werff ten Bosch, J.; Mazingue, F.; Plouvier, E.; Robert, A.; Boutard, P.; et al. Second Neoplasm in Children Treated in EORTC 58881 Trial for Acute Lymphoblastic Malignancies: Low Incidence of CNS Tumours. Pediatr. Blood Cancer 2011, 57, 119–125. [Google Scholar] [CrossRef]
- Schiemegelow, K.; Levinsen, M.F.; Attarbaschi, A.; Baruchel, A.; Devidas, M.; Escherich, G.; Gibson, B.; Heydrich, C.; Horibe, K.; Ishida, Y.; et al. Second Malignant Neoplasms after Treatment of Childhood Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2013, 31, 2469–2476. [Google Scholar] [CrossRef]
- Socié, G.; Curtis, R.E.; Deeg, H.J.; Sobocinski, K.A.; Filipovich, A.H.; Travis, L.B.; Sullivan, K.M.; Rowlings, P.A.; Kingma, D.W.; Banks, P.M.; et al. New Malignant Diseases after Allogeneic Marrow Transplantation for Childhood Acute Leukemia. J. Clin. Oncol. 2000, 18, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Croft, A.P.; Palace, A.M.; Winter, D.L.; Reulen, R.C.; Stiller, C.A.; Stevens, M.C.G.; Hawkins, M.M. Risk of Thyroid Cancer in Survivors of Childhood Cancer: Results from the British Childhood Cancer Survivor Study. Int. J. Cancer 2009, 125, 2400–2405. [Google Scholar] [CrossRef] [PubMed]
- Toret, E.; Aytac, S.; Guzelkucuk, Z.; Celkan, T.; Genc, D.B.; Sezgin-Evim, M.; Cakmakli, H.F.; Bahadir, A.; Karapinar, T.H.; Oren, H.; et al. Prognosis of Second Primary Malignancies in Pediatric Acute Lymphoblastic Leukemia Survivors: A Multicenter Study by the Turkish Pediatric Hematology Society. J. Pediatr. Hematol. Oncol. 2024, 46, e363–e367. [Google Scholar] [CrossRef]
- Veiga, L.H.S.; Bhatti, P.; Ronckers, C.M.; Sigurdson, A.J.; Stovall, M.; Smith, S.A.; Weathers, R.; Leisenring, W.; Mertens, A.C.; Hammond, S.; et al. Chemotherapy and Thyroid Cancer Risk: A Report from the Childhood Cancer Survivor Study. Cancer Epidemiol. Biomark. Prev. 2012, 21, 92–101. [Google Scholar] [CrossRef]
- Williams, D. Twenty Years’ Experience with Post-Chernobyl Thyroid Cancer. Best. Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 1061–1073. [Google Scholar] [CrossRef]
- Prescott, J.D.; Zeiger, M.A. The RET Oncogene in Papillary Thyroid Carcinoma. Cancer 2015, 121, 2137–2146. [Google Scholar] [CrossRef]
- Lorenz, E.; Scholz-Kreisel, P.; Baaken, D.; Pokora, R.; Blettner, M. Radiotherapy for Childhood Cancer and Subsequent Thyroid Cancer Risk: A Systematic Review. Eur. J. Epidemiol. 2018, 33, 1139–1162. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.M.; Bhatia, S.; Casillas, J.; Landier, W. Long-Term Follow-up Care for Childhood, Adolescent, and Young Adult Cancer Survivors. Pediatrics 2021, 148, e2021053127. [Google Scholar] [CrossRef]
- Tonorezos, E.S.; Barnea, D.; Moskowitz, C.S.; Chou, J.F.; Sklar, C.A.; Elkin, E.B.; Wong, R.J.; Li, D.; Tuttle, R.M.; Korenstein, D.; et al. Screening for Thyroid Cancer in Survivors of Childhood and Young Adult Cancer Treated with Neck Radiation. J. Cancer Surviv. 2016, 11, 302. [Google Scholar] [CrossRef]
- Van Iersel, L.; Mulder, R.L.; Denzer, C.; Cohen, L.E.; Spoudeas, H.A.; Meacham, L.R.; Sugden, E.; Schouten-Van Meeteren, A.Y.N.; Hoving, E.W.; Packer, R.J.; et al. Hypothalamic-Pituitary and Other Endocrine Surveillance Among Childhood Cancer Survivors. Endocr. Rev. 2022, 43, 794–823. [Google Scholar] [CrossRef]
- Han, M.A.; Kim, J.H. Diagnostic X-Ray Exposure and Thyroid Cancer Risk: Systematic Review and Meta-Analysis. Thyroid 2018, 28, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Sinnott, B.; Ron, E.; Schneider, A.B. Exposing the Thyroid to Radiation: A Review of Its Current Extent, Risks, and Implications. Endocr. Rev. 2010, 31, 756–773. [Google Scholar] [CrossRef] [PubMed]
- Veiga, L.H.S.; Lubin, J.H.; Anderson, H.; De Vathaire, F.; Tucker, M.; Bhatti, P.; Schneider, A.; Johansson, R.; Inskip, P.; Kleinerman, R.; et al. A Pooled Analysis of Thyroid Cancer Incidence Following Radiotherapy for Childhood Cancer. Radiat. Res. 2012, 178, 365–376. [Google Scholar] [CrossRef]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A. Thyroid Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up†. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef]
- American Cancer Society. Thyroid Cancer Survival Rates, by Type and Stage. Available online: https://www.cancer.org/cancer/types/thyroid-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 7 March 2025).
| Study | Study Setting | Type of Leukemia | Treatment for Leukemia | Sample Size (Total/ Leukemia) | No./Sex of Patients with Second Malignancies | Age at Diagnosis of Leukemia or of Primary Malignancy (Years) a | Time to Thyroid Cancer Diagnosis After Leukemia (Years) | Type of Thyroid Cancer | Treatment for Thyroid Cancer | Reported Outcomes | Survival at 5 Years After Thyroid Cancer Diagnosis | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total/ After Leukemia | Thyroid Cancer After Leukemia | |||||||||||
| Acharya et al., 2003 [12] | USA | ALL and AML | Radiation therapy | 33/3 | 13/3 | 3 F (2 after ALL and 1 after AML) | 5.6 ± 1.0 | 15.3 ± 3.3 | Follicular and papillary | Near-total thyroidectomy and RAI therapy | Thyroid neoplasms after radiation therapy | NR |
| Bebeshko et al., 2021 [13] | Ukraine | ALL | Standard chemotherapy and radiotherapy | 92/92 | 1/1 | 1 F | NR | 11 | Papillary | NR | Incidence of thyroid disease | NR |
| Berger et al., 2011 [14] | France | ALL and CML | TBI (n = 11), allogeneic marrow transplantation (n = 11) | 2907/854 | 54 (28 F)/21 | 3 (2 after ALL and 1 after CML) | 7.8 ± 5.5 | 15.0 ± 5.8 | NS | NR | Incidence of second malignancy and patient outcomes | 3/3 |
| Bhatia et al., 2002 [15] | USA and Canada | ALL | CP (100%), anthracyclines (100%), and radiation (50%) | 8831/8831 | 63/63 | 4 (2F and 2M) | 2.9 (1.5–4.0) | 9.7 (5.5–11.8) | Papillary | NR | Risk of developing second malignancy | 4/4 |
| Borgman et al., 2008 [16] | Germany | ALL | Cranial irradiation, TBI, multi-drug chemotherapy protocols | 1376/1376 | 21/21 | 2 (1F and 1M) | 5.1 ± 0.7 | 15.7 ± 6.9 | NS | NS (treatment based on disease-specific protocol) | Cumulative incidence of secondary malignancy | 2/2 |
| Echecopar et al., 2024 [17] | Spain | ALL | TBI ± HSCT or HSCT alone | 57/57 | 7/7 | 2 | 6.3 ± 3.9 b | NR | Follicular | NR | Survival of ALL patients after TBI | NR |
| Gow et al., 2003 [18] | USA | ALL | Chemotherapy + radiation (70.6%), chemotherapy only (5.9%), radiation only (11.8%) | 17/17 | 17 (13 F)/6 | 6 | NR | 16.2 (0.9–29.2) | Papillary (88.2%) and follicular (11.8%) | Total thyroidectomy, cervical nodal dissection, and RAI ablation | Outcomes of thyroid carcinoma | 6/6 |
| Koh et al., 2016 [19] | Korea | ALL, AML, BAL | Chemotherapy, TBI, and HSCT | 102/18 | 18/18 | 2 | 6.6 (0–19.7) c | 6.8 (4.1–18.5) c | Papillary | Thyroidectomy | Outcomes of second malignancy | 2/2 |
| Martucci et al., 2022 [20] | Italy, Brazil, UK, Argentina, and France | ALL | Radiotherapy | 255/5 | 13/5 | 5 | 14 (1–18.4) c | 9.0 ± 4.8 | Adenoma and papillary | Hemi- or total thyroidectomy | Survival | 5/5 |
| Maule et al., 2007 [21] | Australia, Canada, Sweden, Finland, Denmark, Scotland, Iceland, Norway, Slovenia, Spain, and Singapore | NS | NR | 16,540/ 12,731 | 133/ 67 | 9 | 1–14 | 10–19 | NS | NR | Cumulative incidence of thyroid cancer | NR |
| Oudin et al., 2016 [22] | France | ALL and AML | CNS irradiation, HSCT with or without prior TBI | 502/502 | 26/26 | 26 | 7.4 ± 0.2 b | NR Age at diagnosis of TC: 20.5 (17.1–24.1) | Papillary | RAI therapy and subtotal or total thyroidectomy | Prevalence and risk factors for thyroid cancer | 26/26 |
| Perkins et al., 2013 [23] | USA | ALL and AML | Radiation therapy | 4806/4806 | 82/82 | 18 (16 after ALL; 14 F) | 7.0 (0–18.0) | ~7–35.5 | NS | NR | Survival of primary and second malignancy | 18/18 |
| Renard et al., 2011 [24] | USA | ALL | Radiotherapy and regimens including topoisomerase-II inhibitors, HSCT | 2216/2077 | 22/20 | 2 F | 5.0 ± 1.0 | 5.5 ± 0.9 | Papillary | NR | Incidence of second malignancy | 2/2 |
| Schiemegelow et al., 2013 [25] | North America, Europe, and Asia | ALL | CP, EPT, CNS irradiation | 54,058/54,058 | 642/642 | 32 (17 F) | 5.0 (3.1–6.5) | 10.1 (7.8–13.5) | NS | NR | Incidence of second malignancy and survival | 32/32 |
| Socie’ et al., 2000 [26] | USA | ALL | Allogeneic BMT, conditioning regimen (TBI or limited field irradiation ± CP ± other drugs), SCT | 3182/2022 d | 45/45 | 2 d | 7.4 ± 2.6 e | 7.7 ± 4.5 | Papillary | NR | Risk of developing second malignancy | 2/2 |
| Taylor et al., 2009 [27] | UK | ALL and AML | TBI, cranial radiotherapy, chemotherapy | 17,980/NS | 50 (31 F)/13 | 13 (9 after ALL and 4 after AML) | 2–14 | 8–33 | Papillary, follicular, other | NR | Cumulative incidence of thyroid carcinoma | NR |
| Toret et al., 2024 [28] | Turkey | ALL | Cranial radiotherapy (39%), HSCT (12%), TBI (12%) | 10,360/10,360 | 41/41 | 5 | 5.3 ± 4.3 | 18.9 ± 8.4 f | NS | NR | Survival | NR |
| Veiga et al., 2012 [29] | USA and Canada | NS | Chemotherapy (alkylating agents, anthracyclines, bleomycin, platinum compounds, and EPT), radiotherapy | 12,547/4261 | 119/27 | 27 | 5.7 ± 4.0 | 21.0 ± 7.7 | Papillary and mixed papillary, follicular, other | NR | Cumulative incidence of thyroid cancer according to the treatment regimen | NR |
| Study | Type of Leukemia | Treatment for Leukemia | Radiation Dose (cGy) | Age at Diagnosis of Leukemia or of Primary Malignancy (Years) a | Time to Thyroid Cancer Diagnosis After Leukemia (Years) | Type of Thyroid Cancer | Treatment for Thyroid Cancer | Survival at 5 Years After Thyroid Cancer Diagnosis |
|---|---|---|---|---|---|---|---|---|
| Acharya et al., 2003 [12] | ALL and AML | Radiation therapy | 1000–1800 | 5.6 ± 1.0 | 15.3 ± 3.3 | Follicular and papillary | Near-total thyroidectomy and RAI therapy | NR |
| Bebeshko et al., 2021 [13] | ALL | Standard chemotherapy and radiotherapy | 1200–1800 | NR | 11 | Papillary | NR | NR |
| Berger et al., 2011 [14] | ALL and CML | TBI and allogeneic marrow transplantation | 1200 | 7.8 ± 5.5 | 15.0 ± 5.8 | Papillary | NR | 3/3 |
| Bhatia et al., 2002 [15] | ALL | CP (100%), anthracyclines (100%), and radiation (50%) | 1800–2400 | 2.9 (1.5–4.0) | 9.7 (5.5–11.8) | Papillary | NR | 4/4 |
| Borgman et al., 2008 [16] | ALL | Cranial irradiation, TBI, multi-drug chemotherapy protocols | Cranial: 1800–2400 TBI: 1200 | 5.1 ± 0.7 | 15.7 ± 6.9 | NS | NS (treatment based on disease-specific protocol) | 2/2 |
| Echecopar et al., 2024 [17] | ALL | TBI ± HSCT or HSCT alone | NR | 6.3 ± 3.9 b | NR | Follicular | NR | NR |
| Gow et al., 2003 [18] | ALL | chemotherapy + radiation (70.6%), chemotherapy only (5.9%), radiation only (11.8%) | NS | NR | 16.2 (0.9–29.2) | Papillary (88.2%); follicular (11.8%) | Total thyroidectomy, cervical nodal dissection, and RAI ablation | 6/6 |
| Koh et al., 2016 [19] | ALL, AML, BAL | Chemotherapy, TBI, and HSCT | NR | 6.6 (0–19.7) c | 6.8 (4.1–18.5) c | Papillary | Thyroidectomy | 2/2 |
| Martucci et al., 2022 [20] | ALL | Radiotherapy | NR | 14 (1–18.4) c | 9.0 ± 4.8 | Adenoma and papillary | Hemi- or total thyroidectomy | 5/5 |
| Maule et al., 2007 [21] | NS | NR | NR | 1–14 | 10–19 | NS | NR | NR |
| Oudin et al., 2016 [22] | ALL and AML | CNS irradiation and HSCT with or without prior TBI | 1800 and 2400 | 7.4 ± 0.2 b | NR Age at diagnosis of TC: 20.5 (17.1–24.1) | Papillary | RAI therapy and subtotal or total thyroidectomy | 26/26 |
| Perkins et al., 2013 [23] | ALL and AML | Radiation therapy | NR | 7.0 (0–18.0) | ~7–35.5 | NS | NR | 18/18 |
| Renard et al., 2011 [24] | ALL | Radiotherapy and regimens including topoisomerase-II inhibitors and HSCT | NR | 5.0 ± 1.0 | 5.5 ± 0.9 | Papillary | NR | 2/2 |
| Schiemegelow et al., 2013 [25] | ALL | CP, EPT, CNS irradiation | NR | 5.0 (3.1–6.5) | 10.1 (7.8–13.5) | NS | NR | 32/32 |
| Socie’ et al., 2000 [26] | ALL | Allogeneic BMT, conditioning regimen (TBI or limited field irradiation ± CP ± other drugs), SCT | low dose or high dose (≥1000 single-dose or ≥1300 fractionated) | 7.4 ± 2.6 d | 7.7 ± 4.5 | Papillary | NR | 2/2 |
| Taylor et al., 2009 [27] | ALL and AML | TBI, cranial radiotherapy, chemotherapy | Cranial: 1000–2500 TBI: 1000 | 2–14 | 8–33 | Papillary, follicular, other | NR | NR |
| Toret et al., 2024 [28] | ALL | Cranial radiotherapy (39%), HSCT (12%), TBI (12%) | NR | 5.3 ± 4.3 | 18.9 ± 8.4 e | NS | NR | NR |
| Veiga et al., 2012 [29] | NS | Chemotherapy (alkylating agents, anthracyclines, bleomycin, platinum compounds, and EPT) and radiotherapy | 0–4000 | 5.7 ± 4.0 | 21.0 ± 7.7 | Papillary and mixed papillary, follicular, other | NR | NR |
| Study ID (Authors; Reference) | Selection (Maximum of 3 Stars) | Comparability (Maximum of 2 Stars) | Outcomes (Maximum of 3 Stars) | Quality Assessment a |
|---|---|---|---|---|
| Acharya et al., 2003 [12] | 3 | 1 | 3 | Good |
| Bebeshko et al., 2021 [13] | 2 | 1 | 3 | Fair |
| Berger et al., 2011 [14] | 3 | 2 | 3 | Good |
| Bhatia et al., 2002 [15] | 3 | 1 | 3 | Good |
| Borgman et al., 2008 [16] | 3 | 1 | 3 | Good |
| Echecopar et al., 2024 [17] | 3 | 1 | 3 | Good |
| Gow et al., 2003 [18] | 3 | 1 | 3 | Good |
| Koh et al., 2016 [19] | 3 | 1 | 2 | Good |
| Martucci et al., 2022 [20] | 3 | 1 | 3 | Good |
| Maule et al., 2007 [21] | 3 | 2 | 3 | Good |
| Oudin et al., 2016 [22] | 3 | 2 | 3 | Good |
| Perkins et al., 2013 [23] | 3 | 2 | 3 | Good |
| Renard et al., 2011 [24] | 3 | 1 | 3 | Good |
| Schiemegelow et al., 2013 [25] | 3 | 2 | 3 | Good |
| Socie’ et al., 2000 [26] | 3 | 2 | 3 | Good |
| Taylor et al., 2009 [27] | 3 | 1 | 3 | Good |
| Toret et al., 2024 [28] | 3 | 1 | 3 | Good |
| Veiga et al., 2012 [29] | 3 | 2 | 3 | Good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsinopoulou, V.R.; Kotanidou, E.P.; Kolanis, S.; Tragiannidis, A.; Hatzipantelis, E.; Galli-Tsinopoulou, A. Thyroid Cancer in Childhood Leukemia Survivors: A Systematic Review of the Incidence and Survival Outcomes. J. Clin. Med. 2025, 14, 4248. https://doi.org/10.3390/jcm14124248
Tsinopoulou VR, Kotanidou EP, Kolanis S, Tragiannidis A, Hatzipantelis E, Galli-Tsinopoulou A. Thyroid Cancer in Childhood Leukemia Survivors: A Systematic Review of the Incidence and Survival Outcomes. Journal of Clinical Medicine. 2025; 14(12):4248. https://doi.org/10.3390/jcm14124248
Chicago/Turabian StyleTsinopoulou, Vasiliki Rengina, Eleni P. Kotanidou, Savvas Kolanis, Athanasios Tragiannidis, Emmanouel Hatzipantelis, and Assimina Galli-Tsinopoulou. 2025. "Thyroid Cancer in Childhood Leukemia Survivors: A Systematic Review of the Incidence and Survival Outcomes" Journal of Clinical Medicine 14, no. 12: 4248. https://doi.org/10.3390/jcm14124248
APA StyleTsinopoulou, V. R., Kotanidou, E. P., Kolanis, S., Tragiannidis, A., Hatzipantelis, E., & Galli-Tsinopoulou, A. (2025). Thyroid Cancer in Childhood Leukemia Survivors: A Systematic Review of the Incidence and Survival Outcomes. Journal of Clinical Medicine, 14(12), 4248. https://doi.org/10.3390/jcm14124248










