Serum Presepsin Might Not Detect Periprosthetic Joint Infection After Hip Arthroplasty
Abstract
1. Introduction
2. Material and Method
2.1. Study 1
2.2. Measurement Protocol
2.3. Statistical Analysis
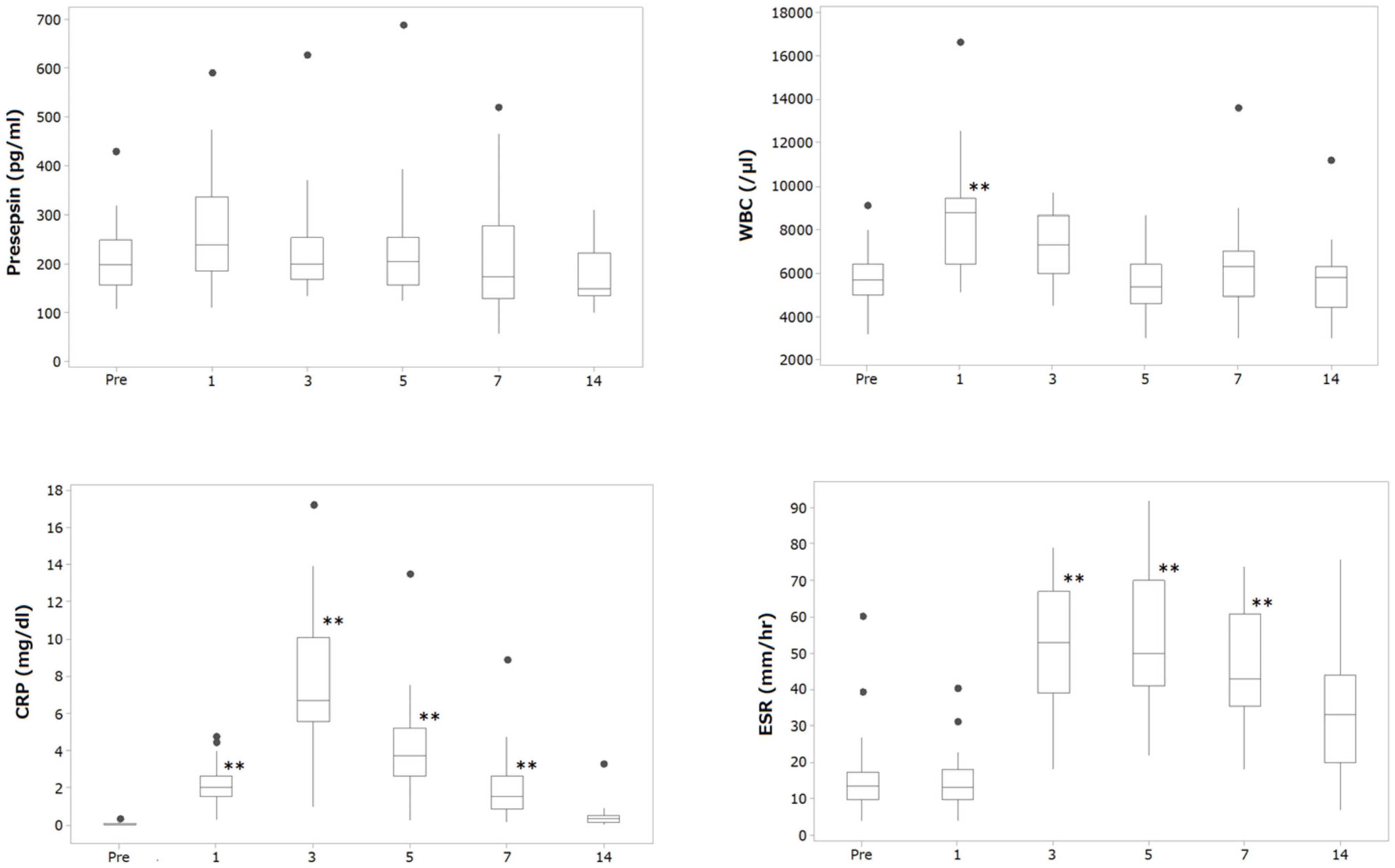
2.4. Result
2.5. Study 2
2.6. Measurement Protocol
2.7. Statistical Analysis
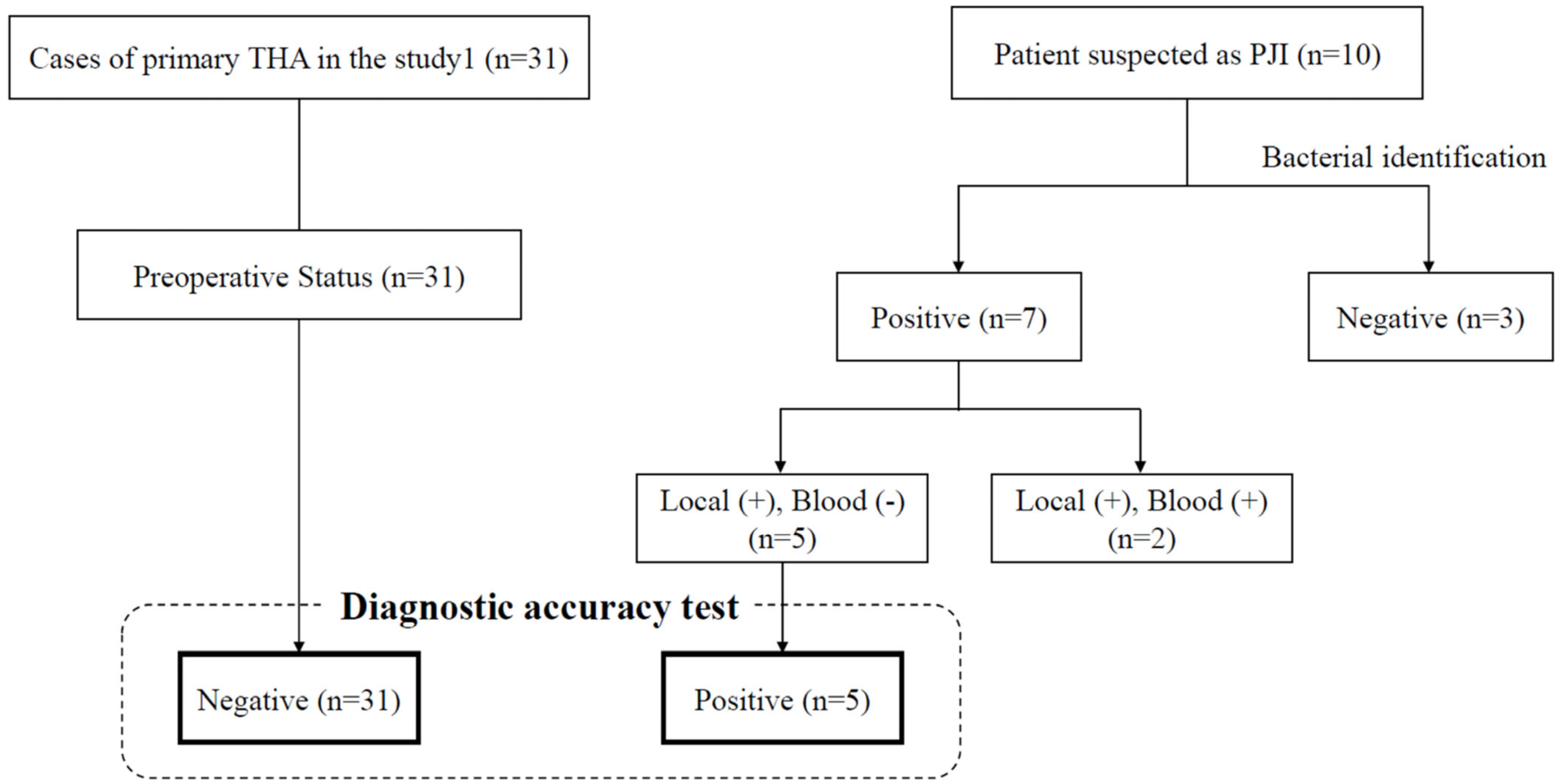
2.8. Result
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ateschrang, A.; Kohl, S.; Keel, M.J.B.; Albers, C.E.; Hirschmann, M.T.; Shaker, A.; Buetikofer, L.; Stöckle, U.; Becker, R.; Maqungo, S.; et al. A meta-analysis of synovial biomarkers in periprosthetic joint infection: Synovasure™ is less effective than the ELISA-based alpha-defensin test. Knee Surgery Sports Traumatol. Arthrosc. 2018, 26, 3039–3047. [Google Scholar] [CrossRef]
- George, J.; Klika, A.K.; Faour, M.; Higuera, C.A.; Saleh, A. Serum biomarkers in periprosthetic joint infections. Bone Jt. Res. 2018, 7, 85–93. [Google Scholar] [CrossRef]
- Parvizi, J.; Zmistowski, B.; Berbari, E.F.; Bauer, T.W.; Springer, B.D.; Della Valle, C.J.; Garvin, K.L.; Mont, M.A.; Wongworawat, M.D.; Zalavras, C.G. New Definition for Periprosthetic Joint Infection: From the Workgroup of the Musculoskeletal Infection Society. Clin. Orthop. Relat. Res. 2011, 469, 2992–2994. [Google Scholar] [CrossRef]
- Spangehl, M.J.; Masri, B.A.; O’connell, J.X.; Duncan, C.P. Prospective Analysis of Preoperative and Intraoperative Investigations for the Diagnosis of Infection at the Sites of Two Hundred and Two Revision Total Hip Arthroplasties*. J. Bone Jt. Surg. Am. 1999, 81, 672–683. [Google Scholar] [CrossRef]
- Yaegashi, Y.; Shirakawa, K.; Sato, N.; Suzuki, Y.; Kojika, M.; Imai, S.; Endo, S. Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. J. Infect. Chemother. Off. J. Jpn Soc. Chemother. 2005, 11, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Matsumoto, N.; Takahashi, G.; Kojika, M.; Okamura, Y.; Shozushima, T. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J. Infect. Chemother. 2011, 17, 764–769. [Google Scholar] [CrossRef]
- Arai, Y.; Mizugishi, K.; Nonomura, K.; Naitoh, K.; Takaori-Kondo, A.; Yamashita, K. Phagocytosis by human monocytes is required for the secretion of presepsin. J. Infect. Chemother. Off. J. Jpn Soc. Chemother. 2015, 21, 564–569. [Google Scholar] [CrossRef]
- Chenevier-Gobeaux, C.; Borderie, D.; Weiss, N.; Mallet-Coste, T.; Claessens, Y.-E. Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clin. Chim. Acta Int. J. Clin. Chem. 2015, 450, 97–103. [Google Scholar] [CrossRef]
- Giavarina, D.; Carta, M. Determination of reference interval for presepsin, an early marker for sepsis. Biochem. Medica Cas. Hrvat. Drus. Med. Biokem./HDMB 2015, 25, 64–68. [Google Scholar] [CrossRef]
- Endo, S.; Suzuki, Y.; Takahashi, G.; Shozushima, T.; Ishikura, H.; Murai, A.; Okamura, Y. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2012, 18, 891–897. [Google Scholar] [CrossRef]
- Tadic, T.; Vodnik, T.; Majkic-Singh, N.; Kaljevic, G. Presepsin (sCD14-ST) in preoperative diagnosis of abdominal sepsis. Clin. Chem. Lab. Med. CCLM/FESCC 2013, 51, 2053–2062. [Google Scholar] [CrossRef]
- Nakamura, M.; Yamasaki, F.; Naito, K.; Takeuchi, T.; Hosaka, Y.; Shirakawa, K.; Furusako, S. Early elevation of plasma soluble CD14 subtype, a novel biomarker for sepsis, in a rabbit cecal ligation and puncture model. Crit. Care 2008, 12, P194. [Google Scholar] [CrossRef]
- Busch, A.; Jäger, M.; Engler, H.; Wasssenaar, D.; Bielefeld, C.; Wegner, A. Diagnostic Accuracy of Synovial Neopterin, TNF-α and Presepsin in Periprosthetic Joint Infection: A Prospective Study. Z. Orthopädie Unfallchirurgie 2022, 160, 299–306. [Google Scholar] [CrossRef]
- Moretti, B.; Vicenti, G.; Pesce, V.; Palmiotto, F.; Carrozzo, M.; Bizzoca, D.; Nappi, V. Perioperative plasmatic presepsin levels in patients undergoing total hip or knee replacement: A preliminary study. J. Biol. Regul. Homeost. Agents 2017, 31, 1081–1086. [Google Scholar]
- Masson, S.; Tognoni, G.; Fumagalli, R.; Isgrò, S.; Fanizza, C.; Romero, M.; Spanuth, E.; Thomae, R.; Sangiorgi, G.; Gattinoni, L.; et al. Presepsin (soluble CD14 subtype) and procalcitonin levels for mortality prediction in sepsis: Data from the Albumin Italian Outcome Sepsis trial. Crit. Care/Soc. Crit. Care Med. 2014, 18, R6. [Google Scholar] [CrossRef]
- International Committee for Standardization in Hematology. Recommendation of measurement of erythrocyte sedimentation rate of human blood. Am. J. Clin. Pathol. 1977, 68, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, H.; Okamura, Y. Development of a point-of-care assay system for measurement of presepsin (sCD14-ST). Clin. Chim. Acta Int. J. Clin. Chem. 2011, 412, 2157–2161. [Google Scholar] [CrossRef]
- Phillips, C.B.; Barrett, J.A.; Losina, E.; Mahomed, N.N.; Lingard, E.A.; Guadagnoli, E.; Baron, J.A.; Harris, W.H.; Poss, R.; Katz, J.N. Incidence rates of dislocation, pulmonary embolism, and deep infection during the first six months after elective total hip replacement. J. Bone Joint Surg. Am. 2003, 85, 20–26. [Google Scholar] [CrossRef]
- Krejcie, R.V.; Morgan, D.W. Determining Sample Size for Research Activities. Educ. Psychol. Meas. 1970, 30, 607–610. [Google Scholar] [CrossRef]
- Della Valle, C.; Higuera, C.; Tan, T.L.; Shohat, N.; Parvizi, J.; Goswami, K.; Chen, A.F. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Infectious Diseases Society of America. Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W.; Lemeshow, S. Assessing the fit of the model. In Applied Logistic Regression; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; pp. 143–202. [Google Scholar]
- Alamanda, V.K.; Springer, B.D. The prevention of infection: 12 modifiable risk factors. Bone Jt. J. 2019, 101-B (Suppl. SA), 3–9. [Google Scholar] [CrossRef]
- Kheir, M.M.; Hansen, H.; Tan, T.L.; Rondon, A.J.; Woolsey, A.; Parvizi, J. Infection Following Total Joint Arthroplasty Is the Main Cause of Litigation: Data from One Metropolitan Area. J. Arthroplast. 2018, 33, 1520–1523. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Crapanzano, C.; Morelli, F.; Marazzi, M.G.; Brioschi, M.; Galliera, E.; Drago, L.; Romanelli, M.M.C.; Banfi, G.; Randelli, F.; et al. Presepsin: A potential biomarker of PJI? A comparative analysis with known and new infection biomarkers. Int. J. Immunopathol. Pharmacol. 2018, 31, 394632017749356. [Google Scholar] [CrossRef]
- Romero, M.; Tognoni, G.; Fanizza, C.; Masson, S.; Thomae, R.; Bernasconi, R.; Noto, A.; Oggioni, R.; Pasetti, G.S.; Caironi, P.; et al. Circulating presepsin (soluble CD14 subtype) as a marker of host response in patients with severe sepsis or septic shock: Data from the multicenter, randomized ALBIOS trial. Intensiv. Care Med. 2014, 41, 12–20. [Google Scholar] [CrossRef]
- Drago, L.; Dozio, E.; De Vecchi, E.; Mattina, R.; Vassena, C.; Corsi, M.; Romano, C. Procalcitonin, C-Reactive Protein, Interleukin-6, and Soluble Intercellular Adhesion Molecule-1 as Markers of Postoperative Orthopaedic Joint Prosthesis Infections. Int. J. Immunopathol. Pharmacol. 2011, 24, 433–440. [Google Scholar] [CrossRef]
- Chang, E.T.; Kurtz, S.M.; Zimmerli, W.; Lau, E.C.; Son, M.-S.; Parvizi, J. Are We Winning or Losing the Battle With Periprosthetic Joint Infection: Trends in Periprosthetic Joint Infection and Mortality Risk for the Medicare Population. J. Arthroplast. 2018, 33, 3238–3245. [Google Scholar] [CrossRef]
| Sex | Age | Consent Obtained |
|---|---|---|
| F | 62 | consent |
| F | 75 | consent |
| F | 61 | consent |
| F | 79 | consent |
| F | 68 | consent |
| F | 83 | consent |
| F | 65 | consent |
| F | 65 | consent |
| F | 67 | consent |
| F | 66 | consent |
| F | 61 | consent |
| F | 53 | consent |
| F | 74 | consent |
| F | 68 | consent |
| F | 75 | consent |
| F | 76 | consent |
| F | 76 | consent |
| F | 65 | consent |
| F | 53 | consent |
| F | 53 | consent |
| F | 68 | consent |
| F | 74 | consent |
| F | 68 | consent |
| F | 53 | consent |
| F | 64 | consent |
| M | 72 | consent |
| M | 52 | consent |
| M | 66 | consent |
| M | 63 | consent |
| M | 55 | consent |
| M | 46 | consent |
| F | 67 | consent |
| M | 74 | consent |
| M | 69 | consent |
| M | 56 | consent |
| M | 74 | consent |
| F | 69 | consent |
| F | 81 | consent |
| M | 69 | consent |
| M | 48 | consent |
| F | 73 | consent |
| Parameter | |
|---|---|
| Number of subjects | 29 |
| Gender (M/F) | 5/24 |
| Number of side (L/R) | 7/24 |
| Age at surgery | 65.4 (9.1) |
| Disease type | |
| Osteoarthritis | 26 |
| Ischemic optic neuropathy | 1 |
| Traumatic hip arthrosis | 3 |
| Rapidly destructive coxarthropathy | 1 |
| Serum Biomarkers | Before Surgery | After Surgery | |||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 5 | Day 7 | Day 14 | |||
| Presepsin (pg/mL) | Mean (SD) | 207.4 (67.4) | 269.9 (109.9) | 222.2 (93.8) | 226.2 (109.6) | 214.6 (113.3) | 178.6 (59.2) |
| IQR | 162.0, 249.5 | 195.0, 323.3 | 168.0, 244.0 | 159.0, 244.8 | 130.5, 271.0 | 137.0, 221.0 | |
| WBC (/μL) | Mean (SD) | 5783.9 (1435.8) | 8446.7 (2387.6) | 7217.4 (1479.1) | 5616.1 (1360.9) | 6240.0 (2014.7) | 5603.4 (1547.5) |
| IQR | 5100, 6350 | 6625, 9400 | 6100, 8250 | 4750, 6350 | 5050, 7000 | 4600, 6300 | |
| CRP (mg/dL) | Mean (SD) | 0.10 (0.09) | 2.10 (1.11) | 7.46 (3.75) | 4.04 (2.51) | 2.06 (1.72) | 0.49 (0.59) |
| IQR | 0.04, 0.13 | 1.64, 2.56 | 5.57, 9.36 | 2.70, 5.13 | 0.90, 2.56 | 0.19, 0.58 | |
| ESR (mm/h) | Mean (SD) | 15.7 (11.1) | 14.4 (7.4) | 50.1 (17.4) | 54.6 (18.9) | 46.2 (16.2) | 31.6 (15.9) |
| IQR | 10.0, 17.0 | 10.3, 17.5 | 39.5, 66.5 | 42.5, 67.5 | 36.3, 60.0 | 20.0, 43.3 | |
| Case No | Age | Sex | Blood Test | Bacterial Identification | Sinus Tract | ||||
|---|---|---|---|---|---|---|---|---|---|
| Presepsin (pg/mL) | WBC (/μL) | CRP (mg/dL) | ESR (mm/h) | Local | Blood | ||||
| 1 | 67 | F | 170 | 4800 | 0.96 | 56 | Enterococcus. faecalis | No growth | − |
| 2 | 74 | M | 170 | 7300 | 1.49 | N/A # | Staphylococcus caprae | No growth | − |
| 3 | 69 | M | 179 | 6000 | 1.06 | 12 | MRCNS (Staphylococcus caprae) | No growth | − |
| 4 | 56 | M | 271 | 15,600 | 5.36 | 56 | MRSA | No growth | + |
| 5 | 74 | M | 246 | 5900 | 0.44 | 48 | MRCNS (Staphylococcus epidermidis) | No growth | + |
| 6 | 48 | M | 244 | 7100 | 2.56 | 54 | MRSA | MRCNS (Staphylococcus haemolyticus) | + |
| 7 | 73 | F | 1700 | 12,200 | 4.97 | 99 | MRCNS (Staphylococcus epidermidis) | MRCNS (Staphylococcus epidermidis) | − |
| Presepsin Level | Sensitivity | Specificity |
|---|---|---|
| 170 | 100% | 29% |
| 174 | 60% | 29% |
| 177 | 60% | 32% |
| 179 | 60% | 35% |
| 184 | 40% | 39% |
| 190 | 40% | 45% |
| 199 | 40% | 48% |
| 201 | 40% | 55% |
| 215 | 40% | 58% |
| 219 | 40% | 61% |
| 235 | 40% | 65% |
| 238 | 40% | 68% |
| 246 | 40% | 71% |
| 249 | 20% | 71% |
| 250 | 20% | 74% |
| 260 | 20% | 77% |
| 268 | 20% | 81% |
| 271 | 20% | 87% |
| 272 | 0% | 87% |
| 278 | 0% | 90% |
| 320 | 0% | 94% |
| 428 | 0% | 97% |
| 429 | 0% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, K.; Morishima, T.; Watanabe, K.; Ikemoto, T.; Nakamura, Y.; Takahashi, N. Serum Presepsin Might Not Detect Periprosthetic Joint Infection After Hip Arthroplasty. J. Clin. Med. 2025, 14, 4246. https://doi.org/10.3390/jcm14124246
Hashimoto K, Morishima T, Watanabe K, Ikemoto T, Nakamura Y, Takahashi N. Serum Presepsin Might Not Detect Periprosthetic Joint Infection After Hip Arthroplasty. Journal of Clinical Medicine. 2025; 14(12):4246. https://doi.org/10.3390/jcm14124246
Chicago/Turabian StyleHashimoto, Kohei, Takkan Morishima, Kazutaka Watanabe, Tatsunori Ikemoto, Yukio Nakamura, and Nobunori Takahashi. 2025. "Serum Presepsin Might Not Detect Periprosthetic Joint Infection After Hip Arthroplasty" Journal of Clinical Medicine 14, no. 12: 4246. https://doi.org/10.3390/jcm14124246
APA StyleHashimoto, K., Morishima, T., Watanabe, K., Ikemoto, T., Nakamura, Y., & Takahashi, N. (2025). Serum Presepsin Might Not Detect Periprosthetic Joint Infection After Hip Arthroplasty. Journal of Clinical Medicine, 14(12), 4246. https://doi.org/10.3390/jcm14124246







