Use of Cell Saver in Elective Coronary Bypass Surgery: What Do We Risk When Saving Blood?
Abstract
1. Introduction
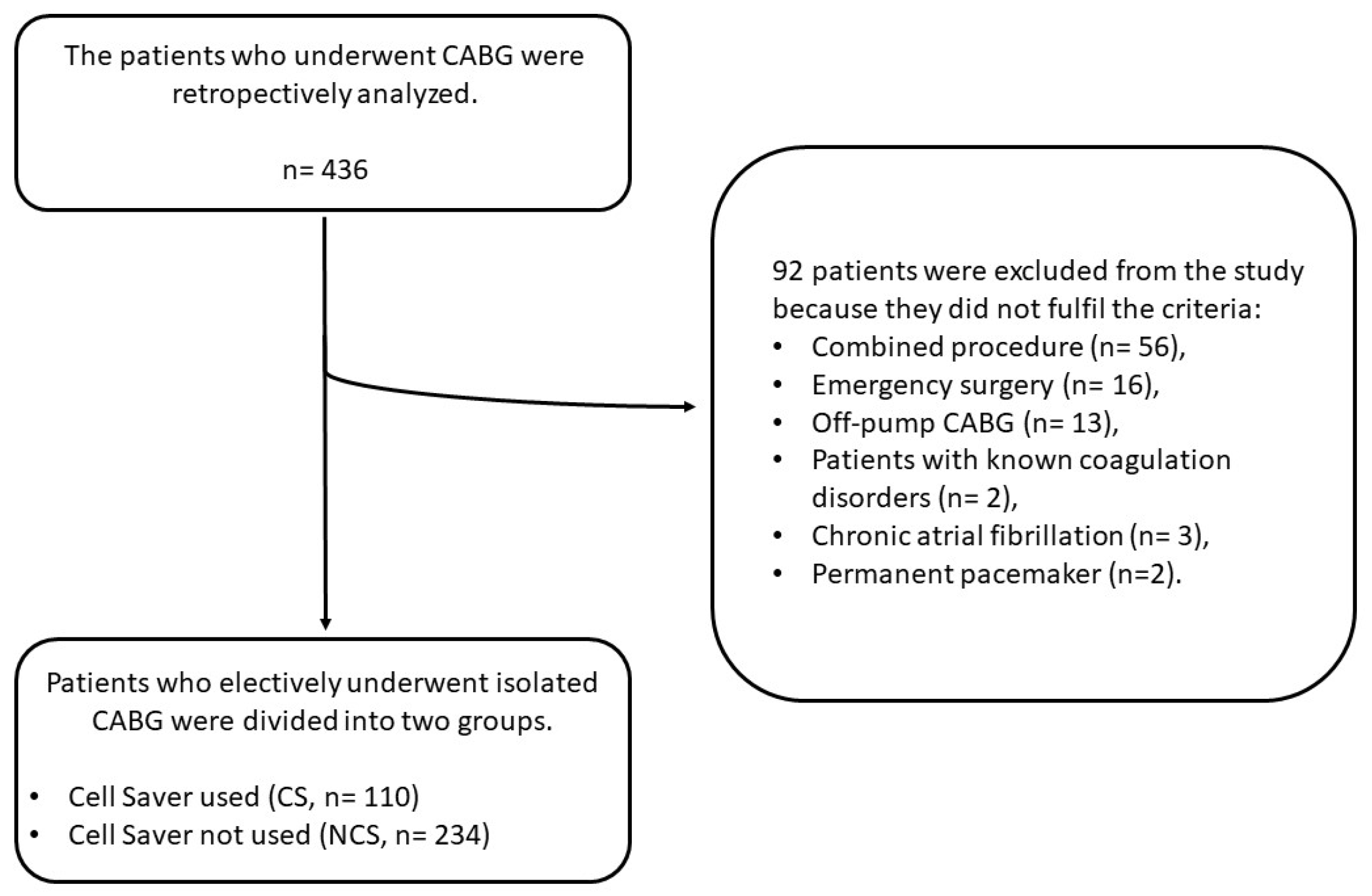
2. Materials and Methods
2.1. The Study Population
2.2. The Use of Cell Saver
2.3. Data Collection
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Aortic cross clamping |
| AKF | Acute kidney failure |
| ACT | Activated clotting time |
| CABG | Coronary artery bypass grafting |
| COPD | Chronic obstructive pulmonary disease |
| CPB | Cardiopulmonary bypass |
| CRP | C-reactive protein |
| CVA | Cerebrovascular accident |
| GFR | Glomerular filtration rate |
| INR | International normalized ratio |
| LA | Left atrium |
| NOAF | New-onset atrial fibrillation |
| RBC | Red blood cell |
References
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Arghami, A.; Habib, R.; Daneshmand, M.A.; Parsons, N.; Elhalabi, Z.; Krohn, C.; Thourani, V.; Bowdish, M.E. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2022 Update on Outcomes and Research. Ann. Thorac. Surg. 2023, 115, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Shander, A.; Moskowitz, D.; Rijhwani, T.S. The safety and efficacy of "bloodless" cardiac surgery. Semin. Cardiothorac. Vasc. Anesth. 2005, 9, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, G.; Torracca, L.; Capestro, F.; Matteucci, M.L.; Rossi, M. Allogenic blood transfusion in cardiac surgery. J. Card. Surg. 2012, 27, 594–599. [Google Scholar] [CrossRef]
- Gaffney, A.M.; Sladen, R.N. Acute kidney injury in cardiac surgery. Curr. Opin. Anaesthesiol. 2015, 28, 50–59. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Liu, Z.; Hu, Z.; Zheng, G. Blood transfusion and risk of atrial fibrillation after coronary artery bypass graft surgery: A meta-analysis of cohort studies. Medicine 2018, 97, e9700. [Google Scholar] [CrossRef]
- Raphael, J.; Mazer, C.D.; Subramani, S.; Schroeder, A.; Abdalla, M.; Ferreira, R.; Roman, P.E.; Patel, N.; Welsby, I.; Greilich, P.E.; et al. Society of Cardiovascular Anesthesiologists Clinical Practice Improvement Advisory for Management of Perioperative Bleeding and Hemostasis in Cardiac Surgery Patients. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2887–2899. [Google Scholar] [CrossRef]
- Blundell, J. Experiments on the Transfusion of Blood by the Syringe. Med. Chir. Trans. 1818, 9 Pt 1, 56–92. [Google Scholar] [CrossRef]
- Klebanoff, G. Early clinical experience with a disposable unit for the intraoperative salvage and reinfusion of blood loss (intraoperative autotransfusion). Am. J. Surg. 1970, 120, 718–722. [Google Scholar] [CrossRef]
- Wang, G.; Bainbridge, D.; Martin, J.; Cheng, D. The efficacy of an intraoperative cell saver during cardiac surgery: A meta-analysis of randomized trials. Anesth. Analg. 2009, 109, 320–330. [Google Scholar] [CrossRef]
- Lloyd, T.D.; Geneen, L.J.; Bernhardt, K.; McClune, W.; Fernquest, S.J.; Brown, T.; Dorée, C.; Brunskill, S.J.; Murphy, M.F.; Palmer, A.J.; et al. Cell salvage for minimising perioperative allogeneic blood transfusion in adults undergoing elective surgery. Cochrane Database Syst. Rev. 2023, 9, Cd001888. [Google Scholar] [CrossRef]
- Reyes, G.; Prieto, M.; Alvarez, P.; Orts, M.; Bustamante, J.; Santos, G.; Sarraj, A.; Planas, A. Cell saving systems do not reduce the need of transfusion in low-risk patients undergoing cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2011, 12, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Djaiani, G.; Fedorko, L.; Borger, M.A.; Green, R.; Carroll, J.; Marcon, M.; Karski, J. Continuous-flow cell saver reduces cognitive decline in elderly patients after coronary bypass surgery. Circulation 2007, 116, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, S.; Nielsen, C.H.; Andersen, L.W.; Bendtzen, K.; Tvede, M.; Steinbrüchel, D.A. Cell saver for on-pump coronary operations reduces systemic inflammatory markers: A randomized trial. Ann. Thorac. Surg. 2010, 89, 1511–1517. [Google Scholar] [CrossRef]
- Al-Mandhari, S.; Maddali, M.M.; Al-Bahrani, M.J. Cell salvage during coronary artery bypass surgery and allogenic blood exposure. Asian Cardiovasc. Thorac. Ann. 2015, 23, 913–916. [Google Scholar] [CrossRef]
- Scrascia, G.; Rotunno, C.; Nanna, D.; Rociola, R.; Guida, P.; Rubino, G.; Schinosa, L.d.L.T.; Paparella, D. Pump blood processing, salvage and re-transfusion improves hemoglobin levels after coronary artery bypass grafting, but affects coagulative and fibrinolytic systems. Perfusion 2012, 27, 270–277. [Google Scholar] [CrossRef]
- Campbell, J.; Holland, C.; Richens, D.; Skinner, H. Impact of cell salvage during cardiac surgery on the thrombelastomeric coagulation profile: A pilot study. Perfusion 2012, 27, 221–224. [Google Scholar] [CrossRef]
- Vymazal, T.; Filaun, M.; Horacek, M. Impact of retransfusion of blood processed in cell-saver on coagulation versus cardiopulmonary bypass: A prospective observational study using thromboelastography. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2015, 159, 131–134. [Google Scholar] [CrossRef]
- Tachias, F.; Samara, E.; Petrou, A.; Karakosta, A.; Siminelakis, S.; Apostolakis, E.; Tzimas, P.; Minervini, G. The Effect of Cell Salvage on Bleeding and Transfusion Needs in Cardiac Surgery. Anesthesiol. Res. Pract. 2022, 2022, 3993452. [Google Scholar] [CrossRef]
- Vonk, A.B.; Meesters, M.I.; Garnier, R.P.; Romijn, J.W.; van Barneveld, L.J.; Heymans, M.W.; Jansen, E.K.; Boer, C. Intraoperative cell salvage is associated with reduced postoperative blood loss and transfusion requirements in cardiac surgery: A cohort study. Transfusion 2013, 53, 2782–2789. [Google Scholar] [CrossRef]
- Vieira, S.D.; Perini, F.d.C.V.; de Sousa, L.C.B.; Buffolo, E.; Chaccur, P.; Arrais, M.; Jatene, F.B. Autologous blood salvage in cardiac surgery: Clinical evaluation, efficacy and levels of residual heparin. Hematol. Transfus. Cell Ther. 2021, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pillay, K.; Perumal, S. Intraoperative Cell Saving: Is the Solution the Actual Problem? J. Extra Corpor. Technol. 2021, 53, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Berbel-Franco, D.; Lopez-Delgado, J.C.; Putzu, A.; Esteve, F.; Torrado, H.; Farrero, E.; Rodríguez-Castro, D.; Carrio, M.L.; Landoni, G. The influence of postoperative albumin levels on the outcome of cardiac surgery. J. Cardiothorac. Surg. 2020, 15, 78. [Google Scholar] [CrossRef]
- Koch, C.G.; Li, L.; Van Wagoner, D.R.; Duncan, A.I.; Gillinov, A.M.; Blackstone, E.H. Red cell transfusion is associated with an increased risk for postoperative atrial fibrillation. Ann. Thorac. Surg. 2006, 82, 1747–1756. [Google Scholar] [CrossRef]
- Whitson, B.A.; Huddleston, S.J.; Savik, K.; Shumway, S.J. Bloodless cardiac surgery is associated with decreased morbidity and mortality. J. Card. Surg. 2007, 22, 373–378. [Google Scholar] [CrossRef]
- Vlahou, A.; Diplaris, K.; Ampatzidou, F.; Karagounnis, L.; Drossos, G. The Role of Blood Transfusion in the Development of Atrial Fibrillation after Coronary Artery Bypass Grafting. Thorac. Cardiovasc. Surg. 2016, 64, 688–692. [Google Scholar] [CrossRef]
- Koçyiğit, M.; Koçyiğit, Ö.I.; Güllü, A.; Şenay, Ş.; Alhan, C. Postoperative Atrial Fibrillation Reduced by Intraoperative and Postoperative Cell Saver System in Coronary Artery Bypass Graft Surgery. Turk. J. Anaesthesiol. Reanim. 2022, 50, 173–177. [Google Scholar] [CrossRef]
- Brascia, D.; Garcia-Medina, N.; Kinnunen, E.M.; Tauriainen, T.; Airaksinen, J.; Biancari, F. Impact of transfusion on stroke after cardiovascular interventions: Meta-analysis of comparative studies. J. Crit. Care 2017, 38, 157–163. [Google Scholar] [CrossRef]
- Karkouti, K. Transfusion and risk of acute kidney injury in cardiac surgery. Br. J. Anaesth. 2012, 109 (Suppl. S1), i29–i38. [Google Scholar] [CrossRef]
| NCS (n = 234) | CS (n = 110) | p | |
|---|---|---|---|
| Age (year) | 63.7 ± 8.9 | 63.6 ± 9.3 | 0.966 |
| BSA (m2) | 1.89 ± 0.17 | 1.89 ± 0.19 | 0.830 |
| EuroScore II | 1.48 ± 0.95 | 1.46 ± 0.92 | 0.849 |
| Biological sex | 0.083 | ||
| Female | 65 (27.8%) | 21 (19.1%) | |
| Male | 169 (72.2%) | 89 (80.9%) | |
| Comorbidities | |||
| Diabetes mellitus | 112 (47.9%) | 52 (47.3%) | 0.919 |
| Hypertension | 168 (71.8%) | 80 (72.7%) | 0.857 |
| COPD | 14 (6%) | 8 (7.3%) | 0.826 |
| CVA | 18 (7.7%) | 9 (8.2%) | >0.99 |
| GFR < 50 mL/min | 43 (18.4%) | 16 (14.5%) | 0.468 |
| Ejection fraction (%) | 54.2 ± 7.4 | 53.4 ± 7.8 | 0.355 |
| LA diameter (mm) | 35.9 ± 4.7 | 36.7 ± 4.6 | 0.201 |
| Number of grafted vessels | 3.2 ± 0.9 | 3.3 ± 0.8 | 0.431 |
| CPB time (min) | 106.16 ± 30.36 | 102.88 ± 24.74 | 0.241 |
| ACC time (min) | 54.81 ± 17.38 | 52.80 ± 14.70 | 0.294 |
| NCS (n = 234) | CS (n = 110) | p | |
|---|---|---|---|
| Cell Saver Volume (mL) | 0 | 568.25 ± 197.94 | <0.001 |
| Drainage in 6 h (mL) | 298.18 ± 155.81 | 388.64 ± 173.62 | <0.001 |
| Drainage Index (mL/m2) | 159.42 ± 85.13 | 207.23 ± 95.78 | <0.001 |
| Drainage in 24 h (mL) | 703.22 ± 320.39 | 827.73 ± 344.69 | 0.001 |
| Drainage Index (mL/m2) | 374.26 ± 172.89 | 440.54 ± 192.08 | 0.002 |
| RBC Transfusion (bag) | 3.82 ± 2.37 | 2.95 ± 2.05 | 0.001 |
| Extubation Time (h) | 6.14 ± 4.08 | 6.29 ± 3.87 | 0.750 |
| Reintubation | 8 (3.5%) | 6 (5.5%) | 0.392 |
| Re-exploration | 6 (2.6%) | 7 (6.4%) | 0.126 |
| Prolonged Drainage | 35 (15.2%) | 31 (28.2%) | 0.004 |
| Pneumonia | 11 (4.7%) | 8 (7.3%) | 0.340 |
| NOAF | 94 (40.2%) | 47 (42.7%) | 0.653 |
| AKF | 9 (3.9%) | 3 (2.8%) | 0.759 |
| CVA | 3 (1.3%) | 0 (0%) | 0.554 |
| ICU Stay (day) | 2.4 ± 2.6 | 2.2 ± 0.7 | 0.470 |
| Hospital Stay (day) | 8.5 ± 4.5 | 9.9 ± 5.5 | 0.014 |
| Rehospitalization | 15 (6.6%) | 3 (2.7%) | 0.142 |
| Mortality | 7 (3%) | 0 (0) | 0.102 |
| NCS (n = 234) | CS (n = 110) | p | |
|---|---|---|---|
| ACT (s) | |||
| Preoperative | 126.01 ± 17.84 | 124.76 ± 15.28 | 0.529 |
| Post-protamine | 116.79 ± 12.81 | 118.92 ± 12.53 | 0.149 |
| Admission to the ICU | 117.25 ± 11.99 | 122.46 ± 13.53 | <0.001 |
| CRP (mg/L) | |||
| Preoperative | 16.21 ± 23.87 | 17.65 ± 26.96 | 0.616 |
| Postoperative 1st day | 75.42 ± 30.88 | 77.70 ± 39.43 | 0.561 |
| Postoperative 4th day | 92.38 ± 43.02 | 96.18 ± 48.93 | 0.467 |
| Albumin (g/dL) | |||
| Preoperative | 4.19 ± 0.43 | 4.14 ± 0.45 | 0.289 |
| Postoperative 1st day | 3.51 ± 0.28 | 3.41 ± 0.32 | 0.003 |
| Postoperative 4th day | 3.29 ± 0.30 | 3.19 ± 0.29 | 0.003 |
| Hemoglobin (g/dL) | |||
| Preoperative | 13.12 ± 1.78 | 13.54 ± 1.73 | 0.039 |
| Postoperative 1st day | 9.54 ± 0.69 | 9.91 ± 0.87 | <0.001 |
| Postoperative 4th day | 10.36 ± 0.99 | 10.48 ± 0.99 | 0.316 |
| Hematocrit (%) | |||
| Preoperative | 38.75 ± 4.99 | 39.83 ± 4.69 | 0.057 |
| Postoperative 1st day | 27.76 ± 2.32 | 28.96 ± 2.59 | <0.001 |
| Postoperative 4th day | 30.82 ± 3.06 | 31.03 ± 2.97 | 0.558 |
| Platelet (103 µL) | |||
| Preoperative | 279.74 ± 83.04 | 270.27 ± 76.86 | 0.313 |
| Postoperative 1st day | 218.66 ± 64.39 | 203.45 ± 51.29 | 0.030 |
| Postoperative 4th day | 218.59 ± 71.29 | 207.51 ± 55.21 | 0.253 |
| INR | |||
| Preoperative | 1.016 ± 0.076 | 1.009 ± 0.078 | 0.453 |
| Postoperative 1st day | 1.099 ± 0.072 | 1.099 ± 0.080 | 0.954 |
| Postoperative 4th day | 1.049 ± 0.083 | 1.052 ± 0.111 | 0.808 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyhancan, A.; Büyükadalı, M.; Koçak, E.; Güçlü, O.; Hüseyin, S.; Canbaz, S. Use of Cell Saver in Elective Coronary Bypass Surgery: What Do We Risk When Saving Blood? J. Clin. Med. 2025, 14, 4230. https://doi.org/10.3390/jcm14124230
Reyhancan A, Büyükadalı M, Koçak E, Güçlü O, Hüseyin S, Canbaz S. Use of Cell Saver in Elective Coronary Bypass Surgery: What Do We Risk When Saving Blood? Journal of Clinical Medicine. 2025; 14(12):4230. https://doi.org/10.3390/jcm14124230
Chicago/Turabian StyleReyhancan, Adem, Mürsel Büyükadalı, Ertuğrul Koçak, Orkut Güçlü, Serhat Hüseyin, and Suat Canbaz. 2025. "Use of Cell Saver in Elective Coronary Bypass Surgery: What Do We Risk When Saving Blood?" Journal of Clinical Medicine 14, no. 12: 4230. https://doi.org/10.3390/jcm14124230
APA StyleReyhancan, A., Büyükadalı, M., Koçak, E., Güçlü, O., Hüseyin, S., & Canbaz, S. (2025). Use of Cell Saver in Elective Coronary Bypass Surgery: What Do We Risk When Saving Blood? Journal of Clinical Medicine, 14(12), 4230. https://doi.org/10.3390/jcm14124230






