Nutrition and Diet Patterns as Key Modulators of Metabolic Reprogramming in Melanoma Immunotherapy
Abstract
1. Introduction
2. Materials and Methods
3. Effect of Melanoma Metabolic Heterogeneity on Immunotherapy
3.1. Glucose Metabolism and the Warburg Effect
3.2. The Role of Mitochondria in Melanoma Survival and Immunotherapy Response
3.3. Amino Acid Metabolism and Immune Regulation
3.4. Purine Metabolism and the Immunomodulatory Role of Uric Acid
3.5. Lipid Metabolism and Therapeutic Implications in Melanoma
4. Macronutrients and Their Role in Melanoma Immunotherapy
4.1. Protein Intake and T-Cell Activation
4.2. Sugars and Fructose Metabolism in Immune Evasion
4.3. Metabolic Competition and Hyperglycaemia in Immunotherapy Response
4.4. Flavonoids and Polyphenols as Potential Adjuvants
4.5. The Role of Vitamins and Other Micronutrients in Melanoma Immunotherapy
5. Impact of Dietary Patterns on Melanoma Progression and Response to Immunotherapy
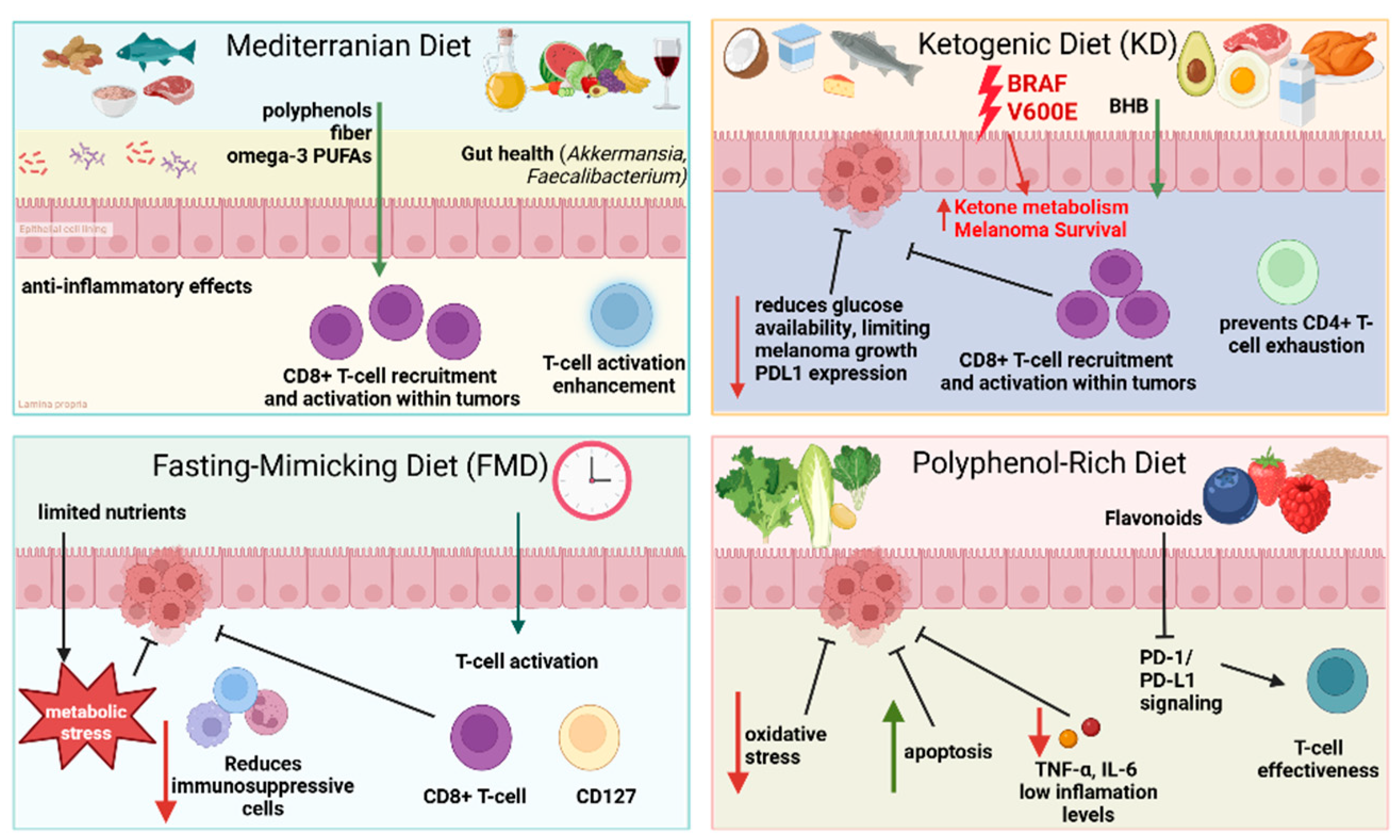
5.1. Mediterranean Diet and Immunotherapy
5.2. Ketogenic Diets and Tumor Metabolism
5.3. Fasting-Mimicking Diets and T-Cell Activation
6. Obesity-Driven Changes in Melanoma Immunotherapy
7. Gut Microbiota and Melanoma Immunotherapy
7.1. Nutrition-Related Clinical Trials
7.2. Personalized Nutrition Strategies for Melanoma Patients
8. Metabolic Influence in Melanoma Immunotherapy: Lessons from Other Cancers
9. Limitations and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dimitriou, F.; Krattinger, R.; Ramelyte, E.; Barysch, M.J.; Micaletto, S.; Dummer, R.; Goldinger, S.M. The World of Melanoma: Epidemiologic, Genetic, and Anatomic Differences of Melanoma Across the Globe. Curr. Oncol. Rep. 2018, 20, 87. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 Inhibitors as a Form of Cancer Immunotherapy: A Comprehensive Review of Registration Trials and Future Considerations. J. Immunother. Cancer 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Hassan, D.; Aldawsari, H.M.; Molugulu, N.; Shukla, R.; Kesharwani, P. Immune Checkpoint Inhibitors: A Promising Anticancer Therapy. Drug Discov. Today 2020, 25, 223–229. [Google Scholar] [CrossRef]
- Lipson, E.J.; Drake, C.G. Ipilimumab: An Anti-CTLA-4 Antibody for Metastatic Melanoma. Clin. Cancer Res. 2011, 17, 6958–6962. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-Driven Biomarkers to Guide Immune Checkpoint Blockade in Cancer Therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.-F.; Testori, A.; Grob, J.-J.; et al. Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef]
- Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. Available online: https://pubmed.ncbi.nlm.nih.gov/26027431/ (accessed on 3 May 2025).
- Grafanaki, K.; Maniatis, A.; Anastogianni, A.; Bania, A.; Petropoulou, A.; Bakoli, D.; Pasmatzi, E.; Stathopoulos, C. A-551—Personalized Nutrition in Melanoma: Optimizing Immunotherapy Through Diet and Metabolism. EJC Ski. Cancer 2025, 3, 100503. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Acevedo, A.; Davidoff, E.J.; Timmins, L.M.; Marrero-Berrios, I.; Patel, M.; White, C.; Lowe, C.; Sherba, J.J.; Hartmanshenn, C.; et al. The Growing Role of Precision and Personalized Medicine for Cancer Treatment. Technology 2018, 6, 79–100. [Google Scholar] [CrossRef]
- Grafanaki, K.; Grammatikakis, I.; Ghosh, A.; Gopalan, V.; Olgun, G.; Liu, H.; Kyriakopoulos, G.C.; Skeparnias, I.; Georgiou, S.; Stathopoulos, C.; et al. Noncoding RNA Circuitry in Melanoma Onset, Plasticity, and Therapeutic Response. Pharmacol. Ther. 2023, 248, 108466. [Google Scholar] [CrossRef]
- Pellegrini, M.; D’Eusebio, C.; Ponzo, V.; Tonella, L.; Finocchiaro, C.; Fierro, M.T.; Quaglino, P.; Bo, S. Nutritional Interventions for Patients with Melanoma: From Prevention to Therapy-An Update. Nutrients 2021, 13, 4018. [Google Scholar] [CrossRef]
- Yu, T.; Dong, T.; Eyvani, H.; Fang, Y.; Wang, X.; Zhang, X.; Lu, X. Metabolic Interventions: A New Insight into the Cancer Immunotherapy. Arch. Biochem. Biophys. 2021, 697, 108659. [Google Scholar] [CrossRef] [PubMed]
- Grzywa, T.M.; Paskal, W.; Włodarski, P.K. Intratumor and Intertumor Heterogeneity in Melanoma. Transl. Oncol. 2017, 10, 956–975. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.M.; Vashisht Gopal, Y.N.; McQuade, J.L.; Peng, W.; DeBerardinis, R.J.; Davies, M.A. Metabolic Strategies of Melanoma Cells: Mechanisms, Interactions with the Tumor Microenvironment, and Therapeutic Implications. Pigment. Cell Melanoma Res. 2018, 31, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, M.R.; Avagliano, A.; Granato, G.; Vigliar, E.; Masone, S.; Montagnani, S.; Arcucci, A. Metabolic Flexibility in Melanoma: A Potential Therapeutic Target. Semin. Cancer Biol. 2019, 59, 187–207. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Li, Y.; Luo, S.; Ma, R.; Liu, J.; Xu, P.; Zhang, H.; Tang, K.; Ma, J.; Zhang, Y.; Liang, X.; et al. Upregulation of Cytosolic Phosphoenolpyruvate Carboxykinase Is a Critical Metabolic Event in Melanoma Cells That Repopulate Tumors. Cancer Res. 2015, 75, 1191–1196. [Google Scholar] [CrossRef]
- Alberghina, L. The Warburg Effect Explained: Integration of Enhanced Glycolysis with Heterogeneous Mitochondria to Promote Cancer Cell Proliferation. Int. J. Mol. Sci. 2023, 24, 15787. [Google Scholar] [CrossRef]
- Henderson, J.; Duffy, L.; Stratton, R.; Ford, D.; O’Reilly, S. Metabolic Reprogramming of Glycolysis and Glutamine Metabolism Are Key Events in Myofibroblast Transition in Systemic Sclerosis Pathogenesis. J. Cell Mol. Med. 2020, 24, 14026–14038. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, X.-R.; Wu, C.-Y.; Liu, Y.-Y.; Chen, M.-B.; Gu, J.-H. PCK1 as a Target for Cancer Therapy: From Metabolic Reprogramming to Immune Microenvironment Remodeling. Cell Death Discov. 2024, 10, 478. [Google Scholar] [CrossRef]
- Ratnikov, B.I.; Scott, D.A.; Osterman, A.L.; Smith, J.W.; Ronai, Z.A. Metabolic Rewiring in Melanoma. Oncogene 2017, 36, 147–157. [Google Scholar] [CrossRef]
- Ho, J.; de Moura, M.B.; Lin, Y.; Vincent, G.; Thorne, S.; Duncan, L.M.; Hui-Min, L.; Kirkwood, J.M.; Becker, D.; Van Houten, B.; et al. Importance of Glycolysis and Oxidative Phosphorylation in Advanced Melanoma. Mol. Cancer 2012, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.; Mignion, L.; Jordan, B.F. Metabolic Profiling to Assess Response to Targeted and Immune Therapy in Melanoma. Int. J. Mol. Sci. 2024, 25, 1725. [Google Scholar] [CrossRef]
- Avagliano, A.; Fiume, G.; Pelagalli, A.; Sanità, G.; Ruocco, M.R.; Montagnani, S.; Arcucci, A. Metabolic Plasticity of Melanoma Cells and Their Crosstalk with Tumor Microenvironment. Front. Oncol. 2020, 10, 722. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.Z.; Shirai, K. What Impact Do the Proton Pump Inhibitors Have on the Efficacy of Immune Check Point Inhibitors in Metastatic Malignant Melanoma? J. Clin. Oncol. 2019, 37, e21040. [Google Scholar] [CrossRef]
- Liu, C.; Guo, H.; Mao, H.; Tong, J.; Yang, M.; Yan, X. An Up-To-Date Investigation Into the Correlation Between Proton Pump Inhibitor Use and the Clinical Efficacy of Immune Checkpoint Inhibitors in Advanced Solid Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 753234. [Google Scholar] [CrossRef]
- Lopes, S.; Pabst, L.; Dory, A.; Klotz, M.; Gourieux, B.; Michel, B.; Mascaux, C. Do Proton Pump Inhibitors Alter the Response to Immune Checkpoint Inhibitors in Cancer Patients? A Meta-Analysis. Front. Immunol. 2023, 14, 1070076. [Google Scholar] [CrossRef]
- Hua, S.; Wang, W.; Yao, Z.; Gu, J.; Zhang, H.; Zhu, J.; Xie, Z.; Jiang, H. The Fatty Acid-Related Gene Signature Stratifies Poor Prognosis Patients and Characterizes TIME in Cutaneous Melanoma. J. Cancer Res. Clin. Oncol. 2024, 150, 40. [Google Scholar] [CrossRef]
- Albers, E. Metabolic Characteristics and Importance of the Universal Methionine Salvage Pathway Recycling Methionine from 5′-Methylthioadenosine. IUBMB Life 2009, 61, 1132–1142. [Google Scholar] [CrossRef]
- Lang, X.; Green, M.D.; Wang, W.; Yu, J.; Choi, J.E.; Jiang, L.; Liao, P.; Zhou, J.; Zhang, Q.; Dow, A.; et al. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 2019, 9, 1673–1685. [Google Scholar] [CrossRef]
- Indini, A.; Grossi, F.; Mandalà, M.; Taverna, D.; Audrito, V. Metabolic Interplay between the Immune System and Melanoma Cells: Therapeutic Implications. Biomedicines 2021, 9, 607. [Google Scholar] [CrossRef]
- Le Gal, K.; Ibrahim, M.X.; Wiel, C.; Sayin, V.I.; Akula, M.K.; Karlsson, C.; Dalin, M.G.; Akyürek, L.M.; Lindahl, P.; Nilsson, J.; et al. Antioxidants Can Increase Melanoma Metastasis in Mice. Sci. Transl. Med. 2015, 7, 308re8. [Google Scholar] [CrossRef]
- Pinto de Almeida, N.; Jánosi, Á.J.; Hong, R.; Rajeh, A.; Nogueira, F.; Szadai, L.; Szeitz, B.; Pla Parada, I.; Doma, V.; Woldmar, N.; et al. Mitochondrial Dysfunction and Immune Suppression in BRAF V600E-Mutated Metastatic Melanoma. Clin. Transl. Med. 2024, 14, e1773. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Tian, Y.; Chen, Y.; Guo, W.; Li, C. Metabolic Rewiring Directs Melanoma Immunology. Front. Immunol. 2022, 13, 909580. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Fingleton, B. Non-Canonical Roles for Metabolic Enzymes and Intermediates in Malignant Progression and Metastasis. Clin. Exp. Metastasis 2019, 36, 211–224. [Google Scholar] [CrossRef]
- Redondo-Muñoz, M.; Rodriguez-Baena, F.J.; Aldaz, P.; Caballé-Mestres, A.; Moncho-Amor, V.; Otaegi-Ugartemendia, M.; Carrasco-Garcia, E.; Olias-Arjona, A.; Lasheras-Otero, I.; Santamaria, E.; et al. Metabolic Rewiring Induced by Ranolazine Improves Melanoma Responses to Targeted Therapy and Immunotherapy. Nat. Metab. 2023, 5, 1544–1562. [Google Scholar] [CrossRef]
- Wang, Q.; Beaumont, K.A.; Otte, N.J.; Font, J.; Bailey, C.G.; van Geldermalsen, M.; Sharp, D.M.; Tiffen, J.C.; Ryan, R.M.; Jormakka, M.; et al. Targeting Glutamine Transport to Suppress Melanoma Cell Growth. Int. J. Cancer 2014, 135, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-B.; Fan, J.; Lin, R.; Elf, S.; Ji, Q.; Zhao, L.; Jin, L.; Seo, J.H.; Shan, C.; Arbiser, J.L.; et al. Metabolic Rewiring by Oncogenic BRAF V600E Links Ketogenesis Pathway to BRAF-MEK1 Signaling. Mol. Cell 2015, 59, 345–358. [Google Scholar] [CrossRef]
- Lu, W.; Luo, Y. Methionine Restriction Sensitizes Cancer Cells to Immunotherapy. Cancer Commun. 2023, 43, 1267–1270. [Google Scholar] [CrossRef]
- Zhao, T.; Lum, J.J. Methionine Cycle-Dependent Regulation of T Cells in Cancer Immunity. Front. Oncol. 2022, 12, 969563. [Google Scholar] [CrossRef]
- Garg, S.; Morehead, L.C.; Bird, J.T.; Graw, S.; Gies, A.; Storey, A.J.; Tackett, A.J.; Edmondson, R.D.; Mackintosh, S.G.; Byrum, S.D.; et al. Characterization of Methionine Dependence in Melanoma Cells. Mol. Omics 2024, 20, 37–47. [Google Scholar] [CrossRef]
- Ji, M.; Xu, Q.; Li, X. Dietary Methionine Restriction in Cancer Development and Antitumor Immunity. Trends Endocrinol. Metab. 2024, 35, 400–412. [Google Scholar] [CrossRef] [PubMed]
- The Metabolic Cross-Talk Between Cancer and T Cells. Available online: https://pubmed.ncbi.nlm.nih.gov/37080875/ (accessed on 24 March 2025).
- Raynor, J.L.; Chi, H. LCK Senses Asparagine for T Cell Activation. Nat. Cell Biol. 2021, 23, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Botticelli, A.; Visconti, I.C.; Angeletti, D.; Fiore, M.; Marchetti, P.; Lambiase, A.; de Vincentiis, M.; Greco, A. Immunotherapy in the Treatment of Metastatic Melanoma: Current Knowledge and Future Directions. J. Immunol. Res. 2020, 2020, 9235638. [Google Scholar] [CrossRef]
- Tang, B.; Yan, X.; Sheng, X.; Si, L.; Cui, C.; Kong, Y.; Mao, L.; Lian, B.; Bai, X.; Wang, X.; et al. Safety and Clinical Activity with an Anti-PD-1 Antibody JS001 in Advanced Melanoma or Urologic Cancer Patients. J. Hematol. Oncol. 2019, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Vanderbilt, C.M.; Cotzia, P.; Arias Stella, J.A.; Chang, J.C.; Chen, Y.; Tang, L.H.; DeLair, D.F.; Yao, J.; Ladanyi, M.; et al. JAK2, PD-L1, and PD-L2 (9p24.1) Amplification in Metastatic Mucosal and Cutaneous Melanomas with Durable Response to Immunotherapy. Hum. Pathol. 2019, 88, 87–91. [Google Scholar] [CrossRef]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef]
- Sivanand, S.; Vander Heiden, M.G. Emerging Roles for Branched-Chain Amino Acid Metabolism in Cancer. Cancer Cell 2020, 37, 147–156. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, J.; Wang, M.; Yi, X.; Guo, S.; Wang, H.; Zhang, H.; Wang, H.; Yang, Y.; Zhang, B.; et al. BCKDHA Contributes to Melanoma Progression by Promoting the Expressions of Lipogenic Enzymes FASN and ACLY. Exp. Dermatol. 2023, 32, 1633–1643. [Google Scholar] [CrossRef]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial Short-Chain Fatty Acids Modulate CD8+ T Cell Responses and Improve Adoptive Immunotherapy for Cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef]
- Yue, C.-F.; Feng, P.-N.; Yao, Z.-R.; Yu, X.-G.; Lin, W.-B.; Qian, Y.-M.; Guo, Y.-M.; Li, L.-S.; Liu, M. High Serum Uric Acid Concentration Predicts Poor Survival in Patients with Breast Cancer. Clin. Chim. Acta 2017, 473, 160–165. [Google Scholar] [CrossRef]
- Rao, H.; Wang, Q.; Zeng, X.; Wen, X.; Huang, L. Analysis of the Prognostic Value of Uric Acid on the Efficacy of Immunotherapy in Patients with Primary Liver Cancer. Clin. Transl. Oncol. 2024, 26, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Baey, C.; Yang, J.; Ronchese, F.; Harper, J.L. Hyperuricaemic UrahPlt2/Plt2 Mice Show Altered T Cell Proliferation and Defective Tumor Immunity after Local Immunotherapy with Poly I:C. PLoS ONE 2018, 13, e0206827. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Fu, T.; Wang, S.; Cai, H.; Xu, F.; Xing, G.; Tong, Y. Fatty Acid Traits Mediate the Effects of Uric Acid on Cancers: A Mendelian Randomization Study. Front. Genet. 2024, 15, 1449205. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shi, D.; Guo, C.; Zhang, W.; Guo, Y.; Yang, F.; Wang, R.; Zhang, J.; Fang, Z.; Yan, Y.; et al. Can Uric Acid Affect the Immune Microenvironment in Bladder Cancer? A Single-Center Multi-Omics Study. Mol. Carcinog. 2024, 63, 461–478. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Su, C.; Peng, B.; Du, J.; Jia, H.; Luo, M.; Fang, C.; Wei, Y. Uric Acid Enhances the Antitumor Immunity of Dendritic Cell-Based Vaccine. Sci. Rep. 2015, 5, 16427. [Google Scholar] [CrossRef]
- Dong, H.; Bullock, T.N.J. Metabolic Influences That Regulate Dendritic Cell Function in Tumors. Front. Immunol. 2014, 5, 24. [Google Scholar] [CrossRef]
- de Vries, I.J.M.; Lesterhuis, W.J.; Scharenborg, N.M.; Engelen, L.P.H.; Ruiter, D.J.; Gerritsen, M.-J.P.; Croockewit, S.; Britten, C.M.; Torensma, R.; Adema, G.J.; et al. Maturation of Dendritic Cells Is a Prerequisite for Inducing Immune Responses in Advanced Melanoma Patients. Clin. Cancer Res. 2003, 9, 5091–5100. [Google Scholar]
- Skočibušić, N.; Belančić, A.; Jovanović, G.K.; Golčić, M.; Herceg, D.; Simetić, L.; Blažičević, K. Personalized Dietary Intervention Based on Mediterranean Diet as a Complementary Strategy to Modify Gut Microbiome, Quality of Life and Outcomes in Patients with Metastatic Melanoma Treated with Immunotherapy: A Study Protocol. Biol. Life Sci. Forum 2023, 29, 23. [Google Scholar] [CrossRef]
- Bolte, L.A.; Lee, K.A.; Björk, J.R.; Leeming, E.R.; Campmans-Kuijpers, M.J.E.; de Haan, J.J.; Vila, A.V.; Maltez-Thomas, A.; Segata, N.; Board, R.; et al. Association of a Mediterranean Diet With Outcomes for Patients Treated with Immune Checkpoint Blockade for Advanced Melanoma. JAMA Oncol. 2023, 9, 705–709. [Google Scholar] [CrossRef]
- Chen, J.; Cui, L.; Lu, S.; Xu, S. Amino Acid Metabolism in Tumor Biology and Therapy. Cell Death Dis. 2024, 15, 42. [Google Scholar] [CrossRef]
- Hsieh, M.-Y.; Hsu, S.-K.; Liu, T.-Y.; Wu, C.-Y.; Chiu, C.-C. Melanoma Biology and Treatment: A Review of Novel Regulated Cell Death-Based Approaches. Cancer Cell Int. 2024, 24, 63. [Google Scholar] [CrossRef] [PubMed]
- Lumaquin-Yin, D.; Montal, E.; Johns, E.; Baggiolini, A.; Huang, T.-H.; Ma, Y.; LaPlante, C.; Suresh, S.; Studer, L.; White, R.M. Lipid Droplets Are a Metabolic Vulnerability in Melanoma. Nat. Commun. 2023, 14, 3192. [Google Scholar] [CrossRef]
- Zhang, Y.; Kurupati, R.; Liu, L.; Zhou, X.Y.; Zhang, G.; Hudaihed, A.; Filisio, F.; Giles-Davis, W.; Xu, X.; Karakousis, G.C.; et al. Enhancing CD8+ T Cell Fatty Acid Catabolism Within a Metabolically Challenging Tumor Microenvironment Increases the Efficacy of Melanoma Immunotherapy. Cancer Cell 2017, 32, 377–391.e9. [Google Scholar] [CrossRef]
- Johns, E.; Ma, Y.; Louphrasitthiphol, P.; Peralta, C.; Hunter, M.V.; Raymond, J.H.; Molina, H.; Goding, C.R.; White, R.M. The Lipid Droplet Protein DHRS3 Is a Regulator of Melanoma Cell State. Pigment Cell Melanoma Res. 2025, 38, e13208. [Google Scholar] [CrossRef] [PubMed]
- Karbanová, J.; Deniz, I.A.; Wilsch-Bräuninger, M.; de Sousa Couto, R.A.; Fargeas, C.A.; Santos, M.F.; Lorico, A.; Corbeil, D. Extracellular Lipidosomes Containing Lipid Droplets and Mitochondria Are Released During Melanoma Cell Division. Cell Commun. Signal. 2024, 22, 57. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8+ T Cells Regulate Tumour Ferroptosis during Cancer Immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, P. The Lipid Droplet: A Conserved Cellular Organelle. Protein Cell 2017, 8, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, U.E.; Law, M.H.; Ong, J.S.; Cust, A.E.; Mann, G.J.; Ward, S.V.; Melanoma Meta-Analysis Consortium; Gharahkhani, P.; Iles, M.M.; MacGregor, S. Polyunsaturated Fatty Acids and Risk of Melanoma: A Mendelian Randomisation Analysis. Int. J. Cancer 2018, 143, 508–514. [Google Scholar] [CrossRef]
- Black, H.S.; Rhodes, L.E. Potential Benefits of Omega-3 Fatty Acids in Non-Melanoma Skin Cancer. J. Clin. Med. 2016, 5, 23. [Google Scholar] [CrossRef]
- Minokawa, Y.; Sawada, Y.; Nakamura, M. The Influences of Omega-3 Polyunsaturated Fatty Acids on the Development of Skin Cancers. Diagnostics 2021, 11, 2149. [Google Scholar] [CrossRef]
- Hong, S.; Lu, Y. Omega-3 Fatty Acid-Derived Resolvins and Protectins in Inflammation Resolution and Leukocyte Functions: Targeting Novel Lipid Mediator Pathways in Mitigation of Acute Kidney Injury. Front. Immunol. 2013, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Saito-Sasaki, N.; Nakamura, M. Omega 3 Fatty Acid and Skin Diseases. Front. Immunol. 2020, 11, 623052. [Google Scholar] [CrossRef] [PubMed]
- Patterson, W.L.; Georgel, P.T. Breaking the Cycle: The Role of Omega-3 Polyunsaturated Fatty Acids in Inflammation-Driven Cancers. Biochem. Cell Biol. 2014, 92, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.C.; Shanahan, E.R.; Batten, M.; Reijers, I.L.M.; Read, M.; Silva, I.P.; Versluis, J.M.; Ribeiro, R.; Angelatos, A.S.; Tan, J.; et al. Diet-Driven Microbial Ecology Underpins Associations between Cancer Immunotherapy Outcomes and the Gut Microbiome. Nat. Med. 2022, 28, 2344–2352. [Google Scholar] [CrossRef]
- D’Mello, R.S.; Mendon, V.; Pai, P.; Das, I.; Sundara, B.K. Exploring the Therapeutic Potential of Oleanolic Acid and Its Derivatives in Cancer Treatment: A Comprehensive Review. 3 Biotech 2025, 15, 56. [Google Scholar] [CrossRef]
- Djeziri, F.Z.; Belarbi, M.; Murtaza, B.; Hichami, A.; Benammar, C.; Khan, N.A. Oleanolic Acid Improves Diet-Induced Obesity by Modulating Fat Preference and Inflammation in Mice. Biochimie 2018, 152, 110–120. [Google Scholar] [CrossRef]
- Dong, Y.; Wei, J.; Yang, F.; Qu, Y.; Huang, J.; Shi, D. Nutrient-Based Approaches for Melanoma: Prevention and Therapeutic Insights. Nutrients 2023, 15, 4483. [Google Scholar] [CrossRef]
- Harel, M.; Ortenberg, R.; Varanasi, S.K.; Mangalhara, K.C.; Mardamshina, M.; Markovits, E.; Baruch, E.N.; Tripple, V.; Arama-Chayoth, M.; Greenberg, E.; et al. Proteomics of Melanoma Response to Immunotherapy Reveals Mitochondrial Dependence. Cell 2019, 179, 236–250.e18. [Google Scholar] [CrossRef]
- Rubio-Patiño, C.; Bossowski, J.P.; De Donatis, G.M.; Mondragón, L.; Villa, E.; Aira, L.E.; Chiche, J.; Mhaidly, R.; Lebeaupin, C.; Marchetti, S.; et al. Low-Protein Diet Induces IRE1α-Dependent Anticancer Immunosurveillance. Cell Metab. 2018, 27, 828–842.e7. [Google Scholar] [CrossRef]
- Golčić, M.; Simetić, L.; Herceg, D.; Blažičević, K.; Kenđel Jovanović, G.; Dražić, I.; Belančić, A.; Skočibušić, N.; Palčevski, D.; Rubinić, I.; et al. Analysis of the Gut Microbiome and Dietary Habits in Metastatic Melanoma Patients with a Complete and Sustained Response to Immunotherapy. Cancers 2023, 15, 3052. [Google Scholar] [CrossRef]
- Na, K.J.; Choi, H.; Oh, H.R.; Kim, Y.H.; Lee, S.B.; Jung, Y.J.; Koh, J.; Park, S.; Lee, H.J.; Jeon, Y.K.; et al. Reciprocal Change in Glucose Metabolism of Cancer and Immune Cells Mediated by Different Glucose Transporters Predicts Immunotherapy Response. Theranostics 2020, 10, 9579–9590. [Google Scholar] [CrossRef]
- Kuehm, L.M.; Khojandi, N.; Piening, A.; Klevorn, L.E.; Geraud, S.C.; McLaughlin, N.R.; Griffett, K.; Burris, T.P.; Pyles, K.D.; Nelson, A.M.; et al. Fructose Promotes Cytoprotection in Melanoma Tumors and Resistance to Immunotherapy. Cancer Immunol. Res. 2021, 9, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Biswas, C.; Shah, N.; Muthu, M.; La, P.; Fernando, A.P.; Sengupta, S.; Yang, G.; Dennery, P.A. Nuclear Heme Oxygenase-1 (HO-1) Modulates Subcellular Distribution and Activation of Nrf2, Impacting Metabolic and Anti-Oxidant Defenses. J. Biol. Chem. 2014, 289, 26882–26894. [Google Scholar] [CrossRef] [PubMed]
- Berberat, P.O.; Dambrauskas, Z.; Gulbinas, A.; Giese, T.; Giese, N.; Künzli, B.; Autschbach, F.; Meuer, S.; Büchler, M.W.; Friess, H. Inhibition of Heme Oxygenase-1 Increases Responsiveness of Pancreatic Cancer Cells to Anticancer Treatment. Clin. Cancer Res. 2005, 11, 3790–3798. [Google Scholar] [CrossRef] [PubMed]
- Fest, S.; Soldati, R.; Christiansen, N.M.; Zenclussen, M.L.; Kilz, J.; Berger, E.; Starke, S.; Lode, H.N.; Engel, C.; Zenclussen, A.C.; et al. Targeting of Heme Oxygenase-1 as a Novel Immune Regulator of Neuroblastoma. Int. J. Cancer 2016, 138, 2030–2042. [Google Scholar] [CrossRef] [PubMed]
- Alaoui-Jamali, M.A.; Bismar, T.A.; Gupta, A.; Szarek, W.A.; Su, J.; Song, W.; Xu, Y.; Xu, B.; Liu, G.; Vlahakis, J.Z.; et al. A Novel Experimental Heme Oxygenase-1-Targeted Therapy for Hormone-Refractory Prostate Cancer. Cancer Res. 2009, 69, 8017–8024. [Google Scholar] [CrossRef]
- Shariff, A.I.; Syed, S.; Shelby, R.A.; Force, J.; Clarke, J.M.; D’Alessio, D.; Corsino, L. Novel Cancer Therapies and Their Association with Diabetes. J. Mol. Endocrinol. 2019, 62, R187–R199. [Google Scholar] [CrossRef]
- Corsello, S.M.; Barnabei, A.; Marchetti, P.; De Vecchis, L.; Salvatori, R.; Torino, F. Endocrine Side Effects Induced by Immune Checkpoint Inhibitors. J. Clin. Endocrinol. Metab. 2013, 98, 1361–1375. [Google Scholar] [CrossRef]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory Cytokine Concentrations Are Acutely Increased by Hyperglycemia in Humans: Role of Oxidative Stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef]
- van de Poll-Franse, L.V.; Houterman, S.; Janssen-Heijnen, M.L.G.; Dercksen, M.W.; Coebergh, J.W.W.; Haak, H.R. Less Aggressive Treatment and Worse Overall Survival in Cancer Patients with Diabetes: A Large Population Based Analysis. Int. J. Cancer 2007, 120, 1986–1992. [Google Scholar] [CrossRef]
- Shahid, R.K.; Ahmed, S.; Le, D.; Yadav, S. Diabetes and Cancer: Risk, Challenges, Management and Outcomes. Cancers 2021, 13, 5735. [Google Scholar] [CrossRef] [PubMed]
- Bjornsdottir, H.H.; Rawshani, A.; Rawshani, A.; Franzén, S.; Svensson, A.-M.; Sattar, N.; Gudbjörnsdottir, S. A National Observation Study of Cancer Incidence and Mortality Risks in Type 2 Diabetes Compared to the Background Population over Time. Sci. Rep. 2020, 10, 17376. [Google Scholar] [CrossRef] [PubMed]
- Yekedüz, E.; Köksoy, E.B.; Yazgan, S.C.; Karataş, G.; Şenler, F.Ç.; Utkan, G.; Akbulut, H.; Demirkazik, A.; Ürün, Y. Chronic Hyperglycemia Based on Diabetes Is Independently Associated with Decreased Survival in Patients with Advanced Cancer Treated with Immune Checkpoint Inhibitors. Anticancer Drugs 2022, 33, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Marsiglio, J.; McPherson, J.P.; Kovacsovics-Bankowski, M.; Jeter, J.; Vaklavas, C.; Swami, U.; Grossmann, D.; Erickson-Wayman, A.; Soares, H.P.; Kerrigan, K.; et al. A Single Center Case Series of Immune Checkpoint Inhibitor-Induced Type 1 Diabetes Mellitus, Patterns of Disease Onset and Long-Term Clinical Outcome. Front. Immunol. 2023, 14, 1229823. [Google Scholar] [CrossRef]
- Cortellini, A.; D’Alessio, A.; Cleary, S.; Buti, S.; Bersanelli, M.; Bordi, P.; Tonini, G.; Vincenzi, B.; Tucci, M.; Russo, A.; et al. Type 2 Diabetes Mellitus and Efficacy Outcomes from Immune Checkpoint Blockade in Patients with Cancer. Clin. Cancer Res. 2023, 29, 2714–2724. [Google Scholar] [CrossRef]
- Feng, H.; Shang, S.; Chen, K.; Sun, X.; Yue, X. Impact of Metformin on Melanoma: A Meta-Analysis and Systematic Review. Front. Oncol. 2024, 14, 1399693. [Google Scholar] [CrossRef]
- Peng, Z.; Cheng, S.; Kou, Y.; Wang, Z.; Jin, R.; Hu, H.; Zhang, X.; Gong, J.-F.; Li, J.; Lu, M.; et al. The Gut Microbiome Is Associated with Clinical Response to Anti-PD-1/PD-L1 Immunotherapy in Gastrointestinal Cancer. Cancer Immunol. Res. 2020, 8, 1251–1261. [Google Scholar] [CrossRef]
- Najjar, Y.G.; Menk, A.V.; Sander, C.; Rao, U.; Karunamurthy, A.; Bhatia, R.; Zhai, S.; Kirkwood, J.M.; Delgoffe, G.M. Tumor Cell Oxidative Metabolism as a Barrier to PD-1 Blockade Immunotherapy in Melanoma. JCI Insight 2019, 4, e124989. [Google Scholar] [CrossRef]
- Conde, E.; Casares, N.; Mancheño, U.; Elizalde, E.; Vercher, E.; Capozzi, R.; Santamaria, E.; Rodriguez-Madoz, J.R.; Prosper, F.; Lasarte, J.J.; et al. FOXP3 Expression Diversifies the Metabolic Capacity and Enhances the Efficacy of CD8 T Cells in Adoptive Immunotherapy of Melanoma. Mol. Ther. 2023, 31, 48–65. [Google Scholar] [CrossRef]
- Peisen, F.; Gerken, A.; Dahm, I.; Nikolaou, K.; Eigentler, T.; Amaral, T.; Moltz, J.H.; Othman, A.E.; Gatidis, S. Pre-Treatment 18F-FDG-PET/CT Parameters as Biomarkers for Progression Free Survival, Best Overall Response and Overall Survival in Metastatic Melanoma Patients Undergoing First-Line Immunotherapy. PLoS ONE 2024, 19, e0296253. [Google Scholar] [CrossRef]
- Ellebaek, E.; Schina, A.; Andersen, R.; Hendel, H.W.; Svane, I.M.; Donia, M. Clinical Value of Routine [18F]2-Fluoro-2-Deoxy-d-Glucose Positron Emission Tomography Scans as a Decision Tool for Early Immunotherapy Discontinuation in Advanced Melanoma. Int. J. Cancer 2022, 150, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.; Neveu, M.-A.; Bouzin, C.; Knezevic, Z.; Gallez, B.; Leucci, E.; Baurain, J.-F.; Mignion, L.; Jordan, B.F. Hyperpolarized 13C-Pyruvate to Assess Response to Anti-PD1 Immune Checkpoint Inhibition in YUMMER 1.7 Melanoma Xenografts. Int. J. Mol. Sci. 2023, 24, 2499. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.C.; Yeh, L.R.; Kuo, Y.T.; Chen, J.H. Imaging Biomarkers for Evaluating Tumor Response: RECIST and Beyond. Biomark. Res. 2021, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Timm, K.N.; Kennedy, B.W.C.; Brindle, K.M. Imaging Tumor Metabolism to Assess Disease Progression and Treatment Response. Clin. Cancer Res. 2016, 22, 5196–5203. [Google Scholar] [CrossRef]
- Bhamidipati, K.; Malleswara Rao Nakka, N.; Ahmed, M.; Javvaji, K.; Banerjee, R.; Puvvada, N.; Sesha Sainath, A.V.; Chakravarty, S. Enhancing Cancer Immunotherapy with Mannose Mimicking Glycopolymer Nanoparticles Induced Activation of Dendritic Cells. Bioorg Chem. 2024, 152, 107711. [Google Scholar] [CrossRef]
- Burton, C.; Bitaraf, A.; Snyder, K.; Zhang, C.; Yoder, S.J.; Avram, D.; Du, D.; Yu, X.; Lau, E.K. The Functional Role of L-Fucose on Dendritic Cell Function and Polarization. Front. Immunol. 2024, 15, 1353570. [Google Scholar] [CrossRef]
- Overvad, K.; Thorling, E.B.; Bjerring, P.; Ebbesen, P. Selenium Inhibits UV-Light-Induced Skin Carcinogenesis in Hairless Mice. Cancer Lett. 1985, 27, 163–170. [Google Scholar] [CrossRef]
- Vinceti, M.; Crespi, C.M.; Malagoli, C.; Bottecchi, I.; Ferrari, A.; Sieri, S.; Krogh, V.; Alber, D.; Bergomi, M.; Seidenari, S.; et al. A Case-Control Study of the Risk of Cutaneous Melanoma Associated with Three Selenium Exposure Indicators. Tumori J. 2012, 98, 287–295. [Google Scholar] [CrossRef]
- Bleys, J.; Navas-Acien, A.; Guallar, E. Serum Selenium Levels and All-Cause, Cancer, and Cardiovascular Mortality among US Adults. Arch. Intern. Med. 2008, 168, 404–410. [Google Scholar] [CrossRef]
- Le, N.T.; Pham, Y.T.-H.; Le, C.T.-K.; Le, L.T.; Le, T.-D.; Dao, H.V.; Ha, T.H.; Kuchipudi, S.V.; Luu, H.N. A U-Shaped Association between Selenium Intake and Cancer Risk. Sci. Rep. 2024, 14, 21378. [Google Scholar] [CrossRef]
- Davoodvandi, A.; Darvish, M.; Borran, S.; Nejati, M.; Mazaheri, S.; Reza Tamtaji, O.; Hamblin, M.R.; Masoudian, N.; Mirzaei, H. The Therapeutic Potential of Resveratrol in a Mouse Model of Melanoma Lung Metastasis. Int. Immunopharmacol. 2020, 88, 106905. [Google Scholar] [CrossRef] [PubMed]
- Diniz, C.; Suliburska, J.; Ferreira, I.M. New Insights into the Antiangiogenic and Proangiogenic Properties of Dietary Polyphenols. Mol. Nutr. Food Res. 2017, 61, 1600912. [Google Scholar] [CrossRef]
- Cunha, C.; Daniel-da-Silva, A.L.; Oliveira, H. Drug Delivery Systems and Flavonoids: Current Knowledge in Melanoma Treatment and Future Perspectives. Micromachines 2022, 13, 1838. [Google Scholar] [CrossRef]
- Coutinho, T.E.; Souto, E.B.; Silva, A.M. Selected Flavonoids to Target Melanoma: A Perspective in Nanoengineering Delivery Systems. Bioengineering 2022, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Splendiani, E.; Besharat, Z.M.; Covre, A.; Maio, M.; Di Giacomo, A.M.; Ferretti, E. Immunotherapy in Melanoma: Can We Predict Response to Treatment with Circulating Biomarkers? Pharmacol. Ther. 2024, 256, 108613. [Google Scholar] [CrossRef]
- Grafanaki, K.; Anastasakis, D.; Kyriakopoulos, G.; Skeparnias, I.; Georgiou, S.; Stathopoulos, C. Translation Regulation in Skin Cancer from a tRNA Point of View. Epigenomics 2019, 11, 215–245. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Y.; Tian, K.; Chen, X.; Zhang, R.; Mu, X.; Wu, Y.; Wang, D.; Wang, S.; Liu, F.; et al. Apigenin Suppresses PD-L1 Expression in Melanoma and Host Dendritic Cells to Elicit Synergistic Therapeutic Effects. J. Exp. Clin. Cancer Res. 2018, 37, 261. [Google Scholar] [CrossRef]
- Liu, K.; Sun, Q.; Liu, Q.; Li, H.; Zhang, W.; Sun, C. Focus on Immune Checkpoint PD-1/PD-L1 Pathway: New Advances of Polyphenol Phytochemicals in Tumor Immunotherapy. Biomed. Pharmacother. 2022, 154, 113618. [Google Scholar] [CrossRef]
- Galus, Ł.; Michalak, M.; Lorenz, M.; Stoińska-Swiniarek, R.; Tusień Małecka, D.; Galus, A.; Kolenda, T.; Leporowska, E.; Mackiewicz, J. Vitamin D Supplementation Increases Objective Response Rate and Prolongs Progression-Free Time in Patients with Advanced Melanoma Undergoing Anti-PD-1 Therapy. Cancer 2023, 129, 2047–2055. [Google Scholar] [CrossRef]
- Giampazolias, E.; Pereira da Costa, M.; Lam, K.C.; Lim, K.H.J.; Cardoso, A.; Piot, C.; Chakravarty, P.; Blasche, S.; Patel, S.; Biram, A.; et al. Vitamin D Regulates Microbiome-Dependent Cancer Immunity. Science 2024, 384, 428–437. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Wang, J.; Lu, H.; Zeng, W.; Zhang, T. Vitamin D3 Regulates NSUN2 Expression and Inhibits Melanoma Cell Proliferation and Migration. Mol. Divers. 2024, 28, 2863–2874. [Google Scholar] [CrossRef]
- Grover, S.; Dougan, M.; Tyan, K.; Giobbie-Hurder, A.; Blum, S.M.; Ishizuka, J.; Qazi, T.; Elias, R.; Vora, K.B.; Ruan, A.B.; et al. Vitamin D Intake Is Associated with Decreased Risk of Immune Checkpoint Inhibitor-Induced Colitis. Cancer 2020, 126, 3758–3767. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, C.B.; Yang, C.; Dickson, K.M.; Shao, H.; Van Booven, D.; Harbour, J.W.; Liu, Z.-J.; Wang, G. Epigenetic Reprogramming of Melanoma Cells by Vitamin C Treatment. Clin. Epigenetics 2015, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Fung, T.T.; Nan, H. An Epidemiological Review of Diet and Cutaneous Malignant Melanoma. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1115–1122. [Google Scholar] [CrossRef]
- Park, S.M.; Li, T.; Wu, S.; Li, W.Q.; Weinstock, M.; Qureshi, A.A.; Cho, E. Niacin Intake and Risk of Skin Cancer in US Women and Men. Int. J. Cancer 2017, 140, 2023–2031. [Google Scholar] [CrossRef]
- van der Pols, J.C.; Russell, A.; Bauer, U.; Neale, R.E.; Kimlin, M.G.; Green, A.C. Vitamin D Status and Skin Cancer Risk Independent of Time Outdoors: 11-Year Prospective Study in an Australian Community. J. Invest. Dermatol. 2013, 133, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Snaidr, V.A.; Damian, D.L.; Halliday, G.M. Nicotinamide for Photoprotection and Skin Cancer Chemoprevention: A Review of Efficacy and Safety. Exp. Dermatol. 2019, 28 (Suppl. 1), 15–22. [Google Scholar] [CrossRef]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernández-Peñas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A.; et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef]
- Castro-Espin, C.; Agudo, A. The Role of Diet in Prognosis among Cancer Survivors: A Systematic Review and Meta-Analysis of Dietary Patterns and Diet Interventions. Nutrients 2022, 14, 348. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network. Cancer Genome Atlas Network Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef]
- Kiuru, M.; Busam, K.J. The NF1 Gene in Tumor Syndromes and Melanoma. Lab. Investig. 2017, 97, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.; Bartok, O.; Patkar, S.; Eli, G.B.; Cohen, S.; Litchfield, K.; Levy, R.; Jiménez-Sánchez, A.; Trabish, S.; Lee, J.S.; et al. UVB-Induced Tumor Heterogeneity Diminishes Immune Response in Melanoma. Cell 2019, 179, 219–235.e21. [Google Scholar] [CrossRef]
- Zou, Z.; Ou, Q.; Ren, Y.; Lv, Q.; Qin, L.; Zhao, L.; Su, S.; Wu, X.; Bao, H.; Wang, A.; et al. Distinct Genomic Traits of Acral and Mucosal Melanomas Revealed by Targeted Mutational Profiling. Pigment Cell Melanoma Res. 2020, 33, 601–611. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, B.; Kong, B.Y.; Zhang, Y.; Igartua, C.; Mohan, L.S.; Quan, V.L.; Panah, E.; Isales, M.C.; Beaubier, N.; et al. Distinct Genomic Features in a Retrospective Cohort of Mucosal, Acral, and Vulvovaginal Melanomas. J. Am. Acad. Dermatol. 2023, 88, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Banik, I.; Shain, A.H.; Yeh, I.; Bastian, B.C. Integrated Genomic Analyses of Acral and Mucosal Melanomas Nominate Novel Driver Genes. Genome Med. 2022, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Schug, Z.T.; Aplin, A.E. Metabolic Alterations and Therapeutic Opportunities in Rare Forms of Melanoma. Trends Cancer 2021, 7, 671–681. [Google Scholar] [CrossRef]
- Giuliani, S.; Accetta, C.; di Martino, S.; De Vitis, C.; Messina, E.; Pescarmona, E.; Fanciulli, M.; Ciliberto, G.; Mancini, R.; Falcone, I. Metabolic Reprogramming in Melanoma: An Epigenetic Point of View. Pharmaceuticals 2025, 18, 853. [Google Scholar] [CrossRef]
- Abildgaard, C.; Guldberg, P. Molecular Drivers of Cellular Metabolic Reprogramming in Melanoma. Trends Mol. Med. 2015, 21, 164–171. [Google Scholar] [CrossRef]
- Pietrzak, B.; Tomela, K.; Olejnik-Schmidt, A.; Galus, Ł.; Mackiewicz, J.; Kaczmarek, M.; Mackiewicz, A.; Schmidt, M. A Clinical Outcome of the Anti-PD-1 Therapy of Melanoma in Polish Patients Is Mediated by Population-Specific Gut Microbiome Composition. Cancers 2022, 14, 5369. [Google Scholar] [CrossRef]
- Weber, D.D.; Aminzadeh-Gohari, S.; Thapa, M.; Redtenbacher, A.S.; Catalano, L.; Capelôa, T.; Vazeille, T.; Emberger, M.; Felder, T.K.; Feichtinger, R.G.; et al. Ketogenic Diets Slow Melanoma Growth in Vivo Regardless of Tumor Genetics and Metabolic Plasticity. Cancer Metab. 2022, 10, 12. [Google Scholar] [CrossRef]
- Ferrere, G.; Tidjani Alou, M.; Liu, P.; Goubet, A.-G.; Fidelle, M.; Kepp, O.; Durand, S.; Iebba, V.; Fluckiger, A.; Daillère, R.; et al. Ketogenic Diet and Ketone Bodies Enhance the Anticancer Effects of PD-1 Blockade. JCI Insight 2021, 6, e145207. [Google Scholar] [CrossRef] [PubMed]
- Pio, R.; Senent, Y.; Tavira, B.; Ajona, D. Fasting and Fasting-Mimicking Conditions in the Cancer Immunotherapy Era. J. Physiol. Biochem. 2024. [Google Scholar] [CrossRef] [PubMed]
- de Gruil, N.; Pijl, H.; van der Burg, S.H.; Kroep, J.R. Short-Term Fasting Synergizes with Solid Cancer Therapy by Boosting Antitumor Immunity. Cancers 2022, 14, 1390. [Google Scholar] [CrossRef]
- Cortellino, S.; Quagliariello, V.; Delfanti, G.; Blaževitš, O.; Chiodoni, C.; Maurea, N.; Di Mauro, A.; Tatangelo, F.; Pisati, F.; Shmahala, A.; et al. Fasting Mimicking Diet in Mice Delays Cancer Growth and Reduces Immunotherapy-Associated Cardiovascular and Systemic Side Effects. Nat. Commun. 2023, 14, 5529. [Google Scholar] [CrossRef]
- Di Biase, S.; Lee, C.; Brandhorst, S.; Manes, B.; Buono, R.; Cheng, C.-W.; Cacciottolo, M.; Martin-Montalvo, A.; de Cabo, R.; Wei, M.; et al. Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell 2016, 30, 136–146. [Google Scholar] [CrossRef]
- Liu, X.-Z.; Pedersen, L.; Halberg, N. Cellular Mechanisms Linking Cancers to Obesity. Cell Stress 2021, 5, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.W.; Menk, A.V.; Rivadeneira, D.B.; Augustin, R.C.; Xu, M.; Li, J.; Wu, X.; Mishra, A.K.; Gide, T.N.; Quek, C.; et al. Obesity Is Associated with Altered Tumor Metabolism in Metastatic Melanoma. Clin. Cancer Res. 2023, 29, 154–164. [Google Scholar] [CrossRef]
- Wang, N.; Wu, Z.; Yang, Z.; Xu, B.; An, Y.; Sun, M.; Li, Y. Association between Body Mass Index and Survival Outcomes for Cancer Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J. Transl. Med. 2020, 18, 235. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of Body-Mass Index and Outcomes in Patients with Metastatic Melanoma Treated with Targeted Therapy, Immunotherapy, or Chemotherapy: A Retrospective, Multicohort Analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef]
- Schneider, G.; Kirschner, M.A.; Berkowitz, R.; Ertel, N.H. Increased Estrogen Production in Obese Men. J. Clin. Endocrinol. Metab. 1979, 48, 633–638. [Google Scholar] [CrossRef]
- Waikar, S.S.; Hodi, F.S.; Haq, R.; Schoenfeld, J.D.; Ott, P.A.; Johnson, A.E.W.; Naik, G.S.; Buchbinder, E.I. Complex Inter-Relationship of Body Mass Index, Gender and Serum Creatinine on Survival: Exploring the Obesity Paradox in Melanoma Patients Treated with Checkpoint Inhibition. J. Immunother. Cancer 2019, 7, 89. [Google Scholar] [CrossRef]
- Hoeller, C.; Richtig, E.; Wolf, I.; Grübler, M.R.; Lange-Asschenfeldt, B.; Schulter, G.; Huegel, R.; Haidn, T.; Heinemann, A.; Richtig, M.; et al. Body Mass Index May Predict the Response to Ipilimumab in Metastatic Melanoma: An Observational Multi-Centre Study. PLoS ONE 2018, 13, e0204729. [Google Scholar] [CrossRef]
- Young, A.C.; Quach, H.T.; Song, H.; Davis, E.J.; Moslehi, J.J.; Ye, F.; Williams, G.R.; Johnson, D.B. Impact of Body Composition on Outcomes from Anti-PD1 +/- Anti-CTLA-4 Treatment in Melanoma. J. Immunother. Cancer 2020, 8, e000821. [Google Scholar] [CrossRef]
- Rinaldi, S.; Fargnoli, M.C.; Berardi, R.; Botticelli, A.; Tinari, N.; Ficorella, C.; Santini, D.; Michiara, M.; Chiari, R.; Cannita, K.; et al. A Multicenter Study of Body Mass Index in Cancer Patients Treated with Anti-PD-1/PD-L1 Immune Checkpoint Inhibitors: When Overweight Becomes Favorable. J. Immunother. Cancer 2019, 7, 57. [Google Scholar] [CrossRef]
- Warner, A.B.; McQuade, J.L. Modifiable Host Factors in Melanoma: Emerging Evidence for Obesity, Diet, Exercise, and the Microbiome. Curr. Oncol. Rep. 2019, 21, 72. [Google Scholar] [CrossRef]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical Effects of Obesity on T Cell Function during Tumor Progression and PD-1 Checkpoint Blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Sun, X.; Yuan, B.; Ge, F.; Gupta, H.B.; Chiang, H.-C.; Li, J.; Hu, Y.; Curiel, T.J.; Li, R. PPARγ Inhibition Boosts Efficacy of PD-L1 Checkpoint Blockade Immunotherapy against Murine Melanoma in a Sexually Dimorphic Manner. Int. J. Biol. Sci. 2020, 16, 1526–1535. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut Microbiome Modulates Response to Anti-PD-1 Immunotherapy in Melanoma Patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Guardamagna, M.; Berciano-Guerrero, M.-A.; Villaescusa-González, B.; Perez-Ruiz, E.; Oliver, J.; Lavado-Valenzuela, R.; Rueda-Dominguez, A.; Barragán, I.; Queipo-Ortuño, M.I. Gut Microbiota and Therapy in Metastatic Melanoma: Focus on MAPK Pathway Inhibition. Int. J. Mol. Sci. 2022, 23, 11990. [Google Scholar] [CrossRef]
- Lam, K.C.; Araya, R.E.; Huang, A.; Chen, Q.; Di Modica, M.; Rodrigues, R.R.; Lopès, A.; Johnson, S.B.; Schwarz, B.; Bohrnsen, E.; et al. Microbiota Triggers STING-Type I IFN-Dependent Monocyte Reprogramming of the Tumor Microenvironment. Cell 2021, 184, 5338–5356.e21. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline Gut Microbiota Predicts Clinical Response and Colitis in Metastatic Melanoma Patients Treated with Ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The Commensal Microbiome Is Associated with Anti-PD-1 Efficacy in Metastatic Melanoma Patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1-Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Jo, J.-H.; Zhang, Z.; MacGibeny, M.A.; Han, J.; Proctor, D.M.; Taylor, M.E.; Che, Y.; Juneau, P.; Apolo, A.B.; et al. Predicting Cancer Immunotherapy Response from Gut Microbiomes Using Machine Learning Models. Oncotarget 2022, 13, 876–889. [Google Scholar] [CrossRef]
- Bender, M.J.; McPherson, A.C.; Phelps, C.M.; Pandey, S.P.; Laughlin, C.R.; Shapira, J.H.; Medina Sanchez, L.; Rana, M.; Richie, T.G.; Mims, T.S.; et al. Dietary Tryptophan Metabolite Released by Intratumoral Lactobacillus Reuteri Facilitates Immune Checkpoint Inhibitor Treatment. Cell 2023, 186, 1846–1862.e26. [Google Scholar] [CrossRef]
- Kumar, P.; Brazel, D.; DeRogatis, J.; Valerin, J.B.G.; Whiteson, K.; Chow, W.A.; Tinoco, R.; Moyers, J.T. The Cure from within? A Review of the Microbiome and Diet in Melanoma. Cancer Metastasis Rev. 2022, 41, 261–280. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; Wang, Y.; Wang, D.; Ke, Y.; Zeng, X. Propionate and Butyrate Produced by Gut Microbiota after Probiotic Supplementation Attenuate Lung Metastasis of Melanoma Cells in Mice. Mol. Nutr. Food Res. 2021, 65, e2100096. [Google Scholar] [CrossRef]
- Rutkowski, M.R.; Stephen, T.L.; Svoronos, N.; Allegrezza, M.J.; Tesone, A.J.; Perales-Puchalt, A.; Brencicova, E.; Escovar-Fadul, X.; Nguyen, J.M.; Cadungog, M.G.; et al. Microbially Driven TLR5-Dependent Signaling Governs Distal Malignant Progression through Tumor-Promoting Inflammation. Cancer Cell 2015, 27, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Kortylewski, M.; Zeng, D.; Pardoll, D.M.; Wang, L.; Yu, H. IL-17 Can Promote Tumor Growth through an IL-6-Stat3 Signaling Pathway. J. Exp. Med. 2009, 206, 1457–1464. [Google Scholar] [CrossRef]
- Li, Y.; Elmén, L.; Tinoco, R.; Ramer-Tait, A.; Bradley, L.M.; Segota, I.; Schmaltz, R.; Zarecki, R.; Xian, Y.; Peterson, S.N.; et al. Prebiotic-Induced Anti-Tumor Immunity Attenuates Tumor Growth. Cell Rep. 2020, 30, 1753–1766.e6. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Kroemer, G.; Zitvogel, L. The Intestinal Microbiota Determines the Clinical Efficacy of Immune Checkpoint Blockers Targeting PD-1/PD-L1. Oncoimmunology 2018, 7, e1434468. [Google Scholar] [CrossRef] [PubMed]
- Derosa, L.; Routy, B.; Desilets, A.; Daillère, R.; Terrisse, S.; Kroemer, G.; Zitvogel, L. Microbiota-Centered Interventions: The Next Breakthrough in Immuno-Oncology? Cancer Discov. 2021, 11, 2396–2412. [Google Scholar] [CrossRef]
- Almonte, A.A.; Thomas, S.; Zitvogel, L. Microbiota-Centered Interventions to Boost Immune Checkpoint Blockade Therapies. J. Exp. Med. 2025, 222, e20250378. [Google Scholar] [CrossRef] [PubMed]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer Immunotherapy by CTLA-4 Blockade Relies on the Gut Microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti-PD-L1 Efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Golonko, A.; Pienkowski, T.; Swislocka, R.; Orzechowska, S.; Marszalek, K.; Szczerbinski, L.; Swiergiel, A.H.; Lewandowski, W. Dietary Factors and Their Influence on Immunotherapy Strategies in Oncology: A Comprehensive Review. Cell Death Dis. 2024, 15, 254. [Google Scholar] [CrossRef]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.-M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal Microbiota Transplant Overcomes Resistance to Anti-PD-1 Therapy in Melanoma Patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Lenehan, J.G.; Miller, W.H.; Jamal, R.; Messaoudene, M.; Daisley, B.A.; Hes, C.; Al, K.F.; Martinez-Gili, L.; Punčochář, M.; et al. Fecal Microbiota Transplantation plus Anti-PD-1 Immunotherapy in Advanced Melanoma: A Phase I Trial. Nat. Med. 2023, 29, 2121–2132. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal Microbiota Transplant Promotes Response in Immunotherapy-Refractory Melanoma Patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Spencer, C.N.; McQuade, J.L.; Gopalakrishnan, V.; McCulloch, J.A.; Vetizou, M.; Cogdill, A.P.; Khan, M.A.W.; Zhang, X.; White, M.G.; Peterson, C.B.; et al. Dietary Fiber and Probiotics Influence the Gut Microbiome and Melanoma Immunotherapy Response. Science 2021, 374, 1632–1640. [Google Scholar] [CrossRef]
- Knisely, A.; Seo, Y.D.; Wargo, J.A.; Chelvanambi, M. Monitoring and Modulating Diet and Gut Microbes to Enhance Response and Reduce Toxicity to Cancer Treatment. Cancers 2023, 15, 777. [Google Scholar] [CrossRef] [PubMed]
- Korfiati, A.; Grafanaki, K.; Kyriakopoulos, G.C.; Skeparnias, I.; Georgiou, S.; Sakellaropoulos, G.; Stathopoulos, C. Revisiting miRNA Association with Melanoma Recurrence and Metastasis from a Machine Learning Point of View. Int. J. Mol. Sci. 2022, 23, 1299. [Google Scholar] [CrossRef]
- Buergel, T.; Steinfeldt, J.; Ruyoga, G.; Pietzner, M.; Bizzarri, D.; Vojinovic, D.; Upmeier Zu Belzen, J.; Loock, L.; Kittner, P.; Christmann, L.; et al. Metabolomic Profiles Predict Individual Multidisease Outcomes. Nat. Med. 2022, 28, 2309–2320. [Google Scholar] [CrossRef]
- Barber, S.; Duggan, D.; Murphy, J.; Markey, G.; O’Connor, D.; Galiauskas, R.; Khan, H.; Ahmed, G.; O’Mahony, D.; Murphy, C.G. Patient Attitudes Regarding the Influence of Diet on Cancer Development and Treatment. J. Clin. Oncol. 2022, 40, e24088. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH). A High-Fiber Diet May Improve the Response of Melanoma Patients to Immunotherapy. Available online: https://www.nih.gov/news-events/news-releases/high-fiber-diet-may-improve-response-melanoma-patients-immunotherapy (accessed on 3 January 2025).
- Ligibel, J.A.; Pierce, L.J.; Bender, C.M.; Crane, T.E.; Dieli-Conwright, C.; Hopkins, J.O.; Masters, G.A.; Schenkel, C.; Garrett-Mayer, E.; Katta, S.; et al. Attention to Diet, Exercise, and Weight in Oncology Care: Results of an American Society of Clinical Oncology National Patient Survey. Cancer 2022, 128, 2817–2825. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. American Cancer Society Guideline for Diet and Physical Activity for Cancer Prevention. Available online: https://www.cancer.org/cancer/risk-prevention/diet-physical-activity/acs-guidelines-nutrition-physical-activity-cancer-prevention.html?utm_source=chatgpt.com (accessed on 3 January 2025).
- Zhang, H.; Li, S.; Wang, D.; Liu, S.; Xiao, T.; Gu, W.; Yang, H.; Wang, H.; Yang, M.; Chen, P. Metabolic Reprogramming and Immune Evasion: The Interplay in the Tumor Microenvironment. Biomark. Res. 2024, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, Z.; Dai, Z.; Zhang, H.; Zhang, J.; Luo, P.; Liu, Z.; Liu, Z.; Yang, K.; Cheng, Q.; et al. Glioblastoma Glycolytic Signature Predicts Unfavorable Prognosis, Immunological Heterogeneity, and ENO1 Promotes Microglia M2 Polarization and Cancer Cell Malignancy. Cancer Gene Ther. 2023, 30, 481–496. [Google Scholar] [CrossRef]
- Wang, B.; Pei, J.; Xu, S.; Liu, J.; Yu, J. A Glutamine Tug-of-War between Cancer and Immune Cells: Recent Advances in Unraveling the Ongoing Battle. J. Exp. Clin. Cancer Res. 2024, 43, 74. [Google Scholar] [CrossRef]
- Tan-Shalaby, J. Ketogenic Diets and Cancer: Emerging Evidence. Fed. Pr. 2017, 34, 37S–42S. [Google Scholar] [CrossRef]
- Song, M.; Chan, A.T.; Sun, J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology 2020, 158, 322–340. [Google Scholar] [CrossRef]
- Xu, M.; Jung, X.; Hines, O.J.; Eibl, G.; Chen, Y. Obesity and Pancreatic Cancer: Overview of Epidemiology and Potential Prevention by Weight Loss. Pancreas 2018, 47, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Patton, E.E.; Mueller, K.L.; Adams, D.J.; Anandasabapathy, N.; Aplin, A.E.; Bertolotto, C.; Bosenberg, M.; Ceol, C.J.; Burd, C.E.; Chi, P.; et al. Melanoma Models for the next Generation of Therapies. Cancer Cell 2021, 39, 610–631. [Google Scholar] [CrossRef] [PubMed]
- Daugaard, N.D.; Tholstrup, R.; Tornby, J.R.; Bendixen, S.M.; Larsen, F.T.; De Zio, D.; Barnkob, M.B.; Ravnskjaer, K.; Brewer, J.R. Characterization of Human Melanoma Skin Cancer Models: A Step towards Model-Based Melanoma Research. Acta Biomater. 2025, 191, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Nebhan, C.A.; Saleh, N.; Shattuck-Brandt, R.; Chen, S.-C.; Ayers, G.D.; Weiss, V.; Richmond, A.; Vilgelm, A.E. Generation of Orthotopic Patient-Derived Xenografts in Humanized Mice for Evaluation of Emerging Targeted Therapies and Immunotherapy Combinations for Melanoma. Cancers 2023, 15, 3695. [Google Scholar] [CrossRef]

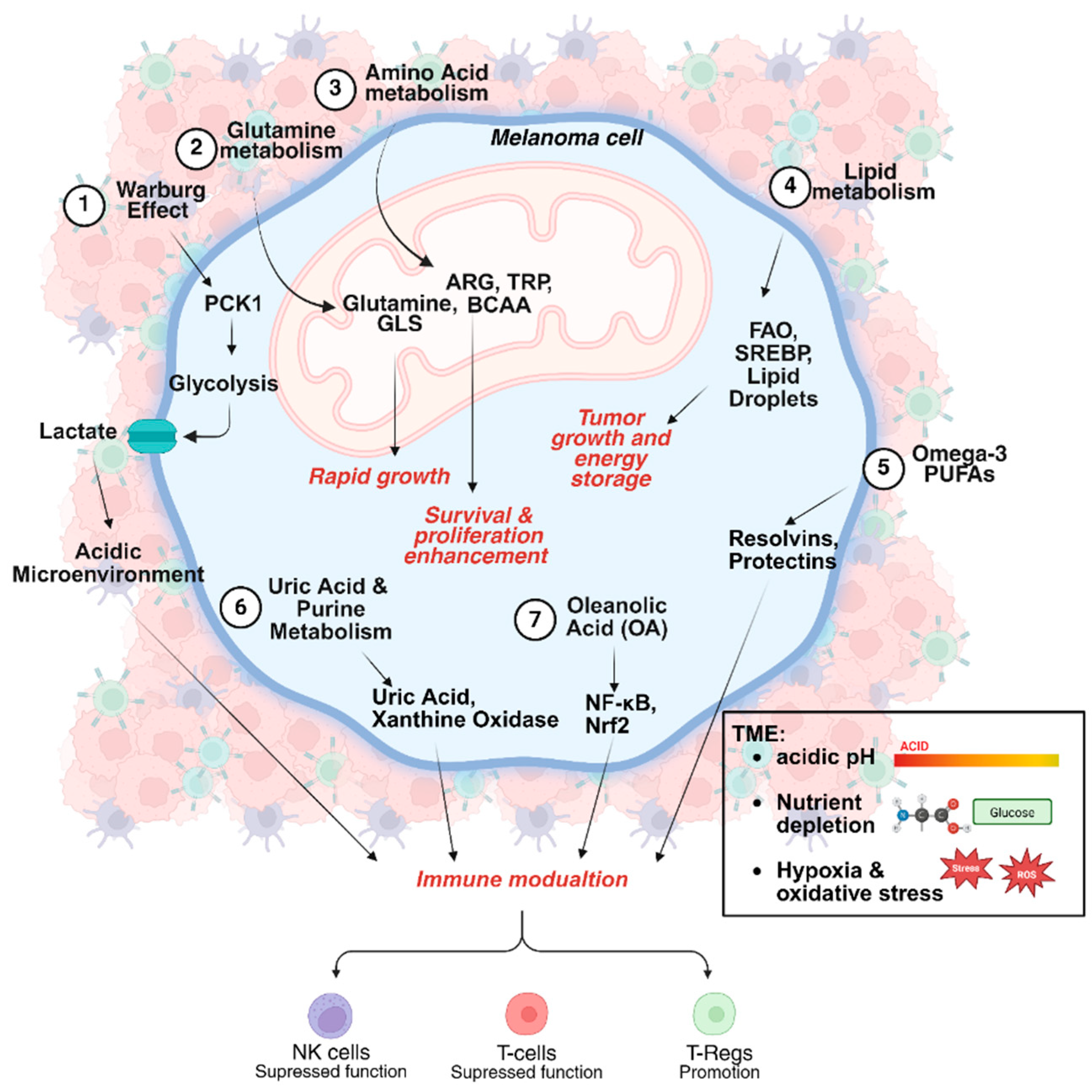

| Biomarker | Associated Pathway/Function | Role in Melanoma Immunotherapy |
|---|---|---|
| PCK1 | Glucose metabolism (glycolysis) | Promotes tumor-repopulating cell growth; overexpression linked to drug resistance; potential ICI target |
| SLC16A1 (MCT1) | Lactate transport | Correlates with immune infiltration (CD8+ T-cells, macrophages); prognostic marker candidate |
| Serum lactate | Glycolysis/ TME acidification | High levels reflect glycolytic flux, linked to an immunosuppressive microenvironment |
| Glutamine dependency | Amino acid metabolism | Fuels tumor growth: Transport inhibition enhances ICI efficacy |
| Arginase | Arginine catabolism | Depletes arginine, impairing T-cell function; inhibition boosts CD8+ T-cell responses |
| Methionine | Methionine salvage pathway | Restriction reprograms T-cell metabolism; sensitizes tumors to ICIs |
| Asparagine | T-cell activation | Essential for CD8+ T-cell proliferation and function |
| BCAAs (Leu, Ile, Val) | Lipid and protein metabolism | Promote immune suppression via metabolic rewiring in the TME |
| Uric acid (UA) | Purine metabolism | High levels impair T-cell immunity, linked to fatty acid metabolism and poor outcomes |
| DHRS3 | Lipid droplet regulation | Controls melanoma cell phenotype; metabolic vulnerability |
| HO-1 | Fructose metabolism/oxidative stress | Induced by fructose; contributes to immune evasion and therapy resistance |
| PD-L1 expression | Immune checkpoint signaling | Predictive biomarker for ICI response |
| Tumor mutational burden (TMB) | Genomic instability | Higher TMB predicts greater ICI responsiveness |
| CD127 | Il-7 receptor on T-cells | Upregulated by fasting-mimicking diets; enhances T-cell survival/function |
| FDG-PET/CT uptake | Glucose uptake/imaging biomarker | High uptake correlates with poor ICI outcomes |
| Hyperpolarized 13C-pyruvate | Glycolytic imaging biomarker | Assesses early ICI therapy response in vivo |
| Microbiota markers | Gut microbiome composition | Faecalibacterium linked to improved PFS; Bacteroidales to reduced response |
| IL-6, IL-17 | Inflammatory cytokines | Lower levels correlate with improved ICI response and prognosis |
| Dietary Pattern | Mechanism | Effect on Immunotherapy | Example Foods |
|---|---|---|---|
| Mediterranean Diet | Enhances microbiota, reduces inflammation | Improves PFS and ORR | Olive oil, fish, nuts, legumes, leafy greens |
| High-Fiber Diet | Supports beneficial gut microbiota, enhances immunity | Enhances ICI efficacy, extends survival | Lentils, oats, beans, and berries |
| Ketogenic Diet Ketogenic Diet + BRAFV600E mutation | Reduces glucose, increases ketones, and CD8+ T-cell function Activates ketone metabolism via Oct1-HMGCL axis | Reduces tumor growth, synergizes with ICIs May induce ICI Resistance, genotype-dependent caution | Avocado, cheese, nuts, and coconut oil As above: Not recommended in BRAF V600E) |
| Fasting-Mimicking Diet | Metabolic stress reduces immunosuppressive cells | Enhances T-cell infiltration, improves ICI response | Vegetable broths, nuts, and zucchini |
| Low-Protein Diet | Triggers the UPR pathway and cytokine signaling via IRE1 and RIG1 | Promotes CD8+ T-cell activation (preclinical) | Green peas, mushrooms, oats |
| Methionine Restriction | Alters T-cell and tumor metabolism | Sensitizes tumors to ICI | Spinach, broccoli, and limited red meat |
| Western/High-Sugar Diet | Promotes lactic acid, increases immunosuppression | Reduces ICI efficacy, increases resistance | To Avoid: Soda, pastries, sugary cereals |
| Flavonoid-Rich Diet | Modulates immune checkpoints, reduces oxidative stress | May enhance anti-tumor immunity | Onions, apples, berries, citrus, green tea |
| Trial Name/ID | Focus/Intervention | Population/Setting | Primary Outcomes | Status/Notes |
|---|---|---|---|---|
| MINI-MD Study NCT06236360 | Mediterranean diet via telehealth coaching | Metastatic melanoma on ICIs | Microbiota, QoL, clinical biomarkers | Ongoing, interventional Phase II |
| DIET Study NCT04645680 | High fiber vs. standard | Stage III–IV melanoma on anti-PD1 | Microbiome composition, immune activation | Phase II, ongoing |
| Camu-Camu Berry Trial NCT05303493 | Camu-Camu supplement to enrich Akkermansia muciniphila | Solid tumors, incl. melanoma | Safety, tolerability, and microbiome enrichment | Phase I, recruiting |
| PreFED Study NCT06466434 | Prebiotic food-enriched diet to increase Faecalibacterium | Patients on ICIs | ICB response, microbiota profiling | Early-phase trial, ongoing |
| Diet + Exercise Trial NCT04866810 | High-fiber, plant-based diet + structured exercise | Melanoma and solid tumors on immunotherapy | Immune markers, fitness, body composition, and ICI efficacy | Active lifestyle intervention study |
| Wheat Germ Supplementation Trial NCT05967533 | Standard wheat germ extract supplementation | Solid tumors, incl. melanoma | Immune activation markers | Phase I, active enrollment |
| Fiber and Probiotic Observational Study (no NCT) | Self-reported fiber and probiotic intake | Melanoma patients on ICIs | ICI response, PFS, microbiome modulation | Observational: probiotics may impair ICI efficacy |
| Strategy/Focus Area | Mechanism/Rationale | Clinical Application/Potential Impact |
|---|---|---|
| Precision Nutrition | Tailors diet to tumor metabolic and immune profiles using metabolomics/microbiome data | Enhances ICI response, reduces resistance, supports personalized treatment plans |
| Metabolic Profiling | Identifies tumor fuel dependencies (e.g., glucose, amino acids, lipids) | Guides diet-based interventions (e.g., ketogenic, methionine-restricted diets) |
| Microbiome Modulation | Alters gut flora through fiber-rich diets, prebiotics, or fecal transplants | Increases ICI efficacy; improves T-cell infiltration and cytokine signalingδ |
| Dietary Pattern Interventions | Uses evidence-based diets (e.g., Mediterranean, FMDs) to reduce inflammation and support immunity | Demonstrates improved ORR and PFS in ongoing clinical trials |
| Nutrient-Specific Modulation | Targets metabolic pathways (e.g., glutamine, methionine, fructose) | Sensitizes tumors to ICIs, reduces immune suppression in the TME |
| Non-Invasive Biomarkers | Uses FDG-PET/CT, hyperpolarized 13C-pyruvate imaging, serum lactate, etc. | Monitors metabolic shifts and early therapy response |
| Sex and BMI Stratification | Accounts for sex-specific metabolism and obesity-related immune modulation | Explains variability in response; supports ‘obesity paradox’ considerations in male patients |
| Diet-Adherence Technologies | Implements mobile apps, digital food logs, and wearable tech | Improves compliance with complex diets and provides real-time feedback |
| Patient-Centered Design | Aligns nutrition strategies with patient preferences, barriers, and quality of life goals | Increases feasibility, adherence, and clinical impact |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grafanaki, K.; Maniatis, A.; Anastogianni, A.; Bania, A.; Pasmatzi, E.; Stathopoulos, C. Nutrition and Diet Patterns as Key Modulators of Metabolic Reprogramming in Melanoma Immunotherapy. J. Clin. Med. 2025, 14, 4193. https://doi.org/10.3390/jcm14124193
Grafanaki K, Maniatis A, Anastogianni A, Bania A, Pasmatzi E, Stathopoulos C. Nutrition and Diet Patterns as Key Modulators of Metabolic Reprogramming in Melanoma Immunotherapy. Journal of Clinical Medicine. 2025; 14(12):4193. https://doi.org/10.3390/jcm14124193
Chicago/Turabian StyleGrafanaki, Katerina, Alexandros Maniatis, Alexandra Anastogianni, Angelina Bania, Efstathia Pasmatzi, and Constantinos Stathopoulos. 2025. "Nutrition and Diet Patterns as Key Modulators of Metabolic Reprogramming in Melanoma Immunotherapy" Journal of Clinical Medicine 14, no. 12: 4193. https://doi.org/10.3390/jcm14124193
APA StyleGrafanaki, K., Maniatis, A., Anastogianni, A., Bania, A., Pasmatzi, E., & Stathopoulos, C. (2025). Nutrition and Diet Patterns as Key Modulators of Metabolic Reprogramming in Melanoma Immunotherapy. Journal of Clinical Medicine, 14(12), 4193. https://doi.org/10.3390/jcm14124193







