Association of Sarcopenia and Visceral Obesity with Clinical Outcomes Among Older Adults with Cardiovascular Disease: A Retrospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Body Composition Analysis and Definitions of Sarcopenia and Visceral Obesity
2.3. Clinical Outcomes and Covariates
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Primary Outcome
3.3. Sarcopenic Obesity
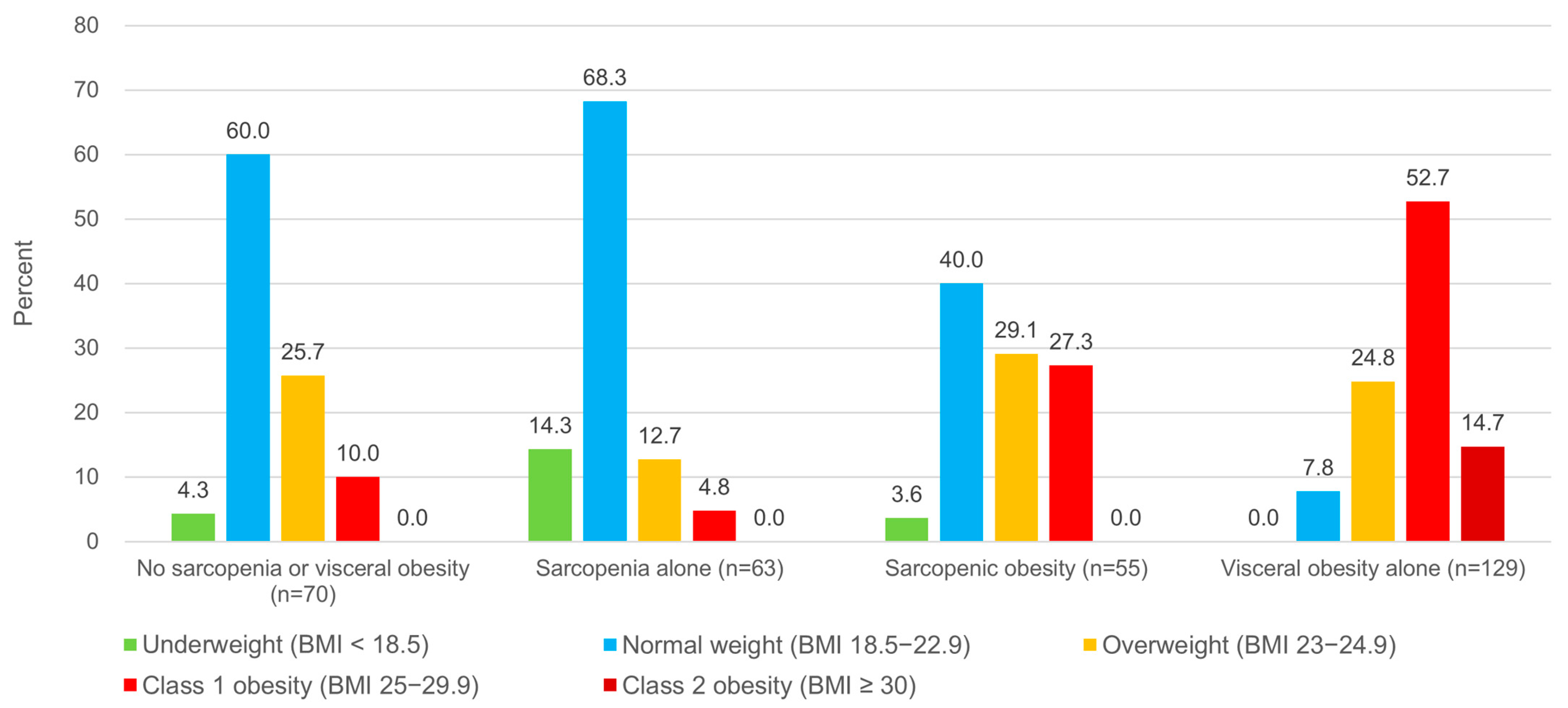
3.4. Sarcopenia, Visceral Obesity, and Body Mass Index
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Han, B.D.; Han, K.; Jung, J.H.; Son, J.W.; Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity. Obesity Fact Sheet in Korea, 2021: Trends in Obesity Prevalence and Obesity-Related Comorbidity Incidence Stratified by Age from 2009 to 2019. J. Obes. Metab. Syndr. 2022, 31, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Bischoff, S.C.; Boirie, Y.; Busetto, L.; Cederholm, T.; Dicker, D.; Toplak, H.; Van Gossum, A.; Yumuk, V.; Vettor, R. Sarcopenic obesity: Time to meet the challenge. Clin. Nutr. 2018, 37, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Choi, K.M. Interplay of skeletal muscle and adipose tissue: Sarcopenic obesity. Metabolism 2023, 144, 155577. [Google Scholar] [CrossRef]
- Kaul, S.; Rothney, M.P.; Peters, D.M.; Wacker, W.K.; Davis, C.E.; Shapiro, M.D.; Ergun, D.L. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity 2012, 20, 1313–1318. [Google Scholar] [CrossRef]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Dahlqvist Leinhard, O. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 1–9. [Google Scholar] [CrossRef]
- Atkins, J.L.; Wannamathee, S.G. Sarcopenic obesity in ageing: Cardiovascular outcomes and mortality. Br. J. Nutr. 2020, 124, 1102–1113. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, X.D.; Shapiro, M.D.; Lip, G.Y.H.; Tilg, H.; Valenti, L.; Somers, V.K.; Byrne, C.D.; Targher, G.; Yang, W.; et al. Global burden of metabolic diseases, 1990–2021. Metabolism 2024, 160, 155999. [Google Scholar] [CrossRef]
- Bahat, G. Sarcopenic obesity: A hot yet under considered evolving concept. Eur. Geriatr. Med. 2022, 13, 1023–1024. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Mantzoros, C.S. Sarcopenia: Still in relative definition-penia and severe treatment-penia. Metabolism 2024, 150, 155717. [Google Scholar] [CrossRef]
- Saad, R.K.; Ghezzawi, M.; Horanieh, R.; Khamis, A.M.; Saunders, K.H.; Batsis, J.A.; Chakhtoura, M. Abdominal Visceral Adipose Tissue and All-Cause Mortality: A Systematic Review. Front Endocrinol. 2022, 13, 922931. [Google Scholar] [CrossRef] [PubMed]
- Benz, E.; Pinel, A.; Guillet, C.; Capel, F.; Pereira, B.; De Antonio, M.; Pouget, M.; Cruz-Jentoft, A.J.; Eglseer, D.; Topinkova, E.; et al. Sarcopenia and Sarcopenic Obesity and Mortality Among Older People. JAMA Netw. Open 2024, 7, e243604. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Cho, S.M.J.; Lee, H.; Baek, J.; Bae, J.H.; Chung, W.J.; Kim, H.C. Korea Heart Disease Fact Sheet 2020: Analysis of Nationwide Data. Korean Circ. J. 2021, 51, 495–503. [Google Scholar] [CrossRef]
- Park, J.J.; Choi, D.J. Current status of heart failure: Global and Korea. Korean J. Intern. Med. 2020, 35, 487–497. [Google Scholar] [CrossRef]
- Lee, C.J.; Lee, H.; Yoon, M.; Chun, K.H.; Kong, M.G.; Jung, M.H.; Kim, I.C.; Cho, J.Y.; Kang, J.; Park, J.J.; et al. Heart Failure Statistics 2024 Update: A Report From the Korean Society of Heart Failure. Int. J. Heart Fail. 2024, 6, 56–69. [Google Scholar] [CrossRef]
- Goh, R.S.J.; Chong, B.; Jayabaskaran, J.; Jauhari, S.M.; Chan, S.P.; Kueh, M.T.W.; Shankar, K.; Li, H.; Chin, Y.H.; Kong, G.; et al. The burden of cardiovascular disease in Asia from 2025 to 2050: A forecast analysis for East Asia, South Asia, South-East Asia, Central Asia, and high-income Asia Pacific regions. Lancet Reg. Health West. Pac. 2024, 49, 101138. [Google Scholar] [CrossRef]
- Damluji, A.A.; Alfaraidhy, M.; AlHajri, N.; Rohant, N.N.; Kumar, M.; Al Malouf, C.; Bahrainy, S.; Ji Kwak, M.; Batchelor, W.B.; Forman, D.E.; et al. Sarcopenia and Cardiovascular Diseases. Circulation 2023, 147, 1534–1553. [Google Scholar] [CrossRef]
- Kim, M.; Won, C.W. Sarcopenia in Korean Community-Dwelling Adults Aged 70 Years and Older: Application of Screening and Diagnostic Tools From the Asian Working Group for Sarcopenia 2019 Update. J. Am. Med. Dir. Assoc. 2020, 21, 752–758. [Google Scholar] [CrossRef]
- Zheng, L.; Sun, A.; Han, S.; Qi, R.; Wang, R.; Gong, X.; Xue, M. Association between visceral obesity and 10-year risk of first atherosclerotic cardiovascular diseases events among American adults: National Health and Nutrition Examination Survey. Front. Cardiovasc. Med. 2023, 10, 1249401. [Google Scholar] [CrossRef]
- Ruiz-Castell, M.; Samouda, H.; Bocquet, V.; Fagherazzi, G.; Stranges, S.; Huiart, L. Estimated visceral adiposity is associated with risk of cardiometabolic conditions in a population based study. Sci. Rep. 2021, 11, 9121. [Google Scholar] [CrossRef]
- Atkins, J.L.; Whincup, P.H.; Morris, R.W.; Lennon, L.T.; Papacosta, O.; Wannamethee, S.G. Sarcopenic obesity and risk of cardiovascular disease and mortality: A population-based cohort study of older men. J. Am. Geriatr. Soc. 2014, 62, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.K.; Peng, L.N.; Lin, M.H.; Loh, C.H.; Lee, W.J.; Hsiao, F.Y.; Chen, L.K. Long-Term Mortality Risk in Older Adults with Sarcopenia: An 11-Year Prospective Cohort Study Comparing AWGS 2014 and AWGS 2019 Guidelines for Enhanced Clinical Utility and Accurate Risk Prediction. J. Nutr. Health Aging 2023, 27, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Cao, L.F.; Ma, W.Z.; Gao, Y.J.; Luo, M.S.; Zhu, J.; Li, T.; Zhou, D. Association between sarcopenia and cardiovascular disease among middle-aged and older adults: Findings from the China health and retirement longitudinal study. EClinicalMedicine 2022, 44, 101264. [Google Scholar] [CrossRef]
- Lundblad, M.W.; Jacobsen, B.K.; Johansson, J.; De Lucia Rolfe, E.; Grimsgaard, S.; Hopstock, L.A. Reference Values for DXA-Derived Visceral Adipose Tissue in Adults 40 Years and Older from a European Population: The Tromso Study 2015-2016. J. Obes. 2021, 2021, 6634536. [Google Scholar] [CrossRef]
- Fulster, S.; Tacke, M.; Sandek, A.; Ebner, N.; Tschope, C.; Doehner, W.; Anker, S.D.; von Haehling, S. Muscle wasting in patients with chronic heart failure: Results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur. Heart J. 2013, 34, 512–519. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Kim, Y.J.; Oh, S.W.; Lee, C.M.; Choi, H.C.; Joh, H.K.; Oh, B.; Hwang, S.S.; Kim, S.J.; Kwon, O.D. Cut-Off Values for Visceral Fat Area Identifying Korean Adults at Risk for Metabolic Syndrome. Korean J. Fam. Med. 2018, 39, 239–246. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia: Sydney, Australia, 2000. [Google Scholar]
- Kim, K.K.; Haam, J.H.; Kim, B.T.; Kim, E.M.; Park, J.H.; Rhee, S.Y.; Jeon, E.; Kang, E.; Nam, G.E.; Koo, H.Y.; et al. Evaluation and Treatment of Obesity and Its Comorbidities: 2022 Update of Clinical Practice Guidelines for Obesity by the Korean Society for the Study of Obesity. J. Obes. Metab. Syndr. 2023, 32, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Kiel, D.P.; Cooper, C.; et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Sales, W.B.; Mâcedo, S.G.G.F.; Gonçalves, R.S.D.S.A.; Andrade, L.E.L.; Ramalho, C.S.T.; de Souza, G.F.; Maciel, Á.C.C. Use of electrical bioimpedance in the assessment of sarcopenia in the older aldults: A scoping review. J. Bodyw. Mov. Ther. 2024, 39, 373–381. [Google Scholar] [CrossRef]
- Hirose, S.; Nakajima, T.; Nozawa, N.; Katayanagi, S.; Ishizaka, H.; Mizushima, Y.; Matsumoto, K.; Nishikawa, K.; Toyama, Y.; Takahashi, R.; et al. Phase Angle as an Indicator of Sarcopenia, Malnutrition, and Cachexia in Inpatients with Cardiovascular Diseases. J. Clin. Med. 2020, 9, 2554. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, H.K.; Lee, M.J.; Bae, S.J.; Choe, J.; Jung, C.H.; Kim, C.H.; Park, J.Y.; Lee, W.J. Sex Differences of Visceral Fat Area and Visceral-to-Subcutaneous Fat Ratio for the Risk of Incident Type 2 Diabetes Mellitus. Diabetes Metab. J. 2022, 46, 486–498. [Google Scholar] [CrossRef]
- Jee, S.H.; Sull, J.W.; Park, J.; Lee, S.Y.; Ohrr, H.; Guallar, E.; Samet, J.M. Body-Mass Index and Mortality in Korean Men and Women. N. Engl. J. Med. 2006, 24, 779–787. [Google Scholar] [CrossRef]
- Global, B.M.I.M.C.; Di Angelantonio, E.; Bhupathiraju Sh, N.; Wormser, D.; Gao, P.; Kaptoge, S.; Berrington de Gonzalez, A.; Cairns, B.J.; Huxley, R.; Jackson Ch, L.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef]
| Variables a | No Sarcopenia or Visceral Obesity (n = 70) | Sarcopenia Alone (n = 63) | Visceral Obesity Alone (n = 129) | Sarcopenic Obesity (n = 55) |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 73.7 ± 6.1 | 77.5 ± 6.0 | 73.9 ± 6.5 | 78.5 ± 6.2 |
| Female | 30 (42.9) | 23 (36.5) | 79 (61.2) | 26 (47.3) |
| Height, cm | 158.5 ± 10.5 | 157.0 ± 9.1 | 157.9 ± 9.6 | 158.2 ± 9.6 |
| Weight, kg | 56.7 ± 8.7 | 51.6 ± 9.0 | 67.0 ± 11.3 | 59.1 ± 10.0 |
| BMI b | 22.5 ± 2.1 | 20.8 ± 2.4 | 26.8 ± 3.2 | 23.5 ± 2.6 |
| Comorbidities | ||||
| Hypertension | 50 (71.4) | 44 (69.8) | 109 (84.5) | 45 (81.8) |
| Diabetes | 30 (42.9) | 28 (44.4) | 48 (37.2) | 24 (43.6) |
| Dyslipidemia | 20 (28.6) | 19 (30.2) | 51 (39.5) | 17 (30.9) |
| Obesity c | 7 (10.0) | 3 (4.8) | 87 (67.4) | 15 (27.3) |
| Chronic kidney disease | 18 (25.7) | 21 (33.3) | 27 (20.9) | 22 (40.0) |
| Body composition | ||||
| Low ASM d | 20 (28.6) | 63 (100) | 6 (4.7) | 55 (100) |
| Low muscle strength e | 32 (45.7) | 51 (81.0) | 71 (55.0) | 39 (70.9) |
| Low physical performance f | 23 (32.9) | 46 (73.0) | 41 (31.8) | 51 (92.7) |
| Trunk-to-total fat ratio, % | 52.5 ± 6.2 | 50.9 ± 7.4 | 59.4 ± 3.8 | 60.0 ± 4.4 |
| Body fat percentage, % | 25.7 ± 6.1 | 26.2 ± 6.4 | 36.1 ± 5.9 | 36.1 ± 5.1 |
| Total fat mass, g | 14,280 ± 3549 | 13,521 ± 4442 | 23,997 ± 5302 | 21,130 ± 3691 |
| VAT mass, g | 601 ± 284 | 607 ± 315 | 1469 ± 551 | 1370 ± 436 |
| VAT area, cm2 | 70.7 ± 31.3 | 72.1 ± 35.2 | 172.9 ± 59.2 | 162.3 ± 45.2 |
| Medication use | ||||
| ACE inhibitor | 7 (10.0) | 15 (23.8) | 11 (8.5) | 7 (12.7) |
| ARB | 24 (34.3) | 17 (27.0) | 71 (55.0) | 26 (47.3) |
| BB | 21 (30.0) | 27 (42.9) | 58 (45.0) | 20 (36.4) |
| CCB | 26 (37.1) | 19 (30.2) | 73 (56.6) | 21 (38.2) |
| Diuretics | 26 (37.1) | 34 (54.0) | 59 (45.7) | 30 (54.5) |
| Aspirin | 25 (35.7) | 20 (31.7) | 42 (32.6) | 14 (25.5) |
| P2Y12 inhibitor | 28 (40.0) | 28 (44.4) | 53 (41.1) | 23 (41.8) |
| Anticoagulant | 21 (30.0) | 17 (27.0) | 35 (27.1) | 20 (36.4) |
| Statin | 58 (82.9) | 50 (79.4) | 121 (93.8) | 48 (87.3) |
| Biochemical characteristics | ||||
| Glycated hemoglobin, % | 6.3 ± 1.0 | 6.4 ± 1.3 | 6.2 ± 0.7 | 6.5 ± 1.0 |
| BUN, mg/dL | 18.7 ± 7.3 | 22.0 ± 11.0 | 18.2 ± 6.1 | 23.1 ± 12.3 |
| Creatinine, mg/dL | 1.0 ± 0.4 | 1.1 ± 0.9 | 0.9 ± 0.3 | 1.3 ± 1.5 |
| GFR, ml/min/1.73 m2 | 75.7 ± 22.3 | 67.1 ± 22.6 | 76.2 ± 18.3 | 61.1 ± 23.0 |
| TC, mg/dL | 137.2 ± 37.4 | 138.1 ± 35.6 | 143.0 ± 32.1 | 131.0 ± 29.5 |
| TG, mg/dL | 111.7 ± 66.6 | 107.5 ± 43.9 | 132.2 ± 52.5 | 133.4 ± 88.7 |
| HDL, mg/dL | 18.3 ± 13.0 | 48.1 ± 16.7 | 47.3 ± 11.7 | 39.8 ± 10.5 |
| LDL, mg/dL | 65.2 ± 27.0 | 68.4 ± 32.3 | 69.4 ± 27.0 | 68.3 ± 24.1 |
| Hs-CRP, mg/dL | 0.3 ± 0.7 | 0.5 ± 2.1 | 0.1 ± 0.3 | 0.7 ± 2.3 |
| Variables | HR | 95% CI | p Value |
|---|---|---|---|
| Sarcopenia | 1.93 | 1.02–3.66 | 0.043 |
| Age | 1.07 | 1.02–1.12 | 0.008 |
| Variables | HR | 95% CI | p Value |
|---|---|---|---|
| Visceral obesity | 2.13 | 0.99–4.57 | 0.052 |
| Weight | 0.96 | 0.94–0.99 | 0.015 |
| Participants | Incidence Rate/1000 Person-Years | Unadjusted Model | Model 1 | Model 2 |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Patients without visceral obesity (n = 63) | 96.5 | 1 (ref) | 1 (ref) | 1 (ref) |
| Patients with visceral obesity (n = 55) | 245.8 | 2.46 (1.05–5.75) | 3.21 (1.24–8.37) | 6.74 (1.81–25.16) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, H.-J.; Park, K.-W.; Seo, Y.-H. Association of Sarcopenia and Visceral Obesity with Clinical Outcomes Among Older Adults with Cardiovascular Disease: A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 4191. https://doi.org/10.3390/jcm14124191
Yoon H-J, Park K-W, Seo Y-H. Association of Sarcopenia and Visceral Obesity with Clinical Outcomes Among Older Adults with Cardiovascular Disease: A Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(12):4191. https://doi.org/10.3390/jcm14124191
Chicago/Turabian StyleYoon, Hye-Jin, Keon-Woo Park, and Young-Hoon Seo. 2025. "Association of Sarcopenia and Visceral Obesity with Clinical Outcomes Among Older Adults with Cardiovascular Disease: A Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 12: 4191. https://doi.org/10.3390/jcm14124191
APA StyleYoon, H.-J., Park, K.-W., & Seo, Y.-H. (2025). Association of Sarcopenia and Visceral Obesity with Clinical Outcomes Among Older Adults with Cardiovascular Disease: A Retrospective Cohort Study. Journal of Clinical Medicine, 14(12), 4191. https://doi.org/10.3390/jcm14124191







