Cardiopulmonary Recovery After Maximal Exercise in Individuals with Neuromuscular Disease and Limited Mobility
Abstract
1. Introduction
2. Materials and Methods
2.1. Cardiopulmonary Exercise Testing (CPET)
2.2. Statistical Analysis
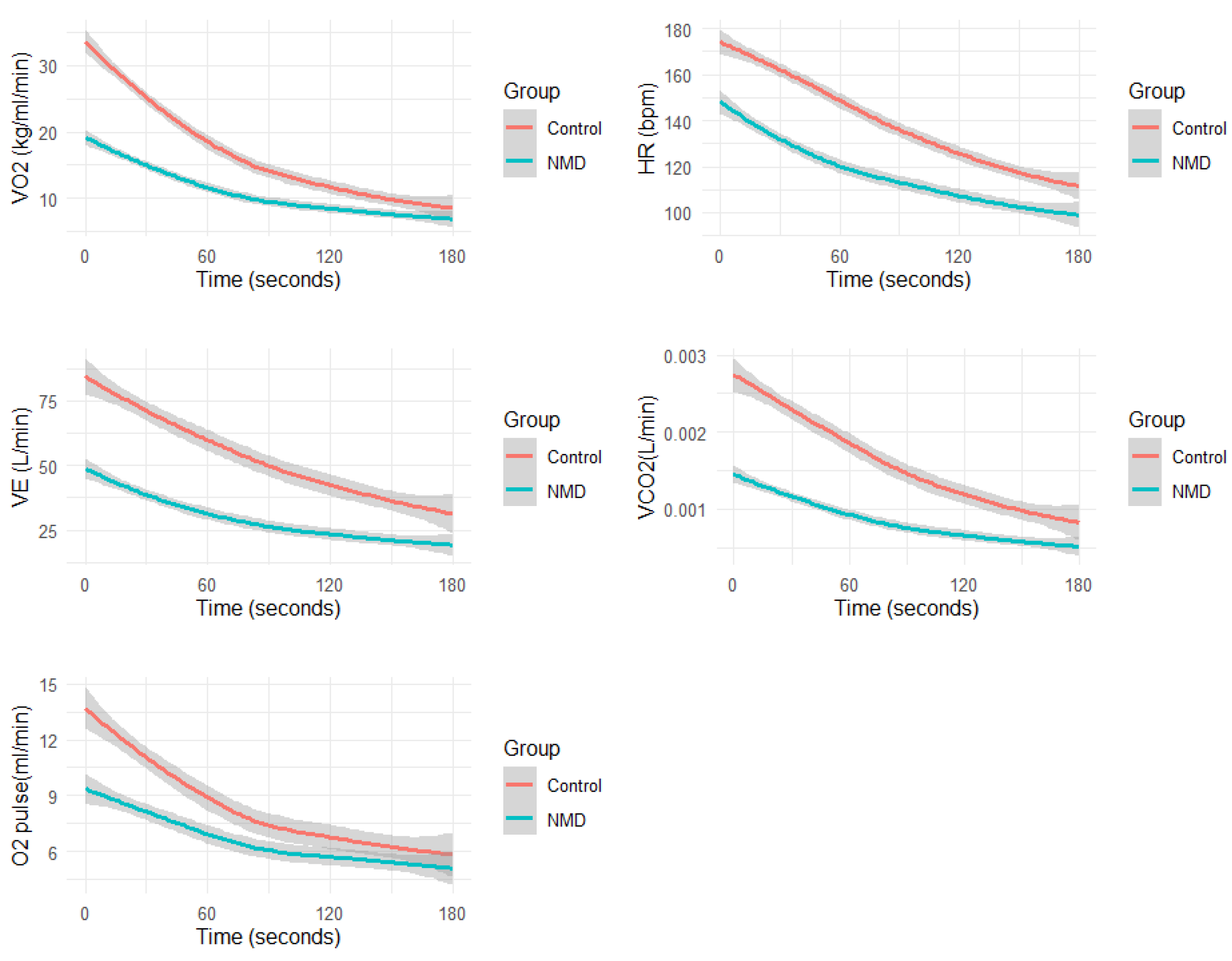
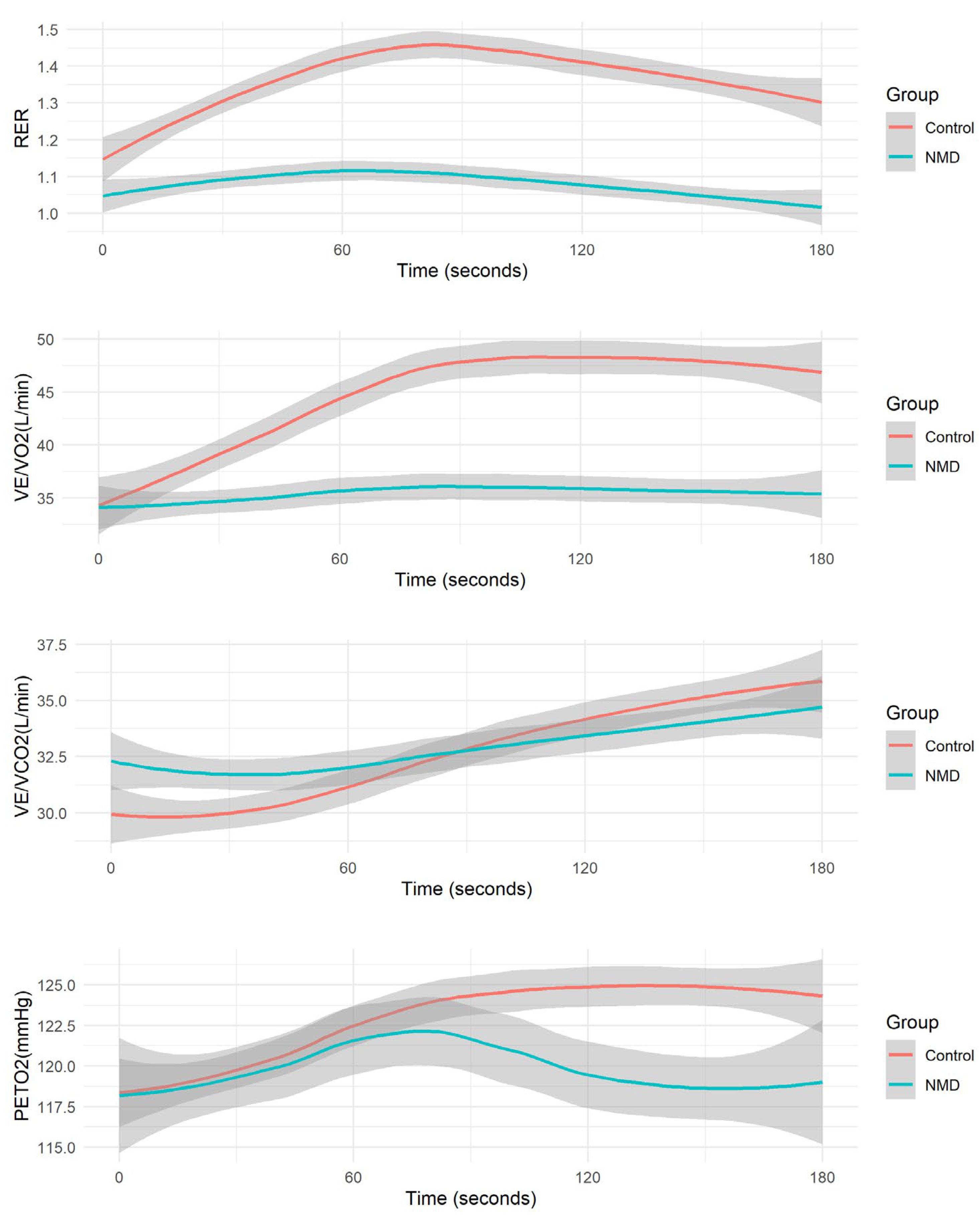
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schillings, M.L.; Kalkman, J.S.; Janssen, H.M.; van Engelen, B.G.; Bleijenberg, G.; Zwarts, M.J. Experienced and physiological fatigue in neuromuscular disorders. Clin. Neurophysiol. 2007, 118, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Torres, R.S.; Uher, D.; Gay, E.L.; Coratti, G.; Dunaway Young, S.; Rohwer, A.; Muni Lofra, R.; De Vivo, D.C.; Hirano, M.; Glynn, N.W.; et al. Measuring Fatigue and Fatigability in Spinal Muscular Atrophy (SMA): Challenges and Opportunities. J. Clin. Med. 2023, 12, 3458. [Google Scholar] [CrossRef] [PubMed]
- Lanfranconi, F.; Marzorati, M.; Tremolizzo, L. Editorial: Strategies to Fight Exercise Intolerance in Neuromuscular Disorders. Front. Physiol. 2020, 11, 968. [Google Scholar] [CrossRef]
- Siciliano, G.; Chico, L.; Lo Gerfo, A.; Simoncini, C.; Schirinzi, E.; Ricci, G. Exercise-Related Oxidative Stress as Mechanism to Fight Physical Dysfunction in Neuromuscular Disorders. Front. Physiol. 2020, 11, 451. [Google Scholar] [CrossRef]
- Markert, C.D.; Case, L.E.; Carter, G.T.; Furlong, P.A.; Grange, R.W. Exercise and Duchenne muscular dystrophy: Where we have been and where we need to go. Muscle Nerve 2012, 45, 746–751. [Google Scholar] [CrossRef]
- Anziska, Y.; Sternberg, A. Exercise in neuromuscular disease. Muscle Nerve 2013, 48, 3–20. [Google Scholar] [CrossRef]
- Voorn, E.L.; Koopman, F.; Nollet, F.; Brehm, M.A. Aerobic exercise in adult neuromuscular rehabilitation: A survey of healthcare professionals. J. Rehabil. Med. 2019, 51, 518–524. [Google Scholar] [CrossRef]
- Peake, J.M. Recovery after exercise: What is the current state of play? Curr. Opin. Physiol. 2019, 10, 17–26. [Google Scholar] [CrossRef]
- Romero, S.A.; Minson, C.T.; Halliwill, J.R. The cardiovascular system after exercise. J. Appl. Physiol. 2017, 122, 925–932. [Google Scholar] [CrossRef]
- Christle, J.W.; Duong, T.; Parker, D.; Stevens, V.; Dunaway Young, S.; Kaufman, B.D.; Tang, W.; Sampson, J.; Myers, J.; Ashley, E.A.; et al. Cardiopulmonary Exercise Testing for Patients With Neuromuscular Disease and Limited Mobility. J. Clin. Exerc. Physiol. 2023, 12, 12–17. [Google Scholar] [CrossRef]
- Arena, R.; Myers, J.; Williams, M.A.; Gulati, M.; Kligfield, P.; Balady, G.J.; Collins, E.; Fletcher, G. Assessment of functional capacity in clinical and research settings: A scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 2007, 116, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.X.; Gyanwali, B.; Soh, J.; Koh, A.S.; Goh, J. The potential benefits of assessing post-cardiopulmonary exercise testing (CPET) in aging: A narrative review. BMC Sports Sci. Med. Rehabil. 2023, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Luttrell, M.J.; Halliwill, J.R. Recovery from exercise: Vulnerable state, window of opportunity, or crystal ball? Front. Physiol. 2015, 6, 204. [Google Scholar] [CrossRef] [PubMed]
- Patti, A.; Blumberg, Y.; Hedman, K.; Neunhauserer, D.; Haddad, F.; Wheeler, M.; Ashley, E.; Moneghetti, K.J.; Myers, J.; Christle, J.W. Respiratory gas kinetics in patients with congestive heart failure during recovery from peak exercise. Clinics 2023, 78, 100225. [Google Scholar] [CrossRef]
- Vecchiato, M.; Neunhaeuserer, D.; Zanardo, E.; Quinto, G.; Battista, F.; Aghi, A.; Palermi, S.; Babuin, L.; Tessari, C.; Guazzi, M.; et al. Respiratory exchange ratio overshoot during exercise recovery: A promising prognostic marker in HFrEF. Clin. Res. Cardiol. 2024, 1–12. [Google Scholar] [CrossRef]
- Vecchiato, M.; Ermolao, A.; Zanardo, E.; Battista, F.; Ruvoletto, G.; Palermi, S.; Quinto, G.; Degano, G.; Gasperetti, A.; Padalino, M.A.; et al. Overshoot of the Respiratory Exchange Ratio during Recovery from Maximal Exercise Testing in Young Patients with Congenital Heart Disease. Children 2023, 10, 521. [Google Scholar] [CrossRef]
- Myers, J.N.; Gujja, P.; Neelagaru, S.; Hsu, L.; Burkhoff, D. Noninvasive measurement of cardiac performance in recovery from exercise in heart failure patients. Clinics 2011, 66, 649–656. [Google Scholar] [CrossRef]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 1999, 341, 1351–1357. [Google Scholar] [CrossRef]
- Sydo, N.; Sydo, T.; Gonzalez Carta, K.A.; Hussain, N.; Farooq, S.; Murphy, J.G.; Merkely, B.; Lopez-Jimenez, F.; Allison, T.G. Prognostic Performance of Heart Rate Recovery on an Exercise Test in a Primary Prevention Population. J. Am. Heart Assoc. 2018, 7, e008143. [Google Scholar] [CrossRef]
- Takahashi, T.; Niizeki, K.; Miyamoto, Y. Respiratory responses to passive and active recovery from exercise. Jpn. J. Physiol. 1997, 47, 59–65. [Google Scholar] [CrossRef]
- Takayanagi, Y.; Koike, A.; Nagayama, O.; Nagamine, A.; Qin, R.; Kato, J.; Nishi, I.; Himi, T.; Kato, Y.; Sato, A.; et al. Clinical significance of the overshoot phenomena of respiratory gas indices during recovery from maximal exercise testing. J. Cardiol. 2017, 70, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.S.; Wooster, L.T.; Buswell, M.; Patel, S.; Pappagianopoulos, P.P.; Bakken, K.; White, C.; Tanguay, M.; Blodgett, J.B.; Baggish, A.L.; et al. Post-Exercise Oxygen Uptake Recovery Delay: A Novel Index of Impaired Cardiac Reserve Capacity in Heart Failure. JACC Heart Fail. 2018, 6, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Takahashi, M.; Hosaka, Y.; Ito, M.; Ito, E.; Suzuki, K. Prolonged recovery of cardiac output after maximal exercise in patients with chronic heart failure. J. Am. Coll. Cardiol. 2000, 35, 1228–1236. [Google Scholar] [CrossRef]
- Feingold, B.; Mahle, W.T.; Auerbach, S.; Clemens, P.; Domenighetti, A.A.; Jefferies, J.L.; Judge, D.P.; Lal, A.K.; Markham, L.W.; Parks, W.J.; et al. Management of Cardiac Involvement Associated With Neuromuscular Diseases: A Scientific Statement From the American Heart Association. Circulation 2017, 136, e200–e231. [Google Scholar] [CrossRef]
- Kaminsky, L.A.; Arena, R.; Myers, J.; Peterman, J.E.; Bonikowske, A.R.; Harber, M.P.; Medina Inojosa, J.R.; Lavie, C.J.; Squires, R.W. Updated Reference Standards for Cardiorespiratory Fitness Measured with Cardiopulmonary Exercise Testing: Data from the Fitness Registry and the Importance of Exercise National Database (FRIEND). Mayo Clin. Proc. 2022, 97, 285–293. [Google Scholar] [CrossRef]
- Cohen-Solal, A.; Laperche, T.; Morvan, D.; Geneves, M.; Caviezel, B.; Gourgon, R. Prolonged kinetics of recovery of oxygen consumption after maximal graded exercise in patients with chronic heart failure. Analysis with gas exchange measurements and NMR spectroscopy. Circulation 1995, 91, 2924–2932. [Google Scholar] [CrossRef]
- Barroso de Queiroz Davoli, G.; Bartels, B.; Mattiello-Sverzut, A.C.; Takken, T. Cardiopulmonary exercise testing in neuromuscular disease: A systematic review. Expert Rev. Cardiovasc. Ther. 2021, 19, 975–991. [Google Scholar] [CrossRef]
- Torri, F.; Lopriore, P.; Montano, V.; Siciliano, G.; Mancuso, M.; Ricci, G. Pathophysiology and Management of Fatigue in Neuromuscular Diseases. Int. J. Mol. Sci. 2023, 24, 5005. [Google Scholar] [CrossRef]
- Patti, A.; Neunhaeuserer, D.; Gasperetti, A.; Baioccato, V.; Vecchiato, M.; Battista, F.; Marchini, F.; Bergamin, M.; Furian, L.; Ermolao, A. Overshoot of the Respiratory Exchange Ratio during Recovery from Maximal Exercise Testing in Kidney Transplant Recipients. Int. J. Environ. Res. Public Health 2021, 18, 9236. [Google Scholar] [CrossRef]
- Finsterer, J.; Stollberger, C. Heart Disease in Disorders of Muscle, Neuromuscular Transmission, and the Nerves. Korean Circ. J. 2016, 46, 117–134. [Google Scholar] [CrossRef]
- Voet, N.B.M. Exercise in neuromuscular disorders: A promising intervention. Acta Myol. 2019, 38, 207–214. [Google Scholar] [PubMed]
| Control (N = 15) | NMD (N = 32) | p | |
|---|---|---|---|
| Age (yrs) | |||
| Median [Min, Max] | 33.3 [22.7, 70.7] | 43.9 [11.8, 78.0] | 0.46 |
| Height | |||
| Median [Min, Max] | 173 [154, 193] | 170 [137, 196] | 0.05 |
| Body Weight (kg) | |||
| Median [Min, Max] | 64.9 [54.0, 96.6] | 67.1 [42.2, 147] | 0.86 |
| BMI (kg/m2) | |||
| Median [Min, Max] | 22.9 [18.5, 30.3] | 23.2 [15.3, 42.6] | |
| Resting HR (bpm) | |||
| Median [Min, Max] | 69 [60, 81] | 70 [58, 84] | 0.57 |
| Disease | |||
| DM1 | - | 7 (21.9%) | |
| DM2 | - | 1 (3.1%) | |
| DMD | - | 1 (3.1%) | |
| Dysferlinopathy | - | 1 (3.1%) | |
| Dystrophinopathy | - | 1 (3.1%) | |
| FSHD | - | 10 (31.3%) | |
| LGMD | - | 1 (3.1%) | |
| Pompe | - | 3 (9.4%) | |
| SMA3 | - | 7 (21.9%) | |
| Control (N = 15) | NMD (N = 32) | p | |
|---|---|---|---|
| Peak Workload (Watt) | |||
| Median [Min, Max] | 172 [140, 282] | 54.5 [10.0, 168] | <0.001 |
| Peak VO2 (L/min) | |||
| Median [Min, Max] | 2.37 [1.39, 4.07] | 1.37 [0.532, 3.04] | <0.001 |
| Peak VO2 (mL/kg/min) | |||
| Median [Min, Max] | 33.0 [19.9, 54.3] | 19.5 [10.8, 34.2] | <0.001 |
| Predicted Peak VO2 (%) | |||
| Median [Min, Max] | 104 [72.0, 182] | 52.4 [26.3, 124] | <0.001 |
| VO2 at AT (mL/kg/min) | |||
| Median [Min, Max] | 17.0 [14.4, 25.1] | 11.2 [8.24, 11.42] | <0.001 |
| Peak RER (no unit) | |||
| Median [Min, Max] | 1.13 [1.01, 1.38] | 1.06 [0.810, 1.39] | 0.02 |
| Peak Heart Rate (bpm) | |||
| Median [Min, Max] | 173 [157, 193] | 150 [95.0, 192] | <0.001 |
| Predicted Heart Rate (%) | |||
| Median [Min, Max] | 93.8 [88.1, 111] | 86.3 [62.7, 108] | <0.001 |
| O2 pulse (mL/bpm) | |||
| Median [Min, Max] | 14.9 [10.5, 23.5] | 8.70 [2.80, 19.8] | <0.001 |
| VE VCO2 Slope (no unit) | |||
| Median [Min, Max] | 32.2 [27.0, 43.3] | 36.7 [27.4, 53.8] | 0.09 |
| VT1 VE VCO2 Slope (no unit) | |||
| Median [Min, Max] | 24.2 [16.4, 29.0] | 27.6 [21.7, 43.5] | 0.06 |
| VT2 VE VCO2 Slope (no unit) | |||
| Median [Min, Max] | 26.9 [24.1, 30.4] | 29.8 [25.6, 50.4] | 0.01 |
| Control (N = 15) | NMD (N = 32) | p | |
|---|---|---|---|
| T1/2 VO2 (second) | |||
| Median [Min, Max] | 60.0 [30.0, 180] | 90.0 [50.0, 210] | 0.02 |
| T1/2 VE (second) | |||
| Median [Min, Max] | 120 [30.0, 230] | 120 [40.0, 280] | 0.94 |
| T1/2 VCO2 (second) | |||
| Median [Min, Max] | 90.0 [50.0, 190] | 100 [40.0, 180] | 0.54 |
| T1/2 O2 Pulse (second) | |||
| Median [Min, Max] | 90.0 [30.0, 260] | 165 [60.0, 330] | <0.001 |
| Overshoot of RER (%) | |||
| Median [Min, Max] | 30.0 [13.7, 43.4] | 16.2 [0.730, 53.6] | <0.001 |
| Overshoot of VE/VO2 (%) | |||
| Median [Min, Max] | 49.5 [22.5, 99.8] | 30.5 [1.85, 124] | 0.01 |
| Overshoot of VE/VCO2 (%) | |||
| Median [Min, Max] | 28.9 [3.03, 59.6] | 15.5 [0.338, 109] | 0.04 |
| Overshoot of PETO2 (%) | |||
| Median [Min, Max] | 8.55 [3.23, 14.0] | 4.76 [0.813, 62.4] | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blumberg, Y.; de Monts, C.; Montalvo, S.; Tang, W.J.; Dunaway Young, S.; Hageman, N.; Sanchis-Gomar, F.; Ashley, E.A.; Amar, D.; Myers, J.; et al. Cardiopulmonary Recovery After Maximal Exercise in Individuals with Neuromuscular Disease and Limited Mobility. J. Clin. Med. 2025, 14, 4190. https://doi.org/10.3390/jcm14124190
Blumberg Y, de Monts C, Montalvo S, Tang WJ, Dunaway Young S, Hageman N, Sanchis-Gomar F, Ashley EA, Amar D, Myers J, et al. Cardiopulmonary Recovery After Maximal Exercise in Individuals with Neuromuscular Disease and Limited Mobility. Journal of Clinical Medicine. 2025; 14(12):4190. https://doi.org/10.3390/jcm14124190
Chicago/Turabian StyleBlumberg, Yair, Constance de Monts, Samuel Montalvo, Whitney J. Tang, Sally Dunaway Young, Nathan Hageman, Fabian Sanchis-Gomar, Euan A. Ashley, David Amar, Jonathan Myers, and et al. 2025. "Cardiopulmonary Recovery After Maximal Exercise in Individuals with Neuromuscular Disease and Limited Mobility" Journal of Clinical Medicine 14, no. 12: 4190. https://doi.org/10.3390/jcm14124190
APA StyleBlumberg, Y., de Monts, C., Montalvo, S., Tang, W. J., Dunaway Young, S., Hageman, N., Sanchis-Gomar, F., Ashley, E. A., Amar, D., Myers, J., Wheeler, M. T., Day, J. W., Duong, T., & Christle, J. W. (2025). Cardiopulmonary Recovery After Maximal Exercise in Individuals with Neuromuscular Disease and Limited Mobility. Journal of Clinical Medicine, 14(12), 4190. https://doi.org/10.3390/jcm14124190





