MAFLD as a Cardiovascular Risk Factor: An Extended Retrospective Study with a Control Group
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Comparison of the Study Group with the Control Group
- RBCs (MD = 0.12; CI 95% [0.10; 0.30]; p < 0.001);
- Hb (MD = 0.68; CI 95% [0.38; 0.98]; p < 0.001);
- HCT (MD = 1.85; CI 95% [1.20; 2.90]; p < 0.001);
- WBCs (MD = 0.62; CI 95% [0.31; 1.10]; p = 0.001);
- PLTs (MD = 11.00; CI 95% [4.00; 18.00]; p = 0.008);
- Fasting glucose (MD = 15.00; CI 95% [8.00; 18.00]; p < 0.001);
- ALT (MD = 4.00; CI 95% [4.00; 7.00]; p < 0.001);
- AST (MD = 2.00; CI 95% [0.00; 3.00]; p = 0.013);
- Triglycerides (MD = 49.00; CI 95% [37.00; 59.00]; p < 0.001);
- Uric acid (MD = 0.80; CI 95% [0.40; 1.00]; p < 0.001).
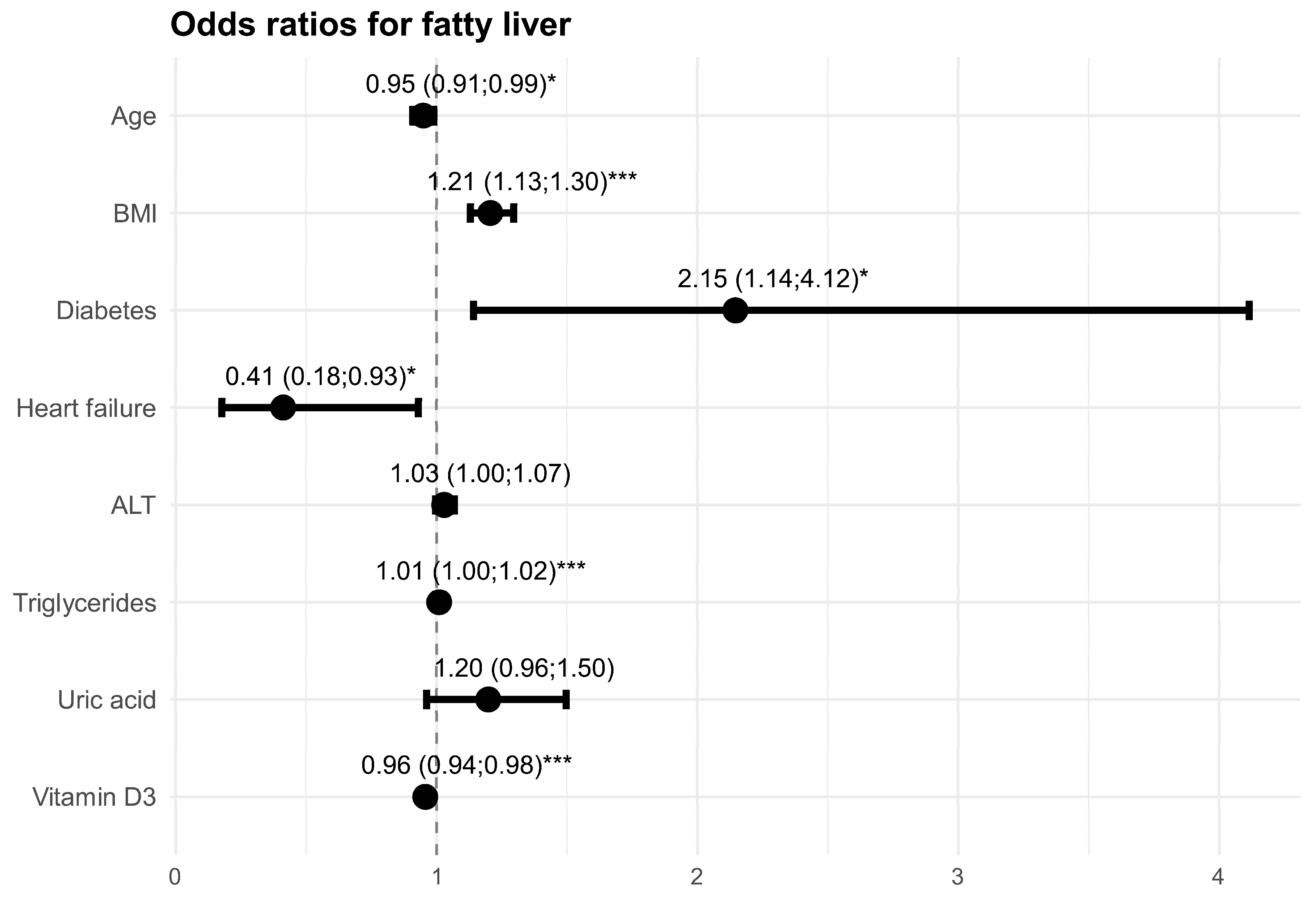
3.2. Factors Affecting the Risk of Liver Steatosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| CVD | Cardiovascular Disease |
| FIB-4 | Fibrosis-4 Index |
| FLD | Fatty Liver Disease |
| GLS | Global Longitudinal Strain |
| GOF | Goodness-of-Fit |
| Hb | Hemoglobin |
| HbA1C | Hemoglobin A1C (Glycated Hemoglobin) |
| HCT | Hematocrit |
| HDL | High-Density Lipoprotein |
| IQR | Interquartile Range |
| IVSd | Interventricular Septal Thickness in Diastole |
| LDL | Low-Density Lipoprotein |
| LVEF | Left Ventricular Ejection Fraction |
| LVPWd | Left Ventricular Posterior Wall Thickness in Diastole |
| LVMI | Left Ventricular Mass Index |
| MAFLD | Metabolic-Associated Fatty Liver Disease |
| M | Mean |
| MD | Mean or Median Difference |
| Me | Median |
| n | Number |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NT-proBNP | N-terminal Pro-B-type Natriuretic Peptide |
| OR | Odds Ratio |
| p | p-Value (Level of Statistical Significance) |
| PLT | Platelet |
| RBC | Red Blood Cell |
| SD | Standard Deviation |
| STE | Speckle Tracking Echocardiography |
| UCH | University Clinical Hospital |
| WBC | White Blood Cell |
| WHO | World Health Organization |
References
- Teng, M.L.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.; Lim, W.H.; Chan, K.E.; Teng, M.L.; Tan, D.J.; Muthiah, M.D.; et al. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S32–S42. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease: Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Roeb, E. Excess body weight and metabolic (dysfunction)-associated fatty liver disease (MAFLD). Visc. Med. 2021, 37, 273–280. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Francque, S.; Lanthier, N.; Verbeke, L.; Reynaert, H.; Van Steenkiste, C.; Vonghia, L.; Kwanten, W.J.; Weyler, J.; Trépo, E.; Cassiman, D.; et al. The Belgian Association for Study of the Liver Guidance Document on the Management of Adult and Paediatric Non-Alcoholic Fatty Liver Disease. Acta Gastroenterol. Belg. 2018, 81, 55–81. [Google Scholar]
- Claypool, K.; Patel, C.J. Prevalence of fatty liver disease is driven by prediabetes and diabetes: US NHANES 2017–2018. Clin. Gastroenterol. Hepatol. 2022, 20, 712–713. [Google Scholar] [CrossRef]
- Tomasiewicz, K.; Flisiak, R.; Halota, W.; Jaroszewicz, J.; Kukla, M.; Lebensztejn, D.; Lisik, W.; Małkowski, P.; Pawłowska, M.; Piekarska, A.; et al. Rekomendacje Grupy Ekspertów Stłuszczeniowej Choroby Wątroby Związanej z Zaburzeniami Metabolicznymi (PGE-MAFLD). Hepatologia 2023, 23, 106988. [Google Scholar]
- Choi, K.M. The impact of organokines on insulin resistance, inflammation, and atherosclerosis. Endocrinol. Metab. 2016, 31, 1–6. [Google Scholar] [CrossRef]
- Ludwig, J.; Viggiano, T.R.; McGill, D.B.; Oh, B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980, 55, 434–438. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H.; et al. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Loria, P.; Lonardo, A.; Carulli, N. Should nonalcoholic fatty liver disease be renamed? Dig. Dis. 2005, 23, 72–82. [Google Scholar] [CrossRef]
- Fouad, Y.; Waked, I.; Bollipo, S.; Gomaa, A.; Ajlouni, Y.; Attia, D. What’s in a name? Renaming ‘NAFLD’ to ‘MAFLD’. Liver Int. 2020, 40, 1254–1261. [Google Scholar] [CrossRef]
- Battistella, S.; D’Arcangelo, F.; Grasso, M.; Zanetto, A.; Gambato, M.; Germani, G.; Senzolo, M.; Russo, F.P.; Burra, P. Liver transplantation for non-alcoholic fatty liver disease: Indications and post-transplant management. Clin. Mol. Hepatol. 2023, 29, S286–S301. [Google Scholar] [CrossRef]
- Frith, J.; Day, C.P.; Henderson, E.; Burt, A.D.; Newton, J.L. Non-alcoholic fatty liver disease in older people. Gerontology 2009, 55, 607–613. [Google Scholar] [CrossRef]
- Prejbisz, A.; Dobrowolski, P. Prewencja chorób sercowo-naczyniowych—Postępy 2023/2024. Med. Prakt. 2024, 7–8, 36–49. [Google Scholar]
- Sattar, N.; Neeland, I.J.; McGuire, D.K. Obesity and cardiovascular disease: A new dawn. Circulation 2024, 149, 1621–1623. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 17 April 2025).
- Rajewski, P.; Cieściński, J. Pacjent ze stłuszczeniową chorobą wątroby związaną z dysfunkcją metaboliczną w praktyce klinicznej. Lekarz POZ 2024, 10. [Google Scholar]
- Bilson, J.; Mantovani, A.; Byrne, C.D.; Targher, G. Steatotic liver disease, MASLD and risk of chronic kidney disease. Diabetes Metab. 2024, 50, 101506. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.; Kim, S.U.; Kim, H.C. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: A nationwide cohort study. Clin. Gastroenterol. Hepatol. 2021, 19, 2138–2147.e10. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.C.; Chen, H.F.; Cheng, H.C.; Li, C.Y. Comparison of all-cause mortality associated with NAFLD and MAFLD in Taiwan MJ cohort. Epidemiol. Health 2024, 46, e2024024. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Li, H.; Wang, C.; Chen, C.; Tang, J.; Zhou, M.; Hong, X.; Cheng, Y.; Wu, Q.; Zhang, X.; et al. Metabolic dysfunction-associated fatty liver disease and cardiovascular disease: A meta-analysis. Front. Endocrinol. 2022, 13, 934225. [Google Scholar] [CrossRef]
- Peng, D.; Yu, Z.; Wang, M.; Shi, J.; Sun, L.; Zhang, Y.; Zhao, W.; Chen, C.; Tang, J.; Wang, C.; et al. Association of metabolic dysfunction-associated fatty liver disease with left ventricular diastolic function and cardiac morphology. Front. Endocrinol. 2022, 13, 935390. [Google Scholar] [CrossRef] [PubMed]
- VanWagner, L.B.; Wilcox, J.E.; Ning, H.; Lewis, C.E.; Carr, J.J.; Rinella, M.E.; Shah, S.J.; Lima, J.A.; Lloyd-Jones, D.M. Longitudinal association of non-alcoholic fatty liver disease with changes in myocardial structure and function: The CARDIA study. J. Am. Heart Assoc. 2020, 9, e014279. [Google Scholar] [CrossRef]
- Gorczyca-Głowacka, I.; Wełnicki, M.; Mamcarz, A.; Filipiak, K.J.; Wożakowska-Kapłon, B.; Barylski, M.; Szymański, F.M.; Kasprzak, J.D.; Tomasiewcz, K. Metabolic associated fatty liver disease and cardiovascular risk: The expert opinion of the Working Group on Cardiovascular Pharmacotherapy of the Polish Cardiac Society. Kardiol. Pol. 2023, 81, 207–214. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Cerini, F.; Fagiani, V.; Nicolosi, G.L.; Rumi, M.G.; Lombardo, M.; Muti, P. Effect of metabolic dysfunction-associated steatotic liver disease (MASLD) on left ventricular mechanics in patients without overt cardiac disease: A systematic review and meta-analysis. J. Clin. Med. 2025, 14, 2690. [Google Scholar] [CrossRef]
| Variable | n (%) | Mean ± SD, Median (IQR) |
|---|---|---|
| Sex, female | 113 (76.4) | |
| Age [years] | 80.72 ± 7.80 | |
| Weight [kg] | 67.36 ± 12.84 | |
| Height [cm] | 161.39 ± 8.71 | |
| BMI [kg/m2] | 25.19 (23.05; 28.09) | |
| Comorbidities: | ||
| type 2 diabetes | 41 (27.7) | |
| hypertension | 110 (74.3) | |
| chronic kidney disease | 13 (8.8) | |
| ischemic heart disease | 41 (27.7) | |
| heart failure | 35 (23.6) | |
| atherosclerosis | 68 (45.9) | |
| hypercholesterolemia | 51 (34.5) | |
| hypertriglyceridemia | 60 (40.5) | |
| MAFLD | - | |
| Laboratory outcomes: | ||
| RBCs [×10⁶/μL] | 4.21 ± 0.45 | |
| Hb [g/dL] | 12.66 ± 1.47 | |
| HCT [%] | 38.60 ± 4.23 | |
| WBCs [×103/μL] | 6.70 (5.06; 8.01) | |
| PLTs [×103/μL] | 216.00 (167.75; 258.50) | |
| glucose [mg/dL] | 94.00 (85.00; 111.25) | |
| ALT [IU/L] | 17.00 (12.00; 20.50) | |
| AST [IU/L] | 19.00 (16.00; 22.00) | |
| cholesterol [mg/dL]: | ||
| total cholesterol | 178.16 ± 50.37 | |
| LDL | 98.00 (72.00; 127.00) | |
| HDL | 56.09 ± 15.93 | |
| triglycerides | 92.00 (70.00; 120.00) | |
| creatinine [mg/dL] | 0.81 (0.73; 1.00) | |
| uric acid [mg/dL] | 5.10 (4.25; 6.35) | |
| vitamin D3 [IU] | 31.60 (22.05; 43.00) | |
| HbA1C [%] | - | |
| IVSd [cm] | 1.14 ± 0.19 | |
| LVPWd [cm] | 1.00 (0.90; 1.00) | |
| (IVSd + LVPWd)/2 [cm] | 1.06 ± 0.16 |
| Variable | Participants (n = 237) | |
|---|---|---|
| Males (n = 79, 33.3%) | Females (n = 158, 66.7%) | |
| Age [years]: | ||
| M ± SD | 77.2 ± 7.1 | 78.4 ± 7.7 |
| Me (IQR) | 75.0 (72.0; 83.0) | 77.5 (72.0; 84.0) |
| BMI [kg/m2]: | ||
| M ± SD | 30.5 ± 5.0 | 31.9 ± 5.6 |
| Me (IQR) | 30.3 (27.3; 33.3) | 32.0 (27.7; 35.8) |
| Variable | Participants (n = 237) | M ± SD | Me (IQR) | |
|---|---|---|---|---|
| n 1 | n (%) | |||
| Sex | 237 | |||
| female | 158 (66.7) | |||
| male | 79 (33.3) | |||
| Age [years] | 237 | 77.96 ± 7.48 | ||
| Weight [kg] | 215 | 84.89 ± 16.49 | ||
| Height [cm] | 204 | 163.75 ± 8.59 | ||
| BMI [kg/m2]: | 204 | 31.46 ± 5.47 | ||
| underweight (<18.49) | 2 | |||
| normal weight (18.5–24.99) | 19 | |||
| overweight (25.0–29.99) | 58 | |||
| obesity (≥30.0) | 125 | |||
| Comorbidities: | 237 | |||
| type 2 diabetes | 118 (49.8) | |||
| hypertension | 186 (78.5) | |||
| chronic kidney disease | 28 (11.8) | |||
| ischemic heart disease | 46 (19.4) | |||
| heart failure | 30 (12.7) | |||
| atherosclerosis | 112 (47.3) | |||
| hypercholesterolemia | 76 (32.1) | |||
| hypertriglyceridemia | 139 (58.6) | |||
| MAFLD | 230 (97.0) | |||
| Laboratory outcomes: | ||||
| RBCs [×10⁶/μL] | 233 | 4.42 (4.13; 4.72) | ||
| Hb [g/dL] | 233 | 13.34 ± 1.46 | ||
| HCT [%] | 233 | 40.50 (37.80; 43.50) | ||
| WBCs [×103/μL] | 233 | 7.32 (6.08; 8.49) | ||
| PLTs [×103/μL] | 233 | 227.00 (192.00; 269.00) | ||
| glucose [mg/dL] | 209 | 109.00 (92.00; 135.00) | ||
| ALT [IU/L] | 233 | 21.00 (16.00; 28.00) | ||
| AST [IU/L] | 233 | 21.00 (17.00; 26.00) | ||
| cholesterol [mg/dL]: | ||||
| total | 229 | 179.00 (151.00; 210.00) | ||
| LDL | 224 | 104.00 (75.75; 129.00) | ||
| HDL | 229 | 46.00 (39.00; 54.00) | ||
| triglycerides [mg/dL] | 228 | 141.00 (106.00; 189.00) | ||
| creatinine [mg/dL] | 232 | 0.84 (0.73; 1.04) | ||
| uric acid [mg/dL] | 218 | 5.90 (5.10; 6.90) | ||
| vitamin D3 [IU] | 221 | 25.90 ± 14.02 | ||
| HbA1C [%] | 122 | 6.80 (6.03; 7.70) | ||
| IVSd [cm] | 173 | 1.22 ± 0.18 | ||
| LVPWd [cm] | 172 | 1.00 (1.00; 1.10) | ||
| (IVSd + LVPWd)/2 [cm] | 172 | 1.10 (1.00; 1.25) | ||
| FIB-4 [pts] | 233 | 1.51 (1.21; 1.98) | ||
| Variable | Patients with Fatty Liver | Patients Without Fatty Liver | MD (95% CI) | p |
|---|---|---|---|---|
| Sex, female | 158 (66.7) | 113 (76.4) | - | 0.056 3 |
| Age [years] | 77.96 ± 7.48 | 80.72 ± 7.80 | −2.77 (−4.33; −1.20) | 0.001 1 |
| Weight [kg] | 84.89 ± 16.49 | 67.36 ± 12.84 | 17.52 (14.49; 20.56) | <0.001 2 |
| Height [cm] | 163.75 ± 8.59 | 161.39 ± 8.71 | 2.35 (0.52; 4.19) | 0.012 1 |
| BMI [kg/m2] | 31.50 (27.67; 34.96) | 25.19 (23.05; 28.09) | 6.30 (4.69; 6.81) | <0.001 |
| Comorbidities: | ||||
| type 2 diabetes | 118 (49.8) | 41 (27.7) | - | <0.001 3 |
| hypertension | 186 (78.5) | 110 (74.3) | - | 0.414 3 |
| chronic kidney disease | 28 (11.8) | 13 (8.8) | - | 0.443 3 |
| ischemic heart disease | 46 (19.4) | 41 (27.7) | - | 0.077 3 |
| heart failure | 30 (12.7) | 35 (23.6) | - | 0.008 3 |
| atherosclerosis | 112 (47.3) | 68 (45.9) | - | 0.884 3 |
| hypercholesterolemia | 76 (32.1) | 51 (34.5) | - | 0.708 3 |
| hypertriglyceridemia | 139 (58.6) | 60 (40.5) | - | 0.001 3 |
| MAFLD | 230 (97.0) | - | - | - |
| Laboratory outcomes: | ||||
| RBCs [×10⁶/μL] | 4.42 (4.13; 4.72) | 4.21 ± 0.45 | 0.12 (0.10; 0.30) | <0.001 |
| Hb [g/dL] | 13.34 ± 1.46 | 12.66 ± 1.47 | 0.68 (0.38; 0.98) | <0.001 1 |
| HCT [%] | 40.50 (37.80; 43.50) | 38.65 (36.00; 41.42) | 1.85 (1.20; 2.90) | <0.001 |
| WBCs [×103/μL] | 7.32 (6.08; 8.49) | 6.70 (5.06; 8.01) | 0.62 (0.31; 1.10) | 0.001 |
| PLTs [×103/μL] | 227.00 (192.00; 269.00) | 216.00 (167.75; 258.50) | 11.00 (4.00; 30.00) | 0.008 |
| glucose [mg/dL] | 109.00 (92.00; 135.00) | 94.00 (85.00; 111.25) | 15.00 (8.00; 18.00) | <0.001 |
| ALT [IU/L] | 21.00 (16.00; 28.00) | 17.00 (12.00; 20.50) | 4.00 (4.00; 7.00) | <0.001 |
| AST [IU/L] | 21.00 (17.00; 26.00) | 19.00 (16.00; 22.00) | 2.00 (0.00; 3.00) | 0.013 |
| cholesterol [mg/dL]: | ||||
| total cholesterol | 179.00 (151.00; 210.00) | 173.50 (141.00; 206.00) | 5.50 (−3.00; 16.00) | 0.172 |
| LDL | 104.00 (75.75; 129.00) | 98.00 (72.00; 127.00) | 6.00 (−4.00; 13.00) | 0.331 |
| HDL | 46.00 (39.00; 54.00) | 54.00 (46.00; 64.00) | −8.00 (−11.00; −5.00) | <0.001 |
| triglycerides | 141.00 (106.00; 189.00) | 92.00 (70.00; 120.00) | 49.00 (37.00; 59.00) | <0.001 |
| creatinine [mg/dL] | 0.84 (0.73; 1.04) | 0.81 (0.73; 1.00) | 0.03 (−0.03; 0.06) | 0.550 |
| uric acid [mg/dL] | 5.90 (5.10; 6.90) | 5.10 (4.25; 6.35) | 0.80 (0.40; 1.00) | <0.001 |
| vitamin D3 [IU] (1) | 24.70 (15.20; 34.40) | 31.60 (22.05; 43.00) | −6.90 (−10.30; −3.90) | <0.001 |
| vitamin D3 [IU] (2) | 24.40 (14.40; 34.00) | 31.60 (22.05; 43.00) | −7.20 (−10.80; −4.30) | <0.001 |
| HbA1C [%] | 6.80 (6.03; 7.70) | - | - | - |
| IVSd [cm] | 1.22 ± 0.18 | 1.14 ± 0.19 | 0.08 (0.04; 0.12) | <0.001 1 |
| (IVSd + LVPWd)/2 [cm] * | 1.09 ± 0.62 | 0.98 ± 0.15 | 0.06 (0.03; 0.09) | <0.001 1 |
| (IVSd + LVPWd)/2 [cm] * | 1.15 ± 0.33 | 1.06 ± 0.16 | 0.07 (0.04; 0.10) | <0.001 1 |
| Variable | Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Sex, female | 1.61 | 1.02–2.59 | 0.044 | - | - | - |
| Age [years] | 0.95 | 0.93–0.98 | <0.001 | 0.95 | 0.91–0.99 | 0.015 |
| Weight [kg] | 1.08 | 1.06–1.11 | <0.001 | - | - | - |
| Height [cm] | 1.03 | 1.01–1.06 | 0.013 | - | - | - |
| BMI [kg/m2] | 1.25 | 1.18–1.32 | <0.001 | 1.21 | 1.13–1.30 | <0.001 |
| Comorbidities: | ||||||
| type 2 diabetes | 2.59 | 1.67–4.05 | <0.001 | 2.15 | 1.14–4.12 | 0.019 |
| hypertension | 1.26 | 0.78–2.04 | 0.347 | - | - | - |
| chronic kidney disease | 1.39 | 0.71–2.86 | 0.350 | - | - | - |
| ischemic heart disease | 0.63 | 0.39–1.02 | 0.059 | - | - | - |
| heart failure | 0.47 | 0.27–0.80 | 0.006 | 0.41 | 0.18–0.93 | 0.036 |
| atherosclerosis | 1.05 | 0.70–1.59 | 0.802 | - | - | - |
| hypercholesterolemia | 0.90 | 0.58–1.39 | 0.627 | - | - | - |
| hypertriglyceridemia | 2.08 | 1.37–3.17 | <0.001 | - | - | - |
| Laboratory outcomes: | ||||||
| RBCs [×10⁶/μL] | 2.45 | 1.59–3.86 | <0.001 | - | - | - |
| Hb [g/dL] | 1.37 | 1.18–1.59 | <0.001 | - | - | - |
| HCT [%] | 1.09 | 1.04–1.15 | <0.001 | - | - | - |
| WBCs [×103/μL] | 1.21 | 1.08–1.36 | 0.001 | - | - | - |
| PLTs [×103/μL] | 1.00 | 1.00–1.01 | 0.035 | - | - | - |
| glucose [mg/dL] | 1.01 | 1.00–1.02 | 0.001 | - | - | - |
| ALT [IU/L] | 1.08 | 1.05–1.11 | <0.001 | 1.03 | 1.00–1.07 | 0.109 |
| AST [IU/L] | 1.03 | 1.00–1.06 | 0.047 | - | - | - |
| cholesterol [mg/dL]: | ||||||
| total cholesterol | 1.00 | 1.00–1.01 | 0.302 | - | - | - |
| LDL | 1.00 | 1.00–1.01 | 0.546 | - | - | - |
| HDL | 0.96 | 0.94–0.97 | <0.001 | - | - | - |
| triglycerides | 1.02 | 1.01–1.03 | <0.001 | 1.01 | 1.00–1.02 | 0.001 |
| creatinine [mg/dL] | 0.78 | 0.47–1.14 | 0.235 | - | - | - |
| uric acid [mg/dL] | 1.39 | 1.19–1.64 | <0.001 | 1.20 | 0.96–1.50 | 0.106 |
| vitamin D3 [IU] (1) | 0.97 | 0.95–0.98 | <0.001 | - | - | - |
| vitamin D3 [IU] (2) | 0.97 | 0.95–0.98 | <0.001 | 0.96 | 0.94–0.98 | <0.001 |
| FIB-4 | 0.63 | 0.48–0.81 | <0.001 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymala-Pędzik, M.; Piersiak, M.; Pachana, M.; Lindner-Pawłowicz, K.; Szczepaniak, W.; Sobieszczańska, M. MAFLD as a Cardiovascular Risk Factor: An Extended Retrospective Study with a Control Group. J. Clin. Med. 2025, 14, 4181. https://doi.org/10.3390/jcm14124181
Szymala-Pędzik M, Piersiak M, Pachana M, Lindner-Pawłowicz K, Szczepaniak W, Sobieszczańska M. MAFLD as a Cardiovascular Risk Factor: An Extended Retrospective Study with a Control Group. Journal of Clinical Medicine. 2025; 14(12):4181. https://doi.org/10.3390/jcm14124181
Chicago/Turabian StyleSzymala-Pędzik, Małgorzata, Marcin Piersiak, Maciej Pachana, Karolina Lindner-Pawłowicz, Wioletta Szczepaniak, and Małgorzata Sobieszczańska. 2025. "MAFLD as a Cardiovascular Risk Factor: An Extended Retrospective Study with a Control Group" Journal of Clinical Medicine 14, no. 12: 4181. https://doi.org/10.3390/jcm14124181
APA StyleSzymala-Pędzik, M., Piersiak, M., Pachana, M., Lindner-Pawłowicz, K., Szczepaniak, W., & Sobieszczańska, M. (2025). MAFLD as a Cardiovascular Risk Factor: An Extended Retrospective Study with a Control Group. Journal of Clinical Medicine, 14(12), 4181. https://doi.org/10.3390/jcm14124181








