Inclisiran: Efficacy in Real World—Systematic Review and Meta-Analysis
Abstract
1. Introduction
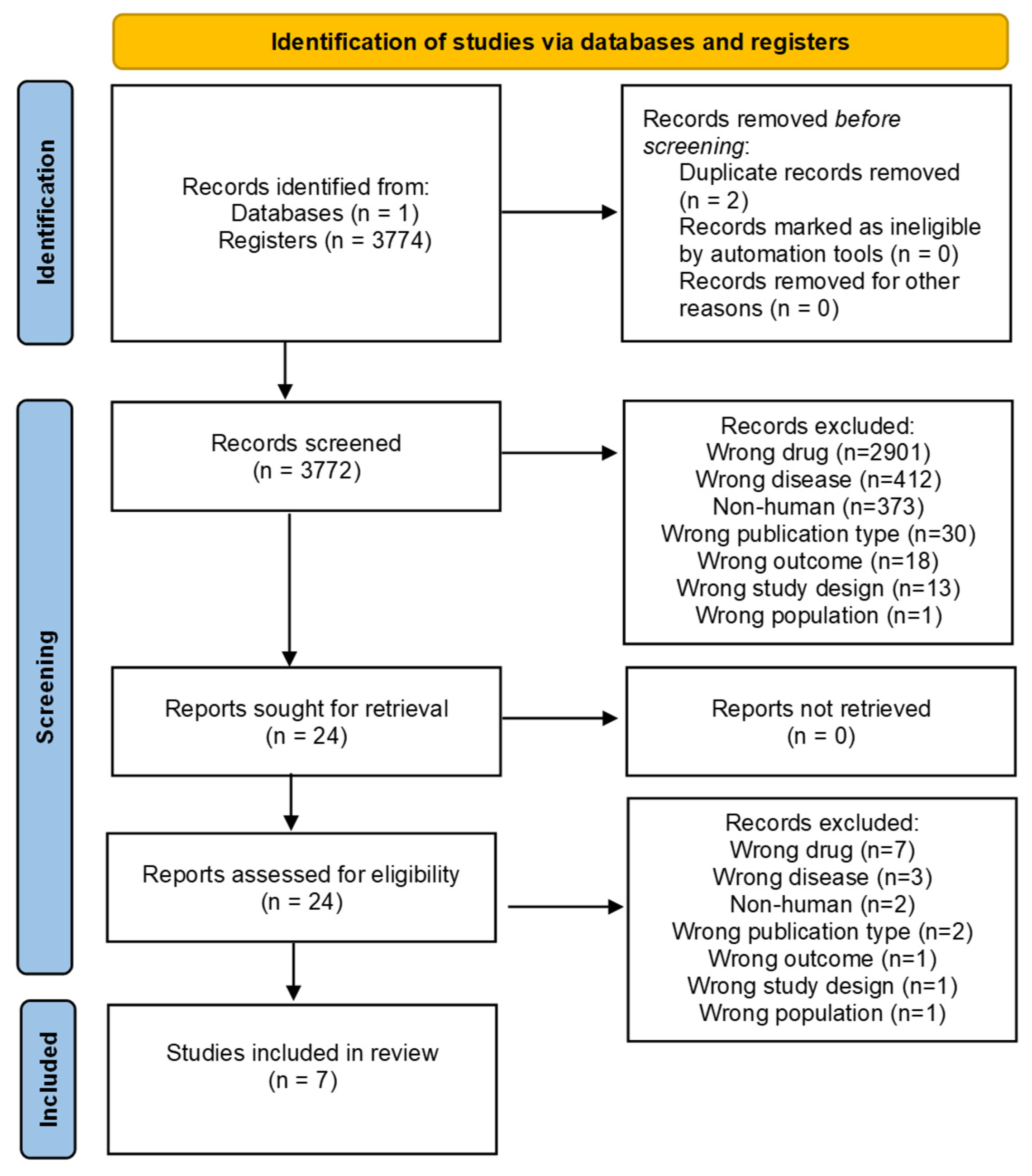
2. Materials and Methods
(“Treatment Outcome”[MeSH] OR “Evidence-Based Medicine”[MeSH] OR “Retrospective Studies”[MeSH] OR “Time Factors”[MeSH] OR “real world” OR “real-world” OR “RWD” OR “RWE” OR “real life” OR “real patient” OR “real practice” OR “real clinical” OR “real population” OR “actual world” OR “actual life” OR “actual patient” OR “actual practice” OR “actual clinical” OR “actual population”)
AND
((ALN PCSsc[Title/Abstract]) OR (ALN 60212[Title/Abstract]) OR (PCSK9si KJX 839[Title/Abstract]) OR (Inclisiran[Title/Abstract]) OR (small interfering RNA[Title/Abstract]) OR (RNAi[Title/Abstract]) OR (SiRNA[Title/Abstract]) OR (RNA, Small Interfering[Title/Abstract])).
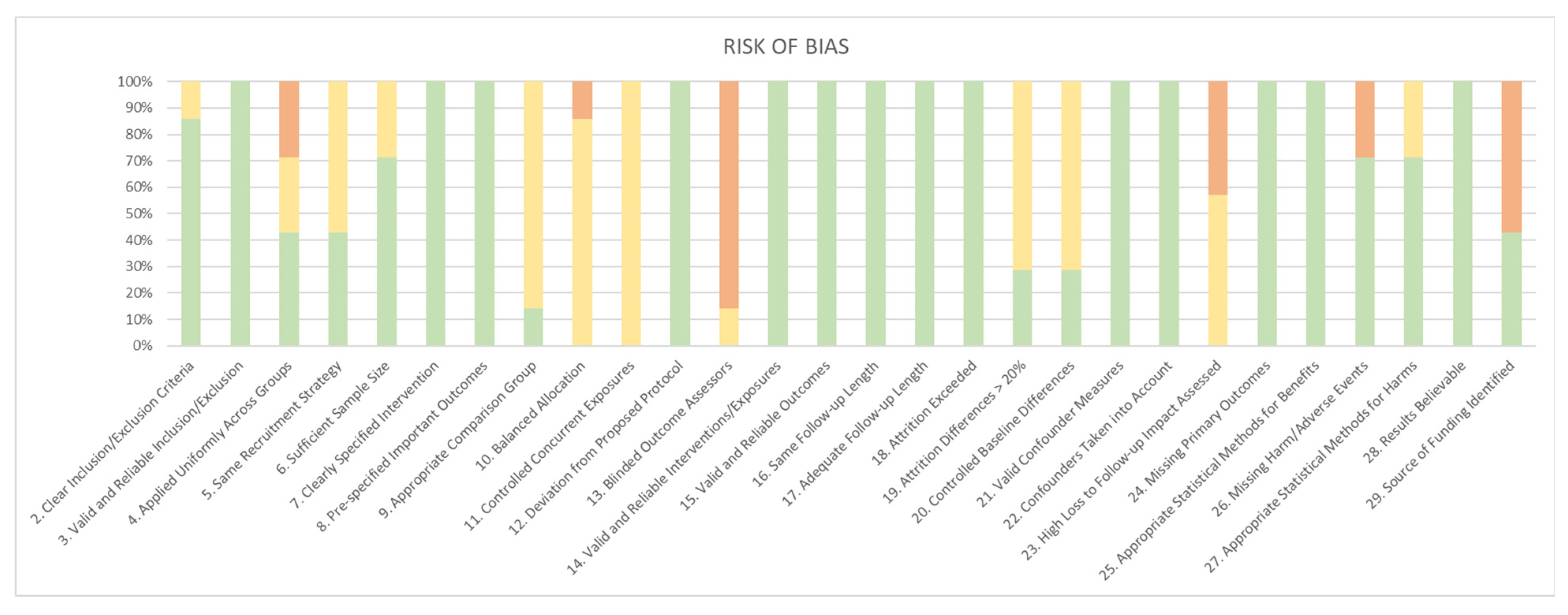
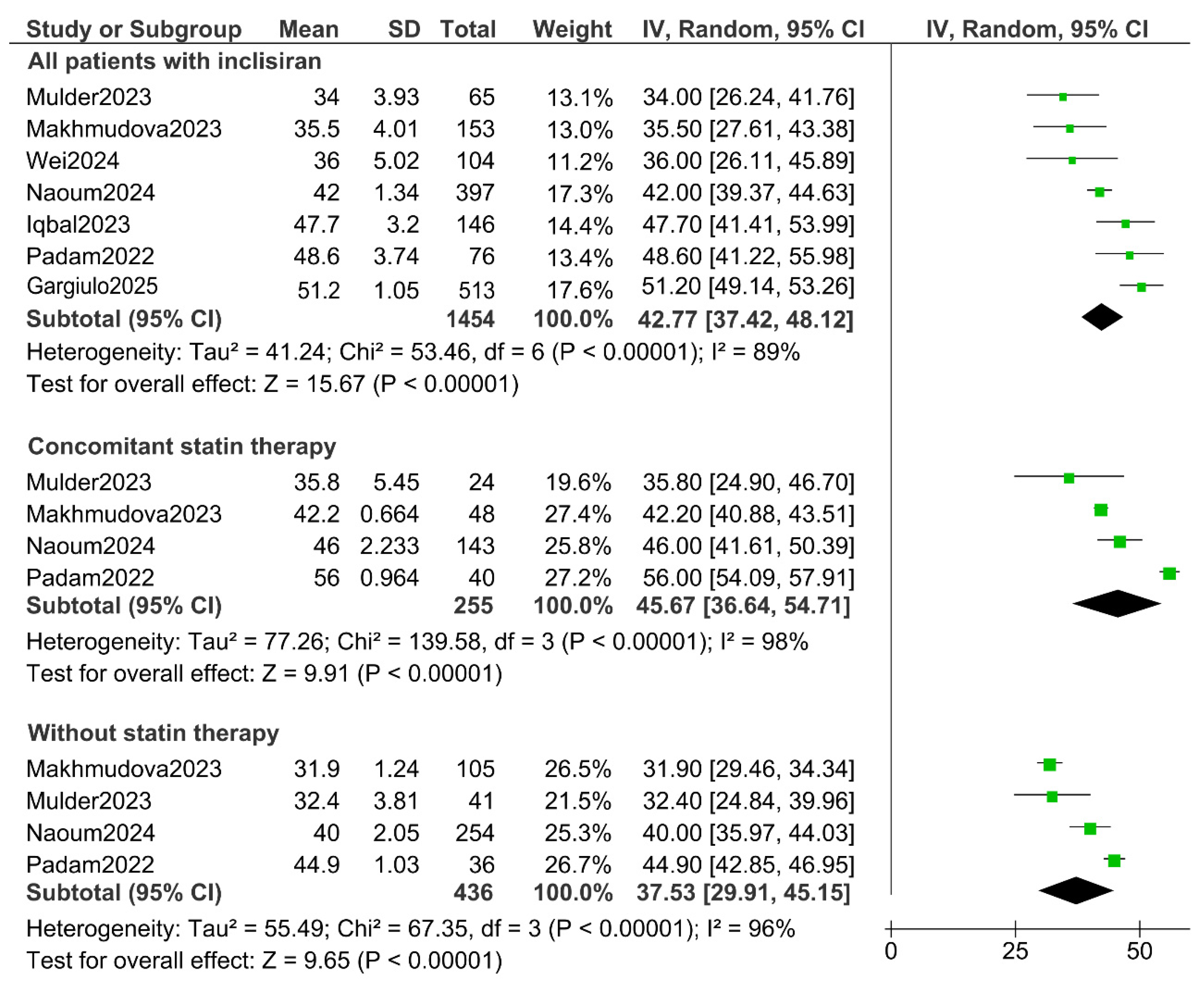
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Heart Report 2023 Confronting the World’s Number One Killer. Available online: https://world-heart-federation.org/wp-content/uploads/World-Heart-Report-2023.pdf (accessed on 18 December 2024).
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [PubMed]
- Ference, B.A.; Graham, I.; Tokgozoglu, L.; Catapano, A.L. Impact of Lipids on Cardiovascular Health: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1141–1156. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.J.; Lepor, N.E.; Michos, E.D. Evolving Management of Low-Density Lipoprotein Cholesterol: A Personalized Approach to Preventing Atherosclerotic Cardiovascular Disease Across the Risk Continuum. J. Am. Heart Assoc. 2023, 12, e028892. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Troquay, R.P.T.; Visseren, F.L.J.; Leiter, L.A.; Wright, R.S.; Vikarunnessa, S.; Talloczy, Z.; Zang, X.; Maheux, P.; Lesogor, A.; et al. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): Results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol. 2023, 11, 109–119. [Google Scholar] [CrossRef]
- Ray, K.K.; Raal, F.J.; Kallend, D.G.; Jaros, M.J.; Koenig, W.; Leiter, L.A.; Landmesser, U.; Schwartz, G.G.; Lawrence, D.; Friedman, A.; et al. Inclisiran and cardiovascular events: A patient-level analysis of phase III trials. Eur. Heart J. 2023, 44, 129–138. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef]
- Ray, K.K.; Kallend, D.; Leiter, L.A.; Raal, F.J.; Koenig, W.; Jaros, M.J.; Schwartz, G.G.; Landmesser, U.; Conde, L.G.; Wright, R.S. Effect of inclisiran on lipids in primary prevention: The ORION-11 trial. Eur. Heart J. 2022, 43, 5047–5057. [Google Scholar] [CrossRef]
- Rothwell, P.M. External validity of randomised controlled trials: “To whom do the results of this trial apply?”. Lancet 2005, 365, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.M.; Givens, T.K.; Tang, E.G.; Schimmer, J.J.; Ramsey, T.; Boyd, K.; Delate, T. Real-World Outcomes of Proprotein Convertase Subtilisin Kexin-9 Inhibitor Use. J. Cardiovasc. Pharmacol. 2023, 81, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Koskinas, K.C.; Siontis, G.C.M.; Piccolo, R.; Mavridis, D.; Räber, L.; Mach, F.; Windecker, S. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: A meta-analysis of randomized trials. Eur. Heart J. 2018, 39, 1172–1180. [Google Scholar] [CrossRef]
- Morton, J.I.; Marquina, C.; Lloyd, M.; Watts, G.F.; Zoungas, S.; Liew, D.; Ademi, Z. Lipid-Lowering Strategies for Primary Prevention of Coronary Heart Disease in the UK: A Cost-Effectiveness Analysis. PharmacoEconomics 2024, 42, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Luo, M.; Liu, Y.; Xu, X.; Liu, K.; Shen, C.; Hu, H.; He, Z.; Wu, F. Efficacy and safety of inclisiran in stroke or cerebrovascular disease prevention: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2023, 14, 1158274. [Google Scholar] [CrossRef]
- Shau, W.-Y.; Setia, S.; Shinde, S.P.; Santoso, H.; Furtner, D. Contemporary Databases in Real-world Studies Regarding the Diverse Health Care Systems of India, Thailand, and Taiwan: Protocol for a Scoping Review. JMIR Res. Protoc. 2022, 11, e43741. [Google Scholar] [CrossRef]
- Rayyan: AI-Powered Systematic Review Management Platform. Available online: https://www.rayyan.ai/ (accessed on 18 December 2024).
- Viswanathan, M.; Berkman, N.D. Item Bank for Assessment of Risk of Bias and Precision for Observational Studies of Interventions or Exposures. In Development of the RTI Item Bank on Risk of Bias and Precision of Observational Studies [Internet]; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK82267/ (accessed on 19 December 2024).
- RevMan: Systematic Review and Meta-Analysis Tool for Researchers Worldwide|Cochrane RevMan. Available online: https://revman.cochrane.org/info (accessed on 9 January 2025).
- Padam, P.; Barton, L.; Wilson, S.; David, A.; Walji, S.; de Lorenzo, F.; Ray, K.K.; Jones, B.; Cegla, J. Lipid lowering with inclisiran: A real-world single-centre experience. Open Heart 2022, 9, e002184. [Google Scholar] [CrossRef]
- Makhmudova, U.; Schatz, U.; Perakakis, N.; Kassner, U.; Schumann, F.; Axthelm, C.; Stürzebecher, P.; Sinning, D.L.; Doevelaar, A.; Rohn, B.; et al. High interindividual variability in LDL-cholesterol reductions after inclisiran administration in a real-world multicenter setting in Germany. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2023, 112, 1639–1649. [Google Scholar] [CrossRef]
- Mulder, J.W.C.M.; Galema-Boers, A.M.H.; van Lennep, J.E.R. First clinical experiences with inclisiran in a real-world setting. J. Clin. Lipidol. 2023, 17, 818–827. [Google Scholar] [CrossRef]
- Iqbal, S.; Sabbour, H.M.; Ashraf, T.; Santos, R.D.; Buckley, A. First Report of Inclisiran Utilization for Hypercholesterolemia Treatment in Real-world Clinical Settings in a Middle East Population. Clin. Ther. 2024, 46, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Naoum, I.; Saliba, W.; Aker, A.; Zafrir, B. Lipid-lowering therapy with inclisiran in the real-world setting: Initial data from a national health care service. J. Clin. Lipidol. 2024, 18, e809–e816. [Google Scholar] [CrossRef]
- Wei, N.M.; Tobin, R.F.; Jacoby, D.S.; Bajaj, A. Real-world experience of inclisiran prescription at the University of Pennsylvania Health Systems. J. Clin. Lipidol. 2024, 18, e1096–e1100. [Google Scholar] [CrossRef]
- Gargiulo, P.; Marzano, F.; Crisci, M.; Marcucci, R.; Bruzzese, D.; Maloberti, A.; Sarullo, F.M.; Galasso, G.; Indolfi, C.; Musumeci, G.; et al. Real-World Efficacy and Safety of Inclisiran: A Single-Country, Multicenter, Observational Study (CHOLINET Registry). J. Am. Coll. Cardiol. 2025, 85, 536–540. [Google Scholar] [CrossRef]
- Wright, R.S.; Ray, K.K.; Raal, F.J.; Kallend, D.G.; Jaros, M.; Koenig, W.; Leiter, L.A.; Landmesser, U.; Schwartz, G.G.; Friedman, A.; et al. Pooled Patient-Level Analysis of Inclisiran Trials in Patients with Familial Hypercholesterolemia or Atherosclerosis. J. Am. Coll. Cardiol. 2021, 77, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Frieden, T.R. Evidence for Health Decision Making—Beyond Randomized, Controlled Trials. N. Engl. J. Med. 2017, 377, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Burnier, M.; Egan, B.M. Adherence in Hypertension. Circ. Res. 2019, 124, 1124–1140. [Google Scholar] [CrossRef]
- Grimes, D.A.; Schulz, K.F. Bias and causal associations in observational research. Lancet 2002, 359, 248–252. [Google Scholar] [CrossRef]
- Sabaté, M.; Vidal, X.; Ballarin, E.; Rottenkolber, M.; Schmiedl, S.; Grave, B.; Huerta, C.; Martin-Merino, E.; Montero, D.; Leon-Muñoz, L.M.; et al. Adherence to Direct Oral Anticoagulants in Patients with Non-Valvular Atrial Fibrillation: A Cross-National Comparison in Six European Countries (2008–2015). Front. Pharmacol. 2021, 12, 682890. [Google Scholar] [CrossRef]
- Hernan, M.A.; Robins, J.M. Causal Inference: What If; Chapman & Hall/CRC: Boca Raton, FL, USA, 2025; 312p. [Google Scholar]
- Concato, J.; Shah, N.; Horwitz, R.I. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N. Engl. J. Med. 2000, 342, 1887–1892. [Google Scholar] [CrossRef]
- Togo, K.; Yonemoto, N. Real world data and data science in medical research: Present and future. Jpn. J. Stat. Data Sci. 2022, 5, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; AFerence, B.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
| Study | Design | Country | Population | Follow-Up |
|---|---|---|---|---|
| Padam (2022) [22] | Retrospective observational | UK | 76 | 2 months |
| Makhmudova (2023) [23] | Retrospective observational | Germany | 153 | 3 months |
| Mulder (2023) [24] | Prospective observational | Netherlands | 65 | 3 months |
| Iqbal (2024) [25] | Retrospective observational | UAE | 146 | 3 months |
| Naoum (2024) [26] | Retrospective observational | Israel | 397 | >2 months |
| Wei (2024) [27] | Retrospective observational | USA | 104 | 3–12 months |
| Gargiulo (2025) [28] | Prospective observational | Italy | 513 | 3 months |
| STUDY | MEAN AGE (SD */IQR **) | FEMALE (%) | c-LDL BASELINE (mmol/L) (SD */IQR **) | |
| Padam (2022) [22] | 64 (10.3 *) | 28 (35%) | 3.5 (1.1 *) | |
| Makhmudova (2023) [23] | 63 (55.70 **) | 66 (43.1%) | 3.6 (2.4 **) | |
| Mulder (2023) [24] | 63 (54.68 **) | 36 (55%) | 3.7 (2.5 **) | |
| Iqbal (2024) [25] | 54.8 (12.12 **) | 64 (43.8%) | 3.0 (1.8 **) | |
| Naoum (2024) [26] | 66 (11 *) | 226 (56.9%) | - | |
| Wei (2024) [27] | 67.5 (11.1 **) | 70 (67.3%) | 3.1 (1.7 *) | |
| Gargiulo (2025) [28] | 63 | 159 (31%) | 2.7 (1.8 **) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaíz, Á.R.; Gudino, L.C.; de la Isla, L.P.; Pardo, H.G.; Calle, D.G.; Miramontes-González, J.P. Inclisiran: Efficacy in Real World—Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 4163. https://doi.org/10.3390/jcm14124163
Alaíz ÁR, Gudino LC, de la Isla LP, Pardo HG, Calle DG, Miramontes-González JP. Inclisiran: Efficacy in Real World—Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(12):4163. https://doi.org/10.3390/jcm14124163
Chicago/Turabian StyleAlaíz, Álvaro Rodrigo, Luis Corral Gudino, Leopoldo Pérez de la Isla, Héctor García Pardo, David González Calle, and José Pablo Miramontes-González. 2025. "Inclisiran: Efficacy in Real World—Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 12: 4163. https://doi.org/10.3390/jcm14124163
APA StyleAlaíz, Á. R., Gudino, L. C., de la Isla, L. P., Pardo, H. G., Calle, D. G., & Miramontes-González, J. P. (2025). Inclisiran: Efficacy in Real World—Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(12), 4163. https://doi.org/10.3390/jcm14124163






