Radiological Insights into UIP Pattern: A Comparison Between IPF and Non-IPF Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Study
- •
- TDiagnosis of Interstitial lung diseases according to the international guidelines published in 2022 [7]; for secondary ILDs related to autoimmune disorders (systemic sclerosis, myositis, Sjögren, etc.), specific rheumatological guidelines were applied.
- •
- At least one HRCT examination—acquired no more than 12 months prior to the Multidisciplinary Discussion; for subjects having multiple HRCT examinations, the latest one was considered for the visual and quantitative assessment.
- •
- Presence of UIP pattern on HRCT examinations.
- •
- Patients were discharged in cases of HRCT examinations with artifacts (motion or respiratory artifacts).
2.2. HRCT Protocol
2.3. Qualitative Evaluation
2.4. Quantitative Evaluation
2.5. Statistical Analysis
3. Results
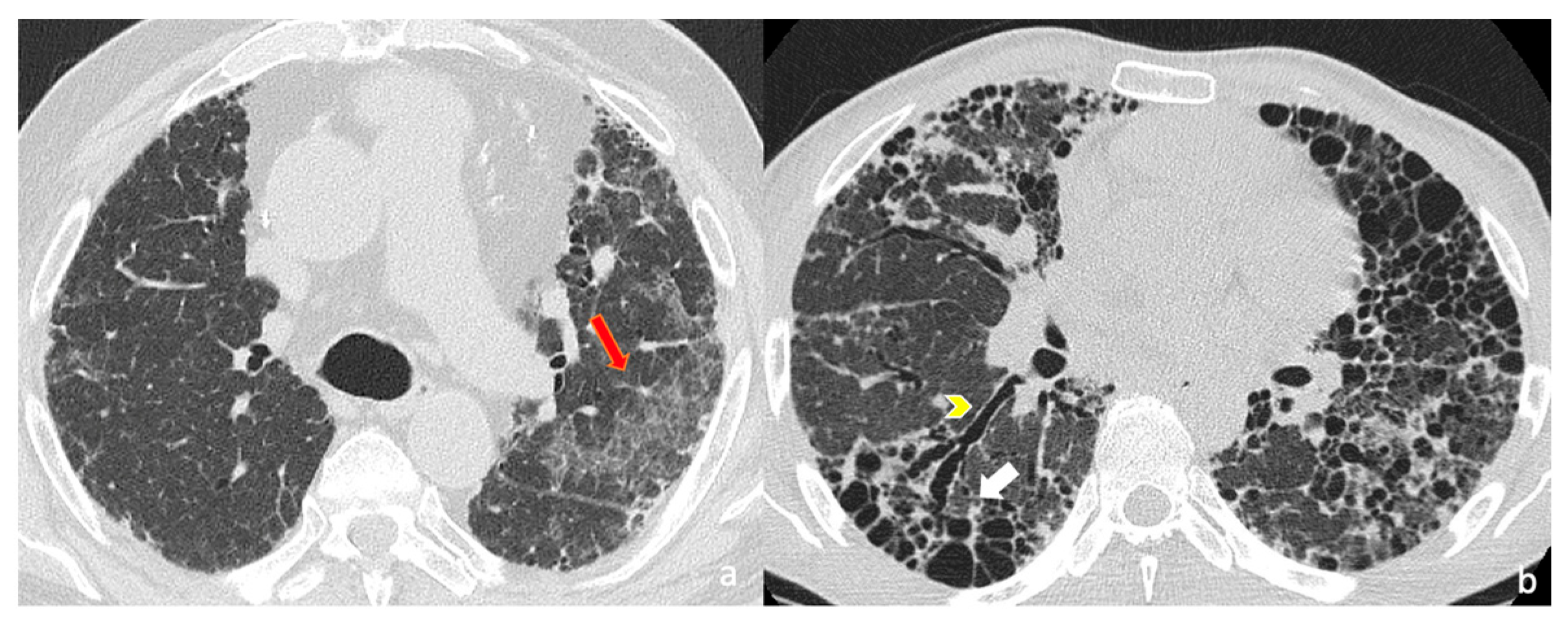
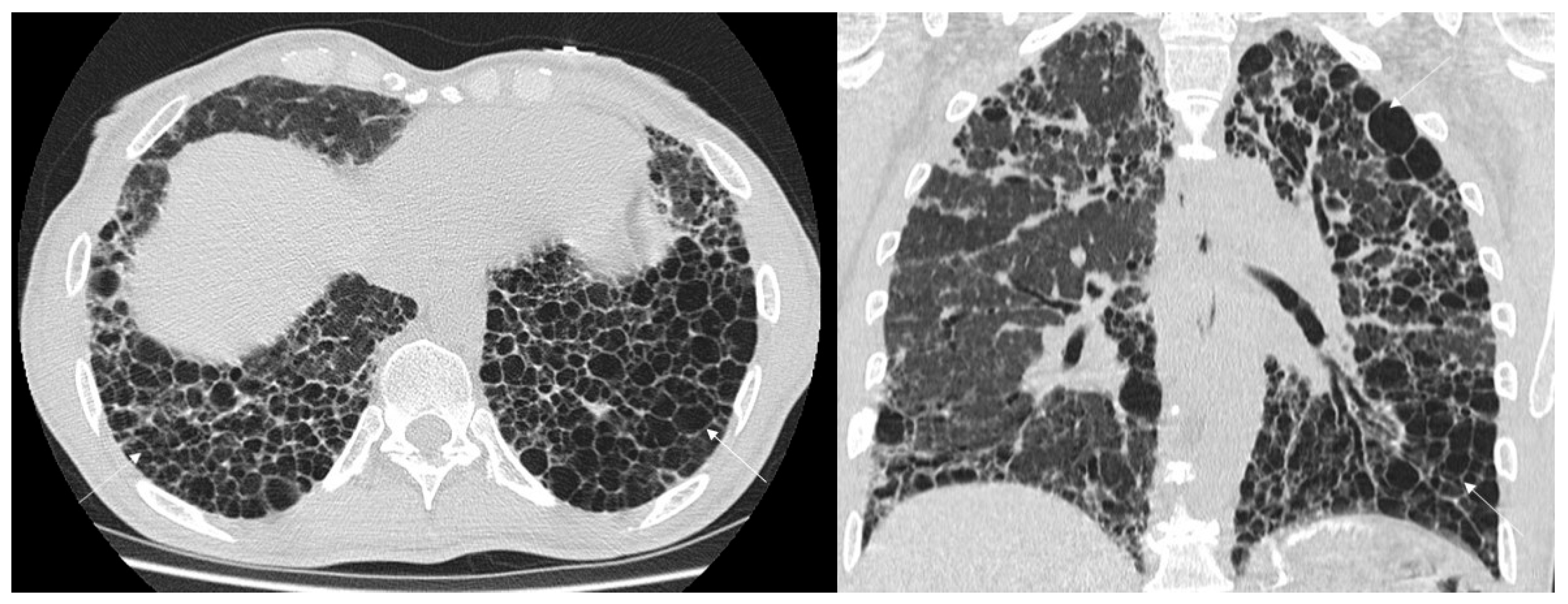
3.1. Qualitative Evaluation
3.2. Diagnostic Accuracy
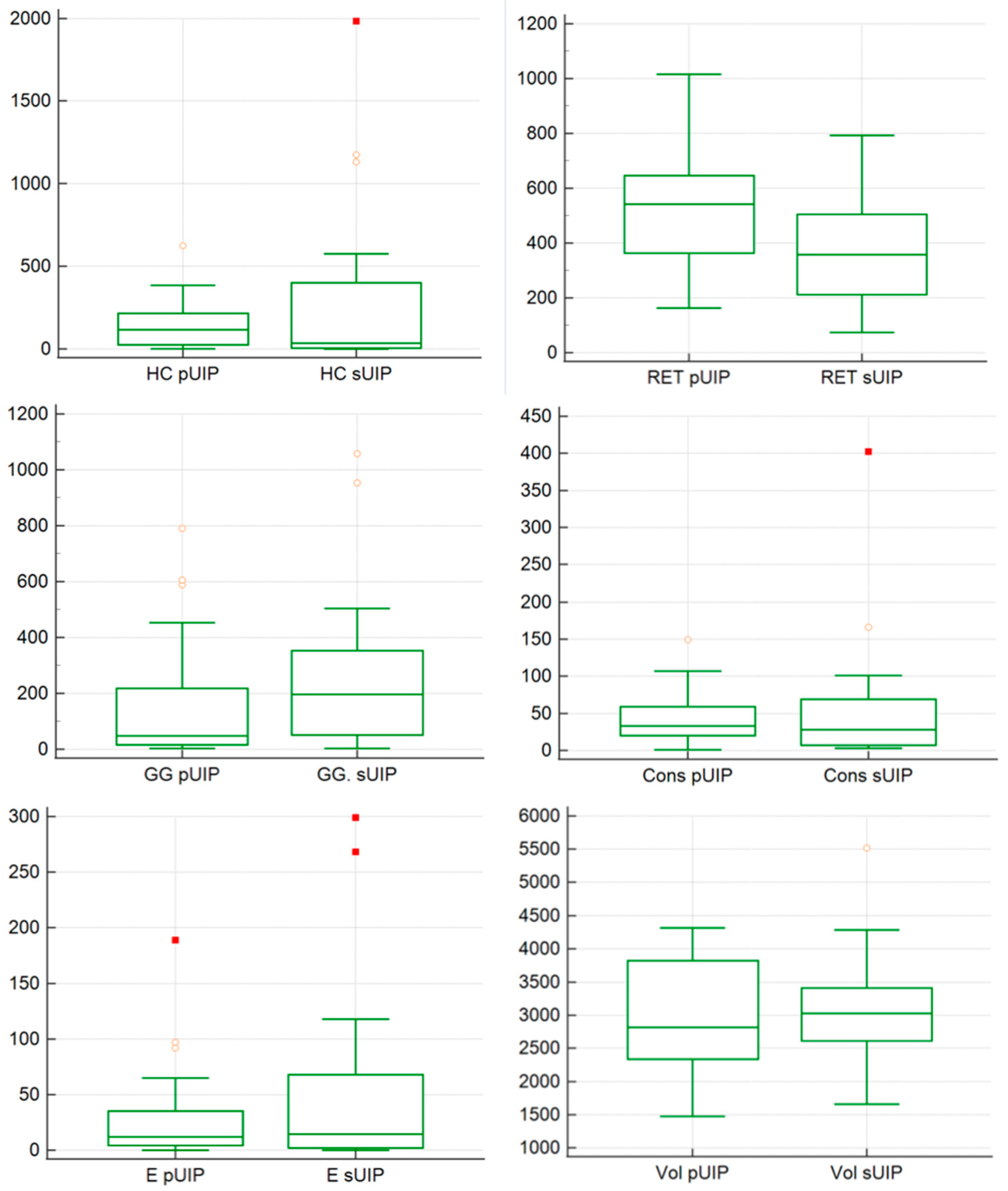
3.3. Quantitative Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UIP | Usual Interstitial Pneumonia |
| HRCT | High Resolution Computed Tomography |
| ILD | Interstitial Lung Disease |
| GGO | Ground Glass Opacity |
| CT | Computed Tomography |
| IPF | Idiopathic Pulmonary Fibrosis |
| HU | Hounsfield Unit |
| CI | Confidence Interval |
| CTD | Connective Tissue Disease |
| IPAF | Interstitial Pneumonia with Autoimmune Feature |
| NSIP | Non Specific Interstitial Pneumonia |
| CHP | Chronic Hypersensitivity Pneumonia |
| fHP | Fibrotic Hypersensitivity Pneumonia |
| AI | Artificial Intelligence |
References
- Yi, E.S.; Wawryko, P.; Ryu, J.H. Diagnosis of interstitial lung diseases: From Averill A. Liebow to artificial intelligence. J. Pathol. Transl. Med. 2024, 58, 1–11. [Google Scholar] [CrossRef]
- Staples, C.A.; Müller, N.L.; Vedal, S.; Abboud, R.; Ostrow, D.; Miller, R.R. Usual interstitial pneumonia: Correlation of CT with clinical, functional, and radiologic findings. Radiology 1987, 162, 377–381. [Google Scholar] [CrossRef]
- Lynch, D.A.; Travis, W.D.; Müller, N.L.; Galvin, J.R.; Hansell, D.M.; Grenier, P.A.; King, T.E.J. Idiopathic Interstitial Pneumonias: CT Features. Radiology 2005, 236, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis (2011). An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Lynch, D.A.; Sverzellati, N.; Travis, W.D.; Brown, K.K.; Colby, T.V.; Galvin, J.R.; Goldin, J.G.; Hansell, D.M.; Inoue, Y.; Johkoh, T.; et al. Diagnostic criteria for idiopathic pulmonary fibrosis: A Fleischner Society White Paper. Lancet Respir. Med. 2018, 6, 138–153. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, W.A.; Cavazza, A.; Rossi, G.; Bonella, F.; Sverzellati, N.; Spagnolo, P. Differential Diagnosis of Usual Interstitial Pneumonia: When Is It Truly Idiopathic? Eur. Respir. Rev. 2014, 23, 308–319. [Google Scholar] [CrossRef]
- Kim, E.A.; Lee, K.S.; Johkoh, T.; Kim, T.S.; Suh, G.Y.; Kwon, O.J.; Han, J. Interstitial Lung Diseases Associated with Collagen Vascular Diseases: Radiologic and Histopathologic Findings. Radiographics 2002, 22, S151–S165. [Google Scholar] [CrossRef]
- Brady, D.; Berkowitz, E.A.; Sharma, A.; Ackman, J.B.; Bernheim, A.; Chung, M.; Veeraraghavan, S.; Little, B.P. CT Morphologic Characteristics and Variant Patterns of Interstitial Pulmonary Fibrosis in Systemic Lupus Erythematosus. Radiol. Cardiothorac. Imaging 2021, 3, e200625. [Google Scholar] [CrossRef]
- Chung, J.H.; Cox, C.W.; Montner, S.M.; Adegunsoye, A.; Oldham, J.M.; Husain, A.N.; Vij, R.; Noth, I.; Lynch, D.A.; Strek, M.E. CT Features of the Usual Interstitial Pneumonia Pattern: Differentiating Connective Tissue Disease-Associated Interstitial Lung Disease From Idiopathic Pulmonary Fibrosis. AJR Am. J. Roentgenol. 2018, 210, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Luppi, F.; Manfredi, A.; Faverio, P.; Andersen, M.B.; Bono, F.; Pagni, F.; Salvarani, C.; Bendstrup, E.; Sebastiani, M. The Usual Interstitial Pneumonia Pattern in Autoimmune Rheumatic Diseases. BMC Pulm. Med. 2023, 23, 501. [Google Scholar] [CrossRef] [PubMed]
- Walkoff, L.; White, D.B.; Chung, J.H.; Asante, D.; Cox, C.W. The Four Corners Sign: A Specific Imaging Feature in Differentiating Systemic Sclerosis-Related Interstitial Lung Disease From Idiopathic Pulmonary Fibrosis. J. Thorac. Imaging 2018, 33, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.F.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef]
- Wells, A.U.; Flaherty, K.R.; Brown, K.K.; Inoue, Y.; Devaraj, A.; Richeldi, L.; Moua, T.; Crestani, B.; Wuyts, W.A.; Stowasser, S.; et al. Nintedanib in Patients with Progressive Fibrosing Interstitial Lung Diseases-Subgroup Analyses by Interstitial Lung Disease Diagnosis in the INBUILD Trial: A Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Trial. Lancet Respir. Med. 2020, 8, 453–460. [Google Scholar] [CrossRef]
- Viswanathan, V.K.; Ghoshal, A.G.; Mohan, A.; Patil, K.; Bhargave, C.; Choudhari, S.; Mehta, S. Patient Profile-Based Management with Nintedanib in Patients with Idiopathic Pulmonary Fibrosis. Pulm. Ther. 2024, 10, 377–409. [Google Scholar] [CrossRef]
- Edey, J.; Devaraj, A.; Barker, R.; Wells, A.; Hansell, D. Fibrotic idiopathic interstitial pneumonias: HRCT findings that predict mortality. Eur. Radiol. 2011, 21, 1586–1593. [Google Scholar] [CrossRef]
- Walsh, S.; Sverzellati, N.; Devaraj, A.; Keir, G.; Wells, A.; Hansell, D. Connective tissue disease related fibrotic lung disease: High resolution computed tomographic and pulmonary function indices as prognostic determinants. Thorax 2014, 69, 216–222. [Google Scholar] [CrossRef]
- Walsh, S.; Sverzellati, N.; Devaraj, A.; Wells, A.; Hansell, D. Chronic hypersensitivity pneumonitis: High resolution computed tomography patterns and pulmonary function indices as prognostic determinants. Eur. Radiol. 2012, 22, 1672–1679. [Google Scholar] [CrossRef]
- Baratella, E.; Borghesi, A.; Calandriello, L.; Cortese, G.; Della Casa, G.; Giraudo, C.; Grassedonio, E.; Larici, A.R.; Palmucci, S.; Romei, C.; et al. Quantification of progressive pulmonary fibrosis by visual scoring of HRCT images: Recommendations from Italian chest radiology experts. Radiol. Med. 2025. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.U.; Brown, K.K.; Flaherty, K.R.; Kolb, M.; Thannickal, V.J.; IPF Consensus Working Group. What’s in a name? That which we call IPF, by any other name would act the same. Eur. Respir. J. 2018, 51, 1800692. [Google Scholar] [CrossRef] [PubMed]
- Gaxiola, M.; Buendía-Roldán, I.; Mejía, M.; Carrillo, G.; Estrada, A.; Navarro, M.C.; Rojas-Serrano, J.; Selman, M. Morphologic diversity of chronic pigeon breeder’s disease: Clinical features and survival. Respir. Med. 2011, 105, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Suda, T.; Kaida, Y.; Kono, M.; Hozumi, H.; Hashimoto, D.; Enomoto, N.; Fujisawa, T.; Inui, N.; Imokawa, S.; et al. Rheumatoid lung disease: Prognostic analysis of 54 biopsy-proven cases. Respir. Med. 2012, 106, 1164–1169. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, D.S.; Park, I.N.; Jang, S.J.; Kitaichi, M.; Nicholson, A.G.; Colby, T.V. Prognosis of fibrotic interstitial pneumonia: Idiopathic versus collagen vascular disease-related subtypes. Am. J. Respir. Crit. Care Med. 2007, 175, 705–711. [Google Scholar] [CrossRef]
- Mei, X.; Liu, Z.; Singh, A.; Lange, M.; Boddu, P.; Gong, J.Q.X.; Lee, J.; DeMarco, C.; Cao, C.; Platt, S.; et al. Interstitial lung disease diagnosis and prognosis using an AI system integrating longitudinal data. Nat. Commun. 2023, 14, 2272. [Google Scholar] [CrossRef]
- Hoffmann, T.; Teichgräber, U.; Lassen-Schmidt, B.; Renz, D.; Brüheim, L.B.; Krämer, M.; Oelzner, P.; Böttcher, J.; Güttler, F.; Wolf, G.; et al. Artificial intelligence-based quantification of pul-monary HRCT (AIqpHRCT) for the evaluation of interstitial lung disease in patients with inflammatory rheumatic diseases. Rheumatol. Int. 2024, 44, 2483–2496. [Google Scholar] [CrossRef]


| pUIPs (n = 31) | sUIPs (n = 22) | |
|---|---|---|
| Age | 73.3 years (range 51–88) | 74.3 years (range 40–86) |
| Gender | 64.5% (20/31) males; 35.5% (11/31) females | 50% (11/22) males; 50% (11/22) females |
| Antibody Positivity | 19.4% (6/31) | 72.3% (16/22) |
| Non Smokers | 38.7% (12/31) | 36.3% (8/22) |
| Current or Former Smokers | 61.2% (19/31) | 63.6% (14/22) |
| Pack-Years (Mean) | 15.1 | 14.6 |
| HRCT Findings | p-UIP | s-UIP |
|---|---|---|
| Cranio-caudal fibrosis distribution | 28/31 (90.3%) | 17/22 (77.3%) |
| Subpleural/peripheral distribution | 29/31 (93.5%) | 19/22 (86.4%) |
| Honeycombing - macro-HC - micro-HC | 27/31 (87.1%) | 19/22 (86.4%) |
| 15/31 (33%) | 11/22 (50%) | |
| 19/31 (40%) | 6/22 (27.3%) | |
| Exhuberant Honeycoming | 1/31 (3.2%) | 7/22 (31.8%) |
| Traction bronchiectasis | 31/31 (100%) | 22/22 (100%) |
| mild | 7/31 (22.6%) | 8/22 (36.4%) |
| moderate | 18/31 (58%) | 12/22 (54.5% |
| severe | 6/31 (19.4%) | 2/22 (9.09%) |
| Reticulations | 28/31 (84.8%) | 22/22 (100%) |
| mild | 13/31 (41.9%) | 9/22 (40.9%) |
| moderate | 14/31 (45.2%) | 13/22 (59.1%) |
| severe | 4/31 (11.8%) | 0/22 (0%) |
| Pulmonary ossifications | 2/31 (6.5%) | 0/22 |
| Wedge-shaped fibrosis | 0/31 | 1/22 (4.5%) |
| Four-corner sign | 1/31 (3.2%) | 2/22 (9%) |
| Oesophagus dilatation | 2/31 (6.5%) * | 2/31 (6.5%) |
| Pulmonary hypertension | 2/31 (6.5%) | 5/22 (22.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmucci, S.; Adorna, M.; Rapisarda, A.; Libra, A.; Fischetti, S.; Sambataro, G.; Mauro, L.A.; David, E.; Foti, P.V.; Mattina, C.; et al. Radiological Insights into UIP Pattern: A Comparison Between IPF and Non-IPF Patients. J. Clin. Med. 2025, 14, 4162. https://doi.org/10.3390/jcm14124162
Palmucci S, Adorna M, Rapisarda A, Libra A, Fischetti S, Sambataro G, Mauro LA, David E, Foti PV, Mattina C, et al. Radiological Insights into UIP Pattern: A Comparison Between IPF and Non-IPF Patients. Journal of Clinical Medicine. 2025; 14(12):4162. https://doi.org/10.3390/jcm14124162
Chicago/Turabian StylePalmucci, Stefano, Miriam Adorna, Angelica Rapisarda, Alessandro Libra, Sefora Fischetti, Gianluca Sambataro, Letizia Antonella Mauro, Emanuele David, Pietro Valerio Foti, Claudia Mattina, and et al. 2025. "Radiological Insights into UIP Pattern: A Comparison Between IPF and Non-IPF Patients" Journal of Clinical Medicine 14, no. 12: 4162. https://doi.org/10.3390/jcm14124162
APA StylePalmucci, S., Adorna, M., Rapisarda, A., Libra, A., Fischetti, S., Sambataro, G., Mauro, L. A., David, E., Foti, P. V., Mattina, C., Spatola, C., Vancheri, C., & Basile, A. (2025). Radiological Insights into UIP Pattern: A Comparison Between IPF and Non-IPF Patients. Journal of Clinical Medicine, 14(12), 4162. https://doi.org/10.3390/jcm14124162









