Thoracic Ultrasound for Acute Dyspnea in Interstitial Lung Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Ultrasound Protocol
2.3. Data Collection
2.4. Statistical Analysis
2.5. Ethics
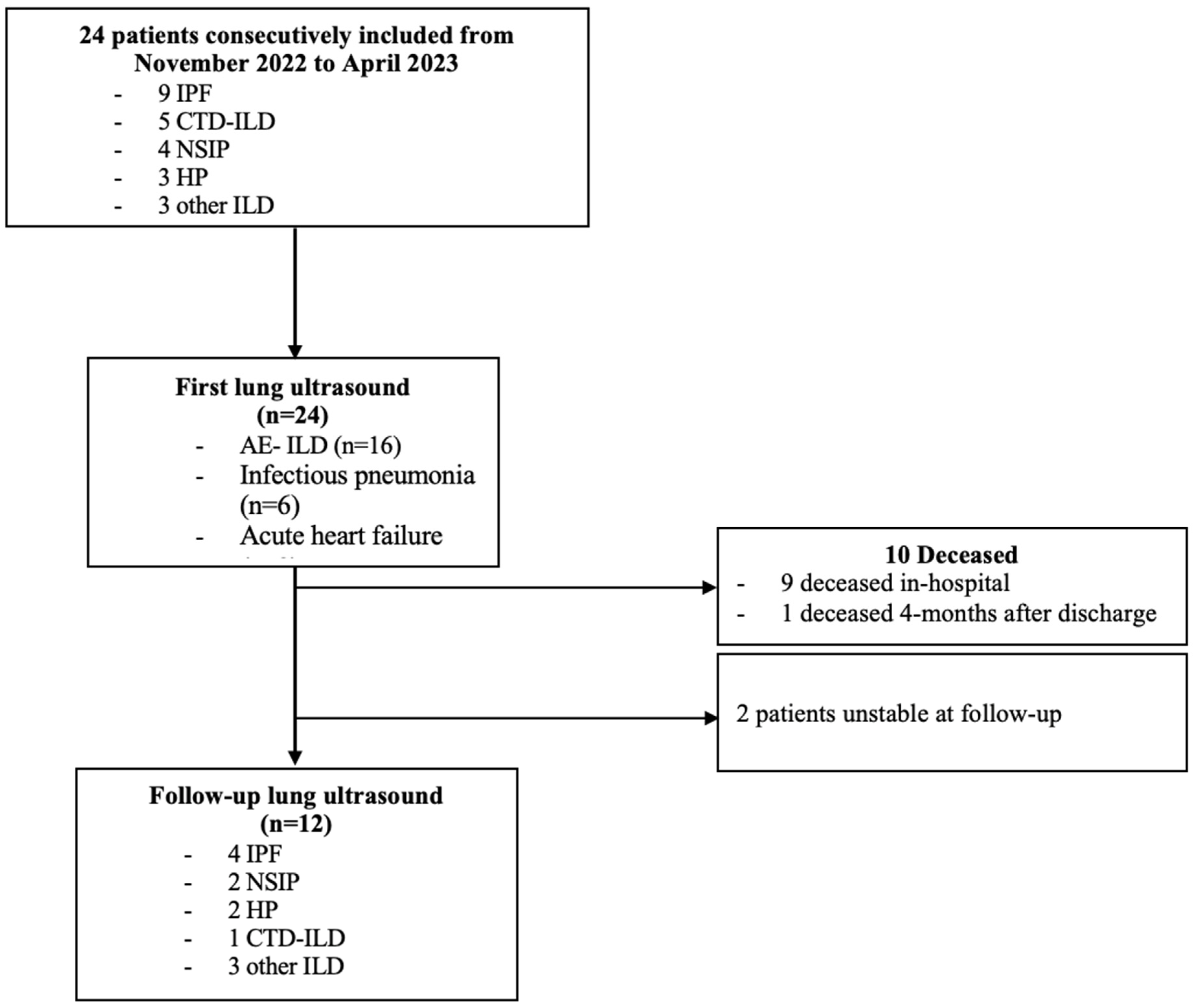
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE-ILD | Acute exacerbation of interstitial lung disease |
| AHF | Acute heart failure |
| BLUE protocol | Bedside Lung Ultrasound in Emergency protocol |
| BMI | Body mass index |
| CT | Computed tomography |
| CTD | Connective tissue disorder |
| CXR | Chest X-ray |
| DLCO | Diffusion capacity of the lungs for carbon monoxide |
| FiO2 | Fraction of inspired oxygen |
| FVC | Forced vital capacity |
| ICU | Intensive care unit |
| ILD | Interstitial lung disease |
| LUS | Lung ultrasound |
| PE | Pulmonary embolism |
| SpO2 | Pulse oxygen saturation |
References
- Morgenthau, A.S.; Padilla, M.L. Spectrum of fibrosing diffuse parenchymal lung disease. Mt. Sinai J. Med. 2009, 76, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Salonen, J.; Vähänikkilä, H.; Purokivi, M.; Kaarteenaho, R. Causes of acute respiratory hospitalizations predict survival in fibrosing interstitial lung diseases. PLoS ONE 2020, 15, e0242860. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Crestani, B.; Cadranel, J.; Cordier, J.F.; Marchand-Adam, S.; Prévot, G.; Marchand-Adam, S.; Nunes, H.; Wémeau-Stervinou, L.; Bergot, E.; et al. French practical guidelines for the diagnosis and management of idiopathic pulmonary fibrosis—2017 update. Full-length version. Rev. Mal. Respir. 2017, 34, 900–968. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.A.; Mezière, G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest 2008, 134, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Bekgoz, B.; Kilicaslan, I.; Bildik, F.; Keles, A.; Demircan, A.; Hakoglu, O.; Coskun, G.; Demir, H.A. BLUE protocol ultrasonography in Emergency Department patients presenting with acute dyspnea. Am. J. Emerg. Med. 2019, 37, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Plantier, L.; Mangiapan, G.; Morel, H.; Marchand-Adam, S.; Legué, S.; Bayeh, B.A.; Flament, T. ThOracic Ultrasound in Idiopathic Pulmonary Fibrosis Evolution (TOUPIE): Research protocol of a multicentric prospective study. BMJ Open 2021, 11, e039078. [Google Scholar]
- Gargani, L.; Doveri, M.; D’Errico, L.; Frassi, F.; Bazzichi, M.L.; Delle Sedie, A.; Scali, M.C.; Monti, S.; Mondillo, S.; Bombardieri, S.; et al. Ultrasound lung comets in systemic sclerosis: A chest sonography hallmark of pulmonary interstitial fibrosis. Rheumatology 2009, 48, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.; Tardella, M.; Rodriguez, L.; Mendoza, J.; Clavijo-Cornejo, D.; García, A.; Bertolazzi, C. Ultrasound as a potential tool for the assessment of interstitial lung disease in rheumatic patients. Where are we now? Radiol. Medica 2019, 124, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gargani, L.; Barskova, T.; Furst, D.E.; Cerinic, M.M. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: A literature review. Arthritis Res. Ther. 2017, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- Vizioli, L.; Ciccarese, F.; Forti, P.; Chiesa, A.M.; Giovagnoli, M.; Mughetti, M.; Zompatori, M.; Zoli, M. Integrated Use of Lung Ultrasound and Chest X-Ray in the Detection of Interstitial Lung Disease. Respiration 2017, 93, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.; Merz, A.A.; Jhund, P.S.; Vazir, A.; Campbell, R.; McMurray, J.J. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: A systematic review. Eur. J. Heart Fail. 2017, 19, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Thoracic Ultrasound; European Respiratory Society: Lausanne, Switzerland, 2018; 265 p. Available online: https://books.ersjournals.com/content/9781849840941/9781849840941 (accessed on 8 June 2025).
- Shimizu, M.; Hashimoto, S. Peripheral oxygen saturation to inspiratory oxygen fraction ratio-based identification of critically ill coronavirus disease patients for early therapeutic interventions. J. Anesth. 2021, 35, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kondoh, Y.; Brown, K.K.; Johkoh, T.; Kataoka, K.; Fukuoka, J.; Kimura, T.; Matsuda, T.; Yokoyama, T.; Fukihara, J.; et al. Acute exacerbations of fibrotic interstitial lung diseases. Respirology 2020, 25, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Moua, T.; Westerly, B.D.; Dulohery, M.M.; Daniels, C.E.; Ryu, J.H.; Lim, K.G. Patients With Fibrotic Interstitial Lung Disease Hospitalized for Acute Respiratory Worsening: A Large Cohort Analysis. Chest 2016, 149, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Pitsidianakis, G.; Vassalou, E.E.; Vasarmidi, E.; Bolaki, M.; Klontzas, M.E.; Xirouchaki, N.; Georgopoulos, D.; Karantanas, A.H.; Tzanakis, N.; Antoniou, K.M. Performance of Lung Ultrasound for Monitoring Interstitial Lung Disease. J. Ultrasound Med. 2022, 41, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.M.; Jouneau, S.; Bendstrup, E.; Kreuter, M.; Miede, C.; Inoue, Y.; Lievens, D.; Crestani, B. Weight loss and outcomes in subjects with progressive pulmonary fibrosis: Data from the INBUILD trial. Respir. Res. 2023, 24, 71. [Google Scholar]
- Mankikian, J.; Caille, A.; Reynaud-Gaubert, M.; Agier, M.S.; Bermudez, J.; Bonniaud, P.; Borie, R.; Brillet, P.-Y.; Cadranel, J.; Court-Fortune, I.; et al. Rituximab and mycophenolate mofetil combination in patients with interstitial lung disease (EVER-ILD): A double-blind, randomised, placebo-controlled trial. Eur. Respir. J. 2023, 61, 2202071. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Qiao, W.; Wang, X.; Yu, X. Association of Lung Ultrasound Score with Mortality and Severity of COVID-19: A Meta-Analysis and Trial Sequential Analysis. Int. J. Infect. Dis. 2021, 108, 603–609. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 24) | Patients with AE-ILD (n = 16) | Patients with Pulmonary Infection (n = 6) | p-Value | |
|---|---|---|---|---|
| Baseline characteristics at admission | ||||
| Age, years | 72 [66–75] | 71.4 [65.8–74.5] | 72.3 [67.8–74.7] | 0.80 |
| Male gender | 18 (75%) | 12 (75%) | 5 (83%) | 1 |
| Type of ILD | ||||
| - IPF | 9 (37.5%) | 5 (31.3%) | 3 (50%) | |
| - Connective tissue-related ILD | 5 (21%) | 4 (25%) | 1 (16.7%) | |
| - Idiopathic NSIP | 4 (17%) | 2 (12.5%) | 1 (16.7%) | |
| - Hypersensitivity pneumonia | 3 (12.5%) | 3 (18.8%) | 0 | |
| - Desquamative interstitial pneumonia - Post-ARDS fibrosis | 1 (4%) 1 (4%) | 0 1 (6.25%) | 1 (16.7%) 0 | |
| - ABCA3 mutation | 1 (4%) | 1 (6.25%) | 0 | |
| Comorbidities at admission | ||||
| Active or previous smokers | 22 (91.8%) | 15 (93.7%) | 5 (83.3%) | 0.48 |
| Smoking, pack-years | 20 [6.5–30] | 17 [6.5–20.5] | 28.5 [21.8–31.5] | 0.16 |
| BMI | 25 [23–29] | 25,7 [23.4–28.5] | 25.8 [23.6–28.4] | 0.91 |
| Immunosuppression | 10 (41%) | 7 (44%) | 2 (33%) | 1 |
| Corticosteroid treatment at admission | 7 (29%) | 5 (31%) | 1 (16.7%) | 0.63 |
| Immunosuppressive treatment | 8 (33%) | 4 (25%) | 3 (50%) | 0.62 |
| Antifibrotic treatment | 10 (42%) | 10 (62.5%) | 4 (66.7%) | 0.35 |
| Most recent pulmonary functional test | ||||
| FVC % predicted | 65 [53–72] | 56 [43–65] | 73.5 [69.5–89.5] | 0.07 |
| DLCO % predicted | 32 [28–38] | 29 [22–40] | 30 [30–37] | 0.42 |
| Long-term supplemental oxygen | 9 (38%) | 8 (50%) | 1 (16.7%) | 0.33 |
| Usual dyspnea (MRC score) | ||||
| 1 | 7 (29%) | 6 (38) | 1 (17%) | |
| 2 | 8 (33%) | 4 (25%) | 3 (50%) | |
| 3 | 6 (25%) | 3 (19%) | 2 (33%) | |
| 4 | 3 (13%) | 3 (19%) | 0 | |
| Symptoms at admission | ||||
| Dyspnea (MRC score) | ||||
| 3 | 4 (17%) | 3 (18.75%) | 1 (16.7%) | |
| 4 | 20 (83%) | 13 (81.25%) | 5 (83.3%) | |
| Cough | 15 (65%) | 9 (56%) | 6 (100%) | 0.12 |
| Sputum | 9 (37%) | 7 (38%) | 3 (50%) | 0.66 |
| Temperature at admission (°C) | 36.4 [36.2–37.3] | 36.4 [36.2–37.5] | 36.95 [36.5–38.1] | 0.17 |
| Recent weight change in kg | −1.5 [−5–0] | −4.25 [−5.93–0.88] | 0 [−0.75–0.45] | 0.03 |
| Maximum oxygen | ||||
| supplementation during hospital stay (SpO2/FiO2) | 197 [118.75–300] | 134 [117–243] | 271 [207–305] | 0.24 |
| Treatments administered | ||||
| Antibiotics | 19 (79%) | 12 (75%) | 6 (100%) | 0.54 |
| Diuretic treatment | 9 (38%) | 7 (44%) | 0 | 0.12 |
| Corticosteroids | 19 (79%) | 16 (100%) | 3 (50%) | 0.01 |
| Immunosuppressant therapy | 2 (8%) | 1 (7%) | 1 (16.7%) | 1 |
| Anticoagulant treatment | 1 (4%) | 1 (6.25%) | 0 | 1 |
| Clinical outcomes | ||||
| In-hospital mortality | 9 (38%) | 8 (50%) | 1 (16.7%) | 0.33 |
| LUS 1 (n = 24) | LUS 2 (n = 12) | |
|---|---|---|
| Lung sliding | 24 (100%) | 12 (100%) |
| Pleural irregularities in all thoracic areas | 21 (87.5%) | 12 (100%) |
| Pleural effusion | 4 (17%) | 0 |
| Consolidation | 2 (8%) | 0 |
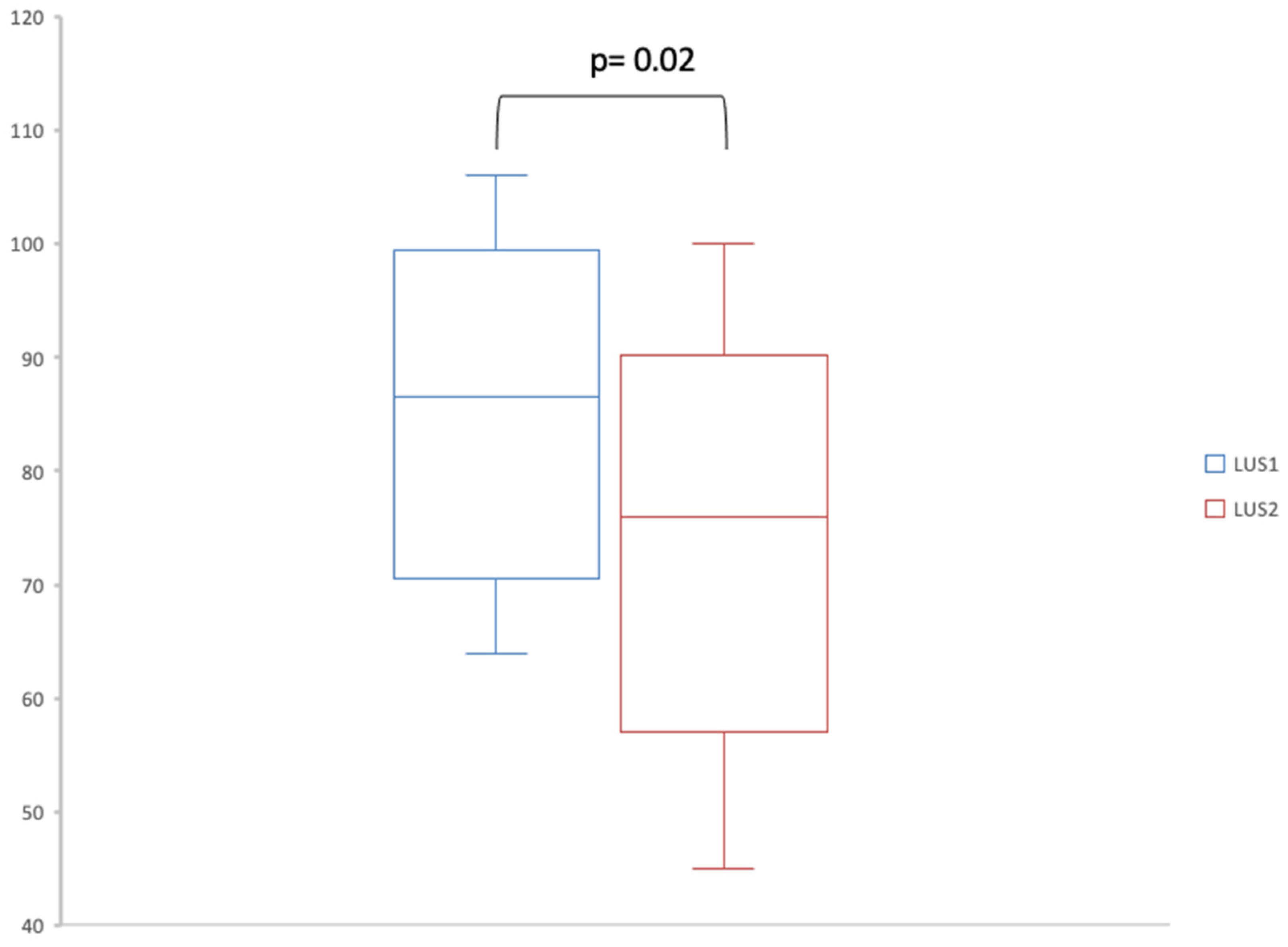
| Sum of A-lines | 0 [0–1] | 1 [0–2.25] |
| Sum of B-lines | 86.5 [71.5–94.5] | 76 [59–86.75] |
| All Patients (n = 24) | Patients with AE-ILD (n = 16) | Patients with Pulmonary Infection (n = 6) | Patients with AHF (n = 2) | |
|---|---|---|---|---|
| Lung sliding | 24 (100%) | 16 (100%) | 6 (100%) | 2 (100%) |
| Pleural irregularities in all thoracic areas | 21 (87.5%) | 14 (87.5) | 5 (83%) | 2 (100%) |
| Pleural effusion | 4 (17%) | 0 | 3 (18.75%) | 1 (50%) |
| Consolidation | 2 (8%) | 1 (6.25%) | 1 (17%) | 0 |
| Sum of A-lines | 0 [0–1] | 0 [0–0] | 1 [0.25–1.75] | 0.5 [0.25–0.75] |
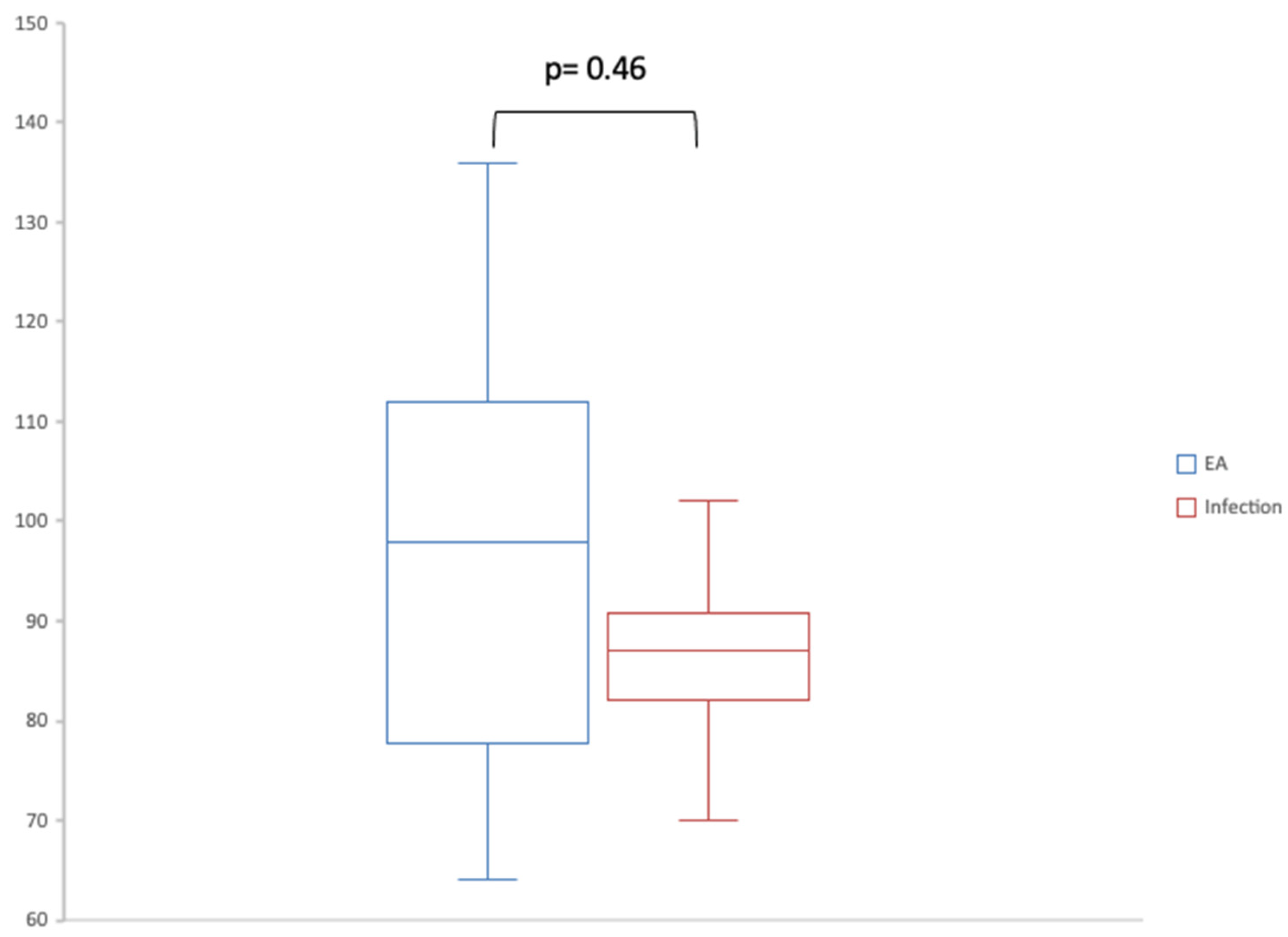
| Sum of B-lines | 87 [81.25–105] | 98 [81.25–110] | 87 [86.25–87] | 79 [75–82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayeh, B.A.; Marchand-Adam, S.; Legué, S.; Luque Paz, D.; Plantier, L.; Flament, T. Thoracic Ultrasound for Acute Dyspnea in Interstitial Lung Disease. J. Clin. Med. 2025, 14, 4159. https://doi.org/10.3390/jcm14124159
Bayeh BA, Marchand-Adam S, Legué S, Luque Paz D, Plantier L, Flament T. Thoracic Ultrasound for Acute Dyspnea in Interstitial Lung Disease. Journal of Clinical Medicine. 2025; 14(12):4159. https://doi.org/10.3390/jcm14124159
Chicago/Turabian StyleBayeh, Betsega A., Sylvain Marchand-Adam, Sylvie Legué, David Luque Paz, Laurent Plantier, and Thomas Flament. 2025. "Thoracic Ultrasound for Acute Dyspnea in Interstitial Lung Disease" Journal of Clinical Medicine 14, no. 12: 4159. https://doi.org/10.3390/jcm14124159
APA StyleBayeh, B. A., Marchand-Adam, S., Legué, S., Luque Paz, D., Plantier, L., & Flament, T. (2025). Thoracic Ultrasound for Acute Dyspnea in Interstitial Lung Disease. Journal of Clinical Medicine, 14(12), 4159. https://doi.org/10.3390/jcm14124159





