CA125 as a Potential Biomarker in Non-Malignant Serous Effusions: Diagnostic and Prognostic Considerations
Abstract
1. Introduction
2. Materials and Methods
3. Results
Pathophysiological Mechanisms of CA125 Synthesis
4. Discussion
4.1. CA125 in the Field of Gynecology
4.2. CA125 in Congestive Heart Failure
4.3. CA125 in Other Cardiac Pathologies
4.4. CA125 in End-Stage Liver Disease
4.5. CA125 in Autoimmune Diseases
4.6. CA125 in Clinical Guidelines
4.7. Strengths and Limitations of the Study
4.8. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA125 | Carbohydrate antigen 125 |
| CA 19-9 | Carbohydrate antigen 19-9 |
| CEA | Carcinoembryonic antigen |
| MUC16 | Mucin16 |
| TAPSE | Tricuspid annular plane systolic excursion |
| AST | Aspartate transaminase |
| ALT | Alanine transaminase |
| LDH | Lactate dehydrogenase |
| STEMI | ST-segment elevation myocardial infarction |
| BNP | B type natriuretic peptide |
| NTproBNP | N-terminal pro-B type natriuretic peptide |
References
- Reynolds, I.S.; Fichtner, M.; McNamara, D.A.; Kay, E.W.; Prehn, J.H.M.; Burke, J.P. Mucin glycoproteins block apoptosis; promote invasion, proliferation, and migration; and cause chemoresistance through diverse pathways in epithelial cancers. Cancer Metastasis Rev. 2019, 38, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Hong, L.L.; Ling, Z.Q. MUC16/CA125 in cancer: New advances. Clin. Chim. Acta 2025, 565, 119981. [Google Scholar] [CrossRef]
- Cundari, G.B.; Feole, L.; Terranova, C.; Nardone, C.D.C.; Montera, R.; Luvero, D.; Guzzo, F.; Martinelli, A.; Di Donato, V.; Angioli, R.; et al. The Role of CA125 and HE4 in Uterine Sarcomas: Beyond Diagnosis and Prognosis-A Systematic Review and Case Series from a Single Institution. Cancers 2025, 17, 1473. [Google Scholar] [CrossRef]
- DynaMedex. Ovarian Cancer/Fallopian Tube Cancer/Primary Peritoneal Cancer: Intraperitoneal CISpla-tin/Intraperitoneal PACLitaxel/IV PACLitaxel. EBSCO Information Services. Available online: https://www.dynamedex.com/chemo-regimen/ovarian-cancer-fallopian-tube-cancer-primary-peritoneal-cancer-ova2#GUID-E47C6C58-4A5A-424B-9F0A-013B2822887E (accessed on 1 March 2025).
- Zhang, X.Y.; Hong, L.L.; Ling, Z.Q. MUC16: Clinical targets with great potential. Clin. Exp. Med. 2024, 24, 101. [Google Scholar] [CrossRef]
- Song, Y.; Yuan, M.; Wang, G. Update value and clinical application of MUC16 (cancer antigen 125). Expert. Opin. Ther. Targets 2023, 27, 745–756. [Google Scholar] [CrossRef]
- Rafiq, M.; Renzi, C.; White, B.; Zakkak, N.; Nicholson, B.; Lyratzopoulos, G.; Barclay, M. Predictive value of abnormal blood tests for detecting cancer in primary care patients with nonspecific abdominal symptoms: A population-based cohort study of 477,870 patients in England. PLoS Med. 2024, 21, e1004426. [Google Scholar] [CrossRef]
- Xiao, W.B.; Liu, Y.L. Elevation of serum and ascites cancer antigen 125 levels in patients with liver cirrhosis. J. Gastroenterol. Hepatol. 2003, 18, 1315–1316. [Google Scholar] [CrossRef]
- Trapé, J.; Fernández-Galán, E.; Auge, J.M.; Carbonell-Prat, M.; Filella, X.; Miró-Cañís, S.; González-Fernández, C.; Oncology Biomarkers Section of the Catalan Association of Clinical Laboratory Science. Factors influencing blood tumor marker concentrations in the absence of neoplasia. Tumour. Biol. 2024, 46 (Suppl. S1), S35–S63. [Google Scholar] [CrossRef]
- Liu, F.; Kong, X.; Dou, Q.; Ye, J.; Xu, D.; Shang, H.; Xu, K.; Song, Y. Evaluation of tumor markers for the differential diagnosis of benign and malignant ascites. Ann. Hepatol. 2014, 13, 357–363. [Google Scholar] [CrossRef]
- Runyon, B.A. Malignancy-Related Ascitis. Available online: https://www.uptodate.com/contents/malignancy-related-ascites?search=ca125%20runyon&source=search_result&selectedTitle=5~108&usage_type=default&display_rank=5 (accessed on 10 June 2025).
- Feng, F.; Tian, Y.; Xu, G.; Liu, Z.; Liu, S.; Zheng, G.; Guo, M.; Lian, X.; Fan, D.; Zhang, H. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer 2017, 17, 737. [Google Scholar] [CrossRef]
- Volarić, D.; Flego, V.; Žauhar, G.; Bulat-Kardum, L. Diagnostic value of tumour markers in pleural effusions. Biochem. Med. 2018, 28, 010706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Liang, B.; Wu, H.; Chen, Y. Diagnosis of malignant pleural effusion with combinations of multiple tumor markers: A comparison study of five machine learning models. Int. J. Biol. Markers 2023, 38, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, J.; Cao, Y. Factors associated with serum CA125 level in women without ovarian cancer in the United States: A population-based study. BMC Cancer 2022, 22, 544. [Google Scholar] [CrossRef] [PubMed]
- Gronnier, M.; Hedhli, K.; Sauzay, C.; Salle, V.; Duhaut, P.; Schmidt, J.; Dernoncourt, A. Relevance of blood tumor markers in inpatients with significant involuntary weight loss and elevated levels of inflammation biomarkers. BMC Cancer 2024, 24, 468. [Google Scholar] [CrossRef]
- Givens, V.; Mitchell, G.E.; Harraway-Smith, C.; Reddy, A.; Maness, D.L. Diagnosis and management of adnexal masses. Am. Fam. Physician 2009, 80, 815–820. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- de la Espriella-Juan, R.; Núñez, E.; Sanchis, J.; Bayés-Genis, A.; Núñez, J. Carbohydrate Antigen-125 in Heart Failure: An Overlooked Biomarker of Congestion. JACC Heart Fail. 2018, 6, 441–442. [Google Scholar] [CrossRef]
- Girerd, N.; Zannad, F.; Rossignol, P.; INI-CRCT, Great Network, and the EF-HF Group. Reply: Carbohydrate Antigen-125 in Heart Failure: An Overlooked Biomarker of Congestion. JACC Heart Fail. 2018, 6, 442–443. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, B.R.; Yang, W.J. Dilution effect of serum CA125 and CA19-9 over a cutoff value, according to obesity. Int. J. Biol. Markers 2015, 30, e122–e126. [Google Scholar] [CrossRef]
- Núñez, J.; de la Espriella, R.; Miñana, G.; Santas, E.; Llácer, P.; Núñez, E.; Palau, P.; Bodí, V.; Chorro, F.J.; Sanchis, J.; et al. Antigen carbohydrate 125 as a biomarker in heart failure: A narrative review. Eur. J. Heart Fail. 2021, 23, 1445–1457. [Google Scholar] [CrossRef]
- Zuckerman, E.; Lanir, A.; Sabo, E.; Rosenvald-Zuckerman, T.; Matter, I.; Yeshurun, D.; Eldar, S. Cancer antigen 125: A sensitive marker of ascites in patients with liver cirrhosis. Am. J. Gastroenterol. 1999, 94, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.L.; Xu, H.; Lv, W.F.; Hua, R.; Du, H.; Zhang, Q.Q. The Significance of Serum CA-125 Elevation in Chinese Patients with Primary Budd-Chiari Syndrome: A Multicenter Study. Gastroenterol. Res. Pract. 2015, 2015, 121060. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Edula, R.G.; Muthukuru, S.; Moroianu, S.; Wang, Y.; Lingiah, V.; Fung, P.; Pyrsopoulos, N.T. CA-125 Significance in Cirrhosis and Correlation with Disease Severity and Portal Hypertension: A Retrospective Study. J. Clin. Transl. Hepatol. 2018, 6, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Júnior, W.V.d.O.; Turani, S.D.; Marinho, M.A.S.; Pinto, S.W.L.; Otoni, A.; Figueiredo, R.C.; Rios, D.R.A. CA-125 and CCL2 may indicate inflammation in peritoneal dialysis patients. J. Bras. Nefrol. 2021, 43, 502–509. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, Z.; Ma, J.; Zhang, L.; Xue, L. Tumour-associated antigens in systemic lupus erythematosus: Association with clinical manifestations and serological indicators. Rheumatology 2024, 63, 235–241. [Google Scholar] [CrossRef]
- Hung, C.-L.; Hung, T.-C.; Liu, C.-C.; Wu, Y.-J.; Kuo, J.-Y.; Hou, C.J.-Y.; Yeh, H.-I. Relation of carbohydrate antigen-125 to left atrial remodeling and its prognostic usefulness in patients with heart failure and preserved left ventricular ejection fraction in women. Am. J. Cardiol. 2012, 110, 993–1000. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; de la Espriella, R.; Núñez, J. CA125 for Fluid Overload Monitoring: A New Life for an Old Tool. J. Am. Coll. Cardiol. 2023, 82, 158–160. [Google Scholar] [CrossRef]
- Akinwunmi, B.O.; Babic, A.; Vitonis, A.F.; Cramer, D.W.; Titus, L.; Tworoger, S.S.; Terry, K.L. Chronic Medical Conditions and CA125 Levels among Women without Ovarian Cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1483–1490. [Google Scholar] [CrossRef]
- Bergmann, J.F.; Bidart, J.M.; George, M.; Beaugrand, M.; Levy, V.G.; Bohuon, C. Elevation of CA 125 in patients with benign and malignant ascites. Cancer 1987, 59, 213–217. [Google Scholar] [CrossRef]
- Wu, H.H.L.; Rakisheva, A.; Ponnusamy, A.; Chinnadurai, R. Hepatocardiorenal syndrome in liver cirrhosis: Recognition of a new entity? World J. Gastroenterol. 2024, 30, 128–136. [Google Scholar] [CrossRef]
- de la Espriella, R.; Bayés-Genís, A.; Llàcer, P.; Palau, P.; Miñana, G.; Santas, E.; Pellicer, M.; González, M.; Górriz, J.L.; Bodi, V.; et al. Prognostic value of NT-proBNP and CA125 across glomerular filtration rate categories in acute heart failure. Eur. J. Intern. Med. 2022, 95, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wijayaratne, D.; Muthuppalaniappan, V.M.; Davenport, A. Serum CA125 a potential marker of volume status for peritoneal dialysis patients? Int. J. Artif. Organs 2021, 44, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.; de la Espriella, R.; Rossignol, P.; Voors, A.A.; Mullens, W.; Metra, M.; Chioncel, O.; Januzzi, J.L.; Mueller, C.; Richards, A.M.; et al. Congestion in heart failure: A circulating biomarker-based perspective. A review from the Biomarkers Working Group of the Heart Failure Association, European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 1751–1766. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Marín, G.; de la Espriella, R.; Santas, E.; Lorenzo, M.; Miñana, G.; Núñez, E.; Bodí, V.; González, M.; Górriz, J.L.; Bonanad, C.; et al. CA125 but not NT-proBNP predicts the presence of a congestive intrarenal venous flow in patients with acute heart failure. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 475–483. [Google Scholar] [CrossRef]
- Kumric, M.; Kurir, T.T.; Bozic, J.; Glavas, D.; Saric, T.; Marcelius, B.; D’aMario, D.; Borovac, J.A. Carbohydrate Antigen 125: A Biomarker at the Crossroads of Congestion and Inflammation in Heart Failure. Card. Fail. Rev. 2021, 7, e19. [Google Scholar] [CrossRef]
- Khanna, A.K.; Rola, P.; Malbrain, M.L.N.G. Biomarkers for intra-abdominal pressure: Another tool in the toolbox? Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 461–463. [Google Scholar] [CrossRef]
- Frigy, A.; Belényi, B.; Kirchmaier, Á.; Fekete, N.; Szabó, I.A. Elevated CA-125 as Humoral Biomarker of Congestive Heart Failure: Illustrative Cases and a Short Review of Literature. Case Rep. Cardiol. 2020, 2020, 1642914. [Google Scholar] [CrossRef]
- Marinescu, M.C.; Oprea, V.D.; Munteanu, S.N.; Nechita, A.; Tutunaru, D.; Nechita, L.C.; Romila, A. Carbohydrate Antigen 125 (CA 125): A Novel Biomarker in Acute Heart Failure. Diagnostics 2024, 14, 795. [Google Scholar] [CrossRef]
- Brann, A.; Selko, S.; Krauspe, E.; Shah, K. Biomarkers of Hemodynamic Congestion in Heart Failure. Curr. Heart Fail. Rep. 2024, 21, 541–553. [Google Scholar] [CrossRef]
- Fudim, M.; Felker, G.M. Biomarkers of Congestion: Emerging Tools in the Management of Heart Failure? JACC Heart Fail. 2020, 8, 398–400. [Google Scholar] [CrossRef]
- Núñez, J.; Bayés-Genís, A.; Revuelta-López, E.; ter Maaten, J.M.; Miñana, G.; Barallat, J.; Cserkóová, A.; Bodi, V.; Fernández-Cisnal, A.; Núñez, E.; et al. Clinical Role of CA125 in Worsening Heart Failure: A BIOSTAT-CHF Study Subanalysis. JACC Heart Fail. 2020, 8, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Pandhi, P.; ter Maaten, J.M.; Anker, S.D.; Ng, L.L.; Metra, M.; Samani, N.J.; Lang, C.C.; Dickstein, K.; de Boer, R.A.; van Veldhuisen, D.J.; et al. Pathophysiologic Processes and Novel Biomarkers Associated With Congestion in Heart Failure. JACC Heart Fail. 2022, 10, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J. Adnexal Mass: Role of Serum Biomarkers in Diagnosing Epithelial Carcinoma of the Ovary, Fallopian Tube or Peritoneum. Available online: https://www.uptodate.com/contents/adnexal-mass-role-of-serum-biomarkers-in-diagnosing-epithelial-carcinoma-of-the-ovary-fallopian-tube-or-peritoneum?search=ca125%20adnexal%20mass&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 25 May 2025).
- González-Martín, A.; Harter, P.; Leary, A.; Lorusso, D.; Miller, R.; Pothuri, B.; Ray-Coquard, I.; Tan, D.; Bellet, E.; Oaknin, A.; et al. Newly diagnosed and relapsed epithelial ovarian cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Vanderpuye, V.D.; Clemenceau, J.R.V.; Temin, S.; Aziz, Z.; Burke, W.M.; Cevallos, N.L.; Chuang, L.T.; Colgan, T.J.; del Carmen, M.G.; Fujiwara, K.; et al. Assessment of Adult Women with Ovarian Masses and Treatment of Epithelial Ovarian Cancer: ASCO Resource-Stratified Guideline. JCO Glob. Oncol. 2021, 7, 1032–1066. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Morice, P.; Lorusso, D.; Prat, J.; Oaknin, A.; Pautier, P.; Colombo, N. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv1–iv18. [Google Scholar] [CrossRef]
- Stephens, A.N.; Hobbs, S.J.; Kang, S.W.; Oehler, M.K.; Jobling, T.W.; Allman, R. ReClassification of Patients with Ambiguous CA125 for Optimised Pre-Surgical Triage. Diagnostics 2024, 14, 671. [Google Scholar] [CrossRef]
- Ali, F.T.; Soliman, R.M.; Hassan, N.S.; Ibrahim, A.M.; El-Gizawy, M.M.; Mandoh, A.A.Y.; Ibrahim, E.A.; Christie, E. Sensitivity and specificity of microRNA-204, CA125, and CA19.9 as biomarkers for diagnosis of ovarian cancer. PLoS ONE 2022, 17, e0272308. [Google Scholar] [CrossRef]
- Carreras-Dieguez, N.; Glickman, A.; Munmany, M.; Casanovas, G.; Agustí, N.; Díaz-Feijoo, B.; Saco, A.; Sánchez, B.; Gaba, L.; Angeles, M.A.; et al. Comparison of HE4, CA125, ROMA and CPH-I for Preoperative Assessment of Adnexal Tumors. Diagnostics 2022, 12, 226. [Google Scholar] [CrossRef]
- Xie, W.T.; Wang, Y.Q.; Xiang, Z.S.; Du, Z.S.; Huang, S.X.; Chen, Y.J.; Tang, L.N. Efficacy of IOTA simple rules, O-RADS, and CA125 to distinguish benign and malignant adnexal masses. J. Ovarian Res. 2022, 15, 15. [Google Scholar] [CrossRef]
- Watrowski, R.; Obermayr, E.; Wallisch, C.; Aust, S.; Concin, N.; Braicu, E.I.; Van Gorp, T.; Hasenburg, A.; Sehouli, J.; Vergote, I.; et al. Biomarker-Based Models for Preoperative Assessment of Adnexal Mass: A Multicenter Validation Study. Cancers 2022, 14, 1780. [Google Scholar] [CrossRef]
- Czekierdowski, A.; Stachowicz, N.; Smolen, A.; Łoziński, T.; Guzik, P.; Kluz, T. Performance of IOTA Simple Rules Risks, ADNEX Model, Subjective Assessment Compared to CA125 and HE4 with ROMA Algorithm in Discriminating between Benign, Borderline and Stage I Malignant Adnexal Lesions. Diagnostics 2023, 13, 885. [Google Scholar] [CrossRef] [PubMed]
- Spagnol, G.; Marchetti, M.; Carollo, M.; Bigardi, S.; Tripepi, M.; Facchetti, E.; De Tommasi, O.; Vitagliano, A.; Cavallin, F.; Tozzi, R.; et al. Clinical Utility and Diagnostic Accuracy of ROMA, RMI, ADNEX, HE4, and CA125 in the Prediction of Malignancy in Adnexal Masses. Cancers 2024, 16, 3790. [Google Scholar] [CrossRef] [PubMed]
- Nithin, K.U.; Sridhar, M.G.; Srilatha, K.; Habebullah, S. CA 125 is a better marker to differentiate endometrial cancer and abnormal uterine bleeding. Afr. Health Sci. 2018, 18, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Shawn LyBarger, K.; Miller, H.A.; Frieboes, H.B. CA125 as a predictor of endometrial cancer lymphovascular space invasion and lymph node metastasis for risk stratification in the preoperative setting. Sci. Rep. 2022, 12, 19783. [Google Scholar] [CrossRef]
- Juang, C.M.; Yen, M.S.; Horng, H.C.; Twu, N.F.; Yu, H.C.; Hsu, W.L. Potential role of preoperative serum CA125 for the differential diagnosis between uterine leiomyoma and uterine leiomyosarcoma. Eur. J. Gynaecol. Oncol. 2006, 27, 370–374. [Google Scholar]
- Yilmaz, N.; Sahin, I.; Kilic, S.; Ozgu, E.; Gungor, T.; Bilge, U. Assessment of the predictivity of preoperative serum CA 125 in the differential diagnosis of uterine leiomyoma and uterine sarcoma in the Turkish female population. Eur. J. Gynaecol. Oncol. 2009, 30, 412–414. [Google Scholar]
- Funston, G.; Hamilton, W.; Abel, G.; Crosbie, E.J.; Rous, B.; Walter, F.M. The diagnostic performance of CA125 for the detection of ovarian and non-ovarian cancer in primary care: A population-based cohort study. PLoS Med. 2020, 17, e1003295. [Google Scholar] [CrossRef]
- Yang, L.; Cai, Y.; Wang, Y.; Huang, Y.; Zhang, C.; Ma, H.; Zhou, J.-G. Fibroblast Growth Factor 23 is a Potential Prognostic Biomarker in Uterine Sarcoma. Technol. Cancer Res. Treat. 2024, 23, 15330338241245924. [Google Scholar] [CrossRef]
- Visnovsky, J.; Kudela, E.; Slavik, P.; Krkoska, M.; Buocik, P.; Szepe, P.; Danko, J. Survival and risk factors associated with uterine sarcomas and carcinosarcomas in stage I and II. Neuro Endocrinol. Lett. 2015, 36, 750–757. [Google Scholar]
- Zhang, Y.Y.; Li, Y.; Qin, M.; Cai, Y.; Jin, Y.; Pan, L.Y. High-grade endometrial stromal sarcoma: A retrospective study of factors influencing prognosis. Cancer Manag. Res. 2019, 11, 831–837. [Google Scholar] [CrossRef]
- Menczer, J.; Schreiber, L.; Berger, E.; Ben-Shem, E.; Golan, A.; Levy, T. CA125 expression in the tissue of uterine leiomyosarcoma. Isr. Med. Assoc. J. 2014, 16, 697–699. [Google Scholar] [PubMed]
- Huchon, C.; Bourdel, N.; Wahab, C.A.; Azaïs, H.; Bendifallah, S.; Bolze, P.-A.; Brun, J.-L.; Canlorbe, G.; Chauvet, P.; Chereau, E.; et al. Borderline ovarian tumors: French guidelines from the CNGOF. Part 1. Epidemiology, biopathology, imaging and biomarkers. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101965. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Casali, P.G.; Croce, S.; Fennessy, F.M.; Fischerova, D.; Jones, R.; Sanfilippo, R.; Zapardiel, I.; Amant, F.; Blay, J.-Y.; et al. ESGO/EURACAN/GCIG guidelines for the management of patients with uterine sarcomas. Int. J. Gynecol. Cancer 2024, 34, 1499–1521. [Google Scholar] [CrossRef]
- Ledermann, J.; Matias-Guiu, X.; Amant, F.; Concin, N.; Davidson, B.; Fotopoulou, C.; González-Martin, A.; Gourley, C.; Leary, A.; Lorusso, D.; et al. ESGO-ESMO-ESP consensus conference recommendations on ovarian cancer: Pathology and molecular biology and early, advanced and recurrent disease. Ann. Oncol. 2024, 35, 248–266. [Google Scholar] [CrossRef]
- Kil, K.; Chung, J.-E.; Pak, H.J.; Jeung, I.-C.; Kim, J.H.; Jo, H.H.; Kim, M.-R. Usefulness of CA125 in the differential diagnosis of uterine adenomyosis and myoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 185, 131–135. [Google Scholar] [CrossRef]
- Babacan, A.; Kizilaslan, C.; Gun, I.; Muhcu, M.; Mungen, E.; Atay, V. CA 125 and other tumor markers in uterine leiomyomas and their association with lesion characteristics. Int. J. Clin. Exp. Med. 2014, 7, 1078–1083. [Google Scholar]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Harada, T.; Taniguchi, F.; Kitajima, M.; Kitawaki, J.; Koga, K.; Momoeda, M.; Mori, T.; Murakami, T.; Narahara, H.; Osuga, Y.; et al. Clinical practice guidelines for endometriosis in Japan (The 3rd edition). J. Obstet. Gynaecol. Res. 2022, 48, 2993–3044. [Google Scholar] [CrossRef]
- Ordás, J.G.; Nuñez, J.; Claret, R.B.; Llacer, P.; Zegri-Reiriz, I.; de la Espriella, R.; Fort, A.; Rubio-Gracia, J.; Blazquez-Bermejo, Z.; Mendez, A.; et al. Usefulness of Antigen Carbohydrate 125 and N-Terminal Pro-B-Type Natriuretic Peptide for Assessing Congestion in Chronic Heart Failure: Insights from the CARDIOREN Registry. Cardiorenal Med. 2024, 14, 543–555. [Google Scholar] [CrossRef]
- Núñez, J.; Bayés-Genís, A.; Revuelta-López, E.; Miñana, G.; Santas, E.; ter Maaten, J.M.; de la Espriella, R.; Carratalá, A.; Lorenzo, M.; Palau, P.; et al. Optimal carbohydrate antigen 125 cutpoint for identifying low-risk patients after admission for acute heart failure. Rev. Esp. Cardiol. (Engl. Ed.) 2022, 75, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; van der Wal, H.H.; Silljé, H.H.W.; Dokter, M.M.; Berg, F.v.D.; Huizinga, L.; Vriesema, M.; Post, J.; Anker, S.D.; Cleland, J.G.; et al. Tumour biomarkers: Association with heart failure outcomes. J. Intern. Med. 2020, 288, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Miñana, G.; de la Espriella, R.; Mollar, A.; Santas, E.; Núñez, E.; Valero, E.; Bodí, V.; Chorro, F.J.; Fernández-Cisnal, A.; Martí-Cervera, J.; et al. Factors associated with plasma antigen carbohydrate 125 and amino-terminal pro-B-type natriuretic peptide concentrations in acute heart failure. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 437–447. [Google Scholar] [CrossRef]
- Núñez, J.; Llàcer, P.; Bertomeu-González, V.; Bosch, M.J.; Merlos, P.; Montagud, V.; Bodí, V.; Bertomeu-Martínez, V.; Pedrosa, V.; Cordero, A.; et al. Carbohydrate Antigen-125-Guided Therapy in Acute Heart Failure: CHANCE-HF: A Randomized Study. JACC Heart Fail. 2016, 4, 833–843. [Google Scholar] [CrossRef]
- García-Blas, S.; Bonanad, C.; Llàcer, P.; Ventura, S.; Núñez, J.M.; Sánchez, R.; Chamorro, C.; Fácila, L.; de la Espriella, R.; Vaquer, J.M.; et al. Diuretic Strategies in Acute Heart Failure and Renal Dysfunction: Conventional vs Carbohydrate Antigen 125-guided Strategy. Clinical Trial Design. Rev. Esp. Cardiol. (Engl. Ed.) 2017, 70, 1067–1073. [Google Scholar] [CrossRef]
- Higgins, A.; Tang, W.H.W. Carbohydrate antigen 125 in heart failure: Congestive kidneys or beyond? Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 484–486. [Google Scholar] [CrossRef]
- Sikaris, K.A. CA125-a test with a change of heart. Heart Lung Circ. 2011, 20, 634–640. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Packer, M.; Sattar, N.; Butler, J.; Pocock, S.J.; Anker, S.D.; Maldonado, S.G.; Panova-Noeva, M.; Sumin, M.; Masson, S.; et al. Carbohydrate antigen 125 concentrations across the ejection fraction spectrum in chronic heart failure: The EMPEROR programme. Eur. J. Heart Fail. 2024, 26, 788–802. [Google Scholar] [CrossRef]
- Pacho, C.; Domingo, M.; Núñez, R.; Lupón, J.; Núñez, J.; Barallat, J.; Moliner, P.; de Antonio, M.; Santesmases, J.; Cediel, G.; et al. Predictive biomarkers for death and rehospitalization in comorbid frail elderly heart failure patients. BMC Geriatr. 2018, 18, 109. [Google Scholar] [CrossRef]
- Menghoum, N.; Badii, M.C.; Deltombe, M.; Lejeune, S.; Roy, C.; Vancraeynest, D.; Pasquet, A.; Gerber, B.L.; Horman, S.; Gruson, D.; et al. Carbohydrate antigen 125: A useful marker of congestion, fibrosis, and prognosis in heart failure with preserved ejection fraction. ESC Heart Fail. 2024, 11, 1493–1505. [Google Scholar] [CrossRef]
- Rubio-Gracia, J.; Giménez-López, I.; Josa-Laorden, C.; Sánchez-Marteles, M.; Garcés-Horna, V.; Ruiz-Laiglesia, F.; Legarre, P.S.; Juana, E.B.; Amores-Arriaga, B.; Pérez-Calvo, J. Prognostic value of multimodal assessment of congestion in acute heart failure. Rev. Clin. Esp. 2021, 221, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, P.; Cunha, F.M.; Elias, C.; Fernandes, C.; Barroso, I.; Guimarães, J.T.; Bettencourt, P. CA-125 variation in acute heart failure: A single-centre analysis. ESC Heart Fail. 2022, 9, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Amorim, S.; Campelo, M.; Moura, B.; Martins, E.; Rodrigues, J.; Barroso, I.; Faria, M.; Guimarães, T.; Macedo, F.; Silva-Cardoso, J.; et al. The role of biomarkers in dilated cardiomyopathy: Assessment of clinical severity and reverse remodeling. Rev. Port. Cardiol. 2017, 36, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, Z.; Tudahun, I.; Liu, N.; Lin, Q.; Liu, J.; Wang, Y.; Chen, M.; Chen, Y.; Qi, N.; et al. High Serum Carbohydrate Antigen (CA) 125 Level Is Associated with Poor Prognosis in Patients with Light-Chain Cardiac Amyloidosis. Front. Cardiovasc. Med. 2021, 8, 692083. [Google Scholar] [CrossRef]
- Wu, B.; Shi, J.; Yu, F.; Wu, Y.; Tao, X.; Xuan, T.; Yang, J.; Wang, X. Association of Cancer Antigen 125 with Long-Term Prognosis in Light-Chain Cardiorenal Amyloidosis. Cardiorenal Med. 2023, 13, 19–25. [Google Scholar] [CrossRef]
- Kang, Y.; Hwang, H.Y. Carbohydrate antigen 125; A biomarker not only for medical patients but also for surgical patients with heart failure. Int. J. Cardiol. 2023, 373, 80. [Google Scholar] [CrossRef]
- Nan, Y.; Tiemuerniyazi, X.; Chen, L.; Song, Y.; Feng, W.; Xu, F. Prognostic value of carbohydrate antigen 125 in patients undergoing surgical left ventricular reconstruction. Int. J. Cardiol. 2023, 371, 377–383. [Google Scholar] [CrossRef]
- Falcão, F.; Oliveira, F.; Cantarelli, F.; Cantarelli, R.; Brito-Júnior, P.; Lemos, H.; Silva, P.; Camboim, I.; Freire, M.; Carvalho, O.; et al. Carbohydrate antigen 125 predicts pulmonary congestion in patients with ST-segment elevation myocardial infarction. Braz. J. Med. Biol. Res. 2019, 52, e9124. [Google Scholar] [CrossRef]
- Yndigegn, T.; Gu, T.; Grufman, H.; Erlinge, D.; Mokhtari, A.; Ekelund, U.; Magnusson, M.; Gustafsson, E.; Nilsson, J.; Goncalves, I.; et al. Elevated carbohydrate antigen 125 (CA125) is associated with incident heart failure and mortality in acute coronary syndrome. ESC Heart Fail. 2024, 11, 4325–4334. [Google Scholar] [CrossRef]
- Cordero, A.; Velasco, I.; Flores, E.; López-Ayala, J.M.; Sánchez-Munuera, S.; Muñoz-Villalba, M.P.; Selva-Mora, A.; Galán-Giménez, F.; de la Espriella, R.; Nuñez, J. Heart failure biomarkers and prediction of early left ventricle remodeling after acute coronary syndromes. Clin. Biochem. 2024, 131–132, 110814. [Google Scholar] [CrossRef]
- Sekiguchi, H.; Shimamoto, K.; Takano, M.; Kimura, M.; Takahashi, Y.; Tatsumi, F.; Watanabe, E.; Jujo, K.; Ishizuka, N.; Kawana, M.; et al. Cancer antigen-125 plasma level as a biomarker of new-onset atrial fibrillation in postmenopausal women. Heart 2017, 103, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Arbault-Biton, C.; Chenevier-Gobeaux, C.; Legallois, D.; Msadek, S.; Boubaya, M.; Roule, V.; Boukertouta, T.; Goudot, F.-X.; Beygui, F.; Meune, C. Multiple biomarkers measurement to estimate the duration of atrial fibrillation. Ann. Clin. Biochem. 2021, 58, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Dudink, E.A.; Weijs, B.; Tull, S.; Luermans, J.G.; Fabritz, L.; Chua, W.; Rienstra, M.; Van Gelder, I.C.; Schotten, U.; Kirchhof, P.; et al. The Biomarkers NT-proBNP and CA-125 are Elevated in Patients with Idiopathic Atrial Fibrillation. J. Atr. Fibrillation 2018, 11, 2058. [Google Scholar] [CrossRef]
- DiBaise, J.K.; Donovan, J.P. Markedly elevated CA125 in hepatic cirrhosis: Two case illustrations and review of the literature. J. Clin. Gastroenterol. 1999, 28, 159–161. [Google Scholar] [CrossRef]
- Rubin, J.; Rockey, D.C. Cirrhotic ascites, ovarian carcinoma, and CA-125. South. Med. J. 1999, 92, 248–250. [Google Scholar] [CrossRef]
- Collazos, J.; Genolla, J.; Ruibal, A. CA 125 serum levels in patients with non-neoplastic liver diseases. A clinical and laboratory study. Scand. J. Clin. Lab. Investig. 1992, 52, 201–206. [Google Scholar] [CrossRef]
- Qureshi, M.O.; Dar, F.S.; Khokhar, N. Cancer Antigen-125 as a marker of ascites in patients with liver cirrhosis. J. Coll. Physicians Surg. Pak. 2014, 24, 232–235. [Google Scholar]
- Bergmann, J.F.; Beaugrand, M.; Labadie, H.; Bidart, J.M.; Bohuon, C. CA 125 (ovarian tumour-associated antigen) in ascitic liver diseases. Clin. Chim. Acta 1986, 155, 163–165. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Xiubin, Z.; Wei, H.; Chenghao, G. Cancer antigen-125 and ICAM-1 are together responsible for ascites in liver cirrhosis. Clin. Lab. 2014, 60, 653–658. [Google Scholar] [CrossRef]
- Aguilar-Reina, J.; Rey-Romero, C.; Ortega-Viñas, M.; Hernandez-Pascual, A.; Sayago-Mota, M. Cancer antigen 125 levels in serum can predict the recurrence of ascites in patients with cirrhosis of the liver. Hepatogastroenterology 1990, 37 (Suppl. S2), 163–165. [Google Scholar]
- Trape, J.; Gurt, G.; Franquesa, J.; Montesinos, J.; Arnau, A.; Sala, M.; Sant, F.; Casado, E.; Ordeig, J.M.; Bergos, C.; et al. Diagnostic Accuracy of Tumor Markers CYFRA21-1 and CA125 in the Differential Diagnosis of Ascites. Anticancer. Res. 2015, 35, 5655–5660. [Google Scholar] [PubMed]
- Devarbhavi, H.; Kaese, D.; Williams, A.W.; Rakela, J.; Klee, G.G.; Kamath, P.S. Cancer antigen 125 in patients with chronic liver disease. Mayo Clin. Proc. 2002, 77, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Lander, E.; Karachristos, A.; Daly, E.; Dowling, P.; Patel, V.; Maloo, M.; Jain, A. Elevation of CA 125 and CA 19-9 in patients with end-stage liver disease. Int. J. Biol. Markers 2012, 27, e147–e151. [Google Scholar] [CrossRef] [PubMed]
- Baskiran, D.Y.; Sarigoz, T.; Baskiran, A.; Yilmaz, S. The Significance of Serum Tumor Markers CEA, Ca 19-9, Ca 125, Ca 15-3, and AFP in Patients Scheduled for Orthotopic Liver Transplantation: Do Elevated Levels Really Mean Malignancy? J. Gastrointest. Cancer 2023, 54, 442–446. [Google Scholar] [CrossRef]
- Li, Q.; Tian, B. Case report: Three cases of systemic lupus erythematosus presenting primarily with massive ascites and significantly elevated CA-125 levels and a review of pseudo-pseudo Meigs’ syndrome in literature. Front. Immunol. 2024, 15, 1423631. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef]
- Biggins, S.W.; Angeli, P.; Garcia-Tsao, G.; Ginès, P.; Ling, S.C.; Nadim, M.K.; Wong, F.; Kim, W.R. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1014–1048. [Google Scholar] [CrossRef]
- Xu, X.; Ding, H.; Jia, J.; Wei, L.; Duan, Z.; Tang, C.; Linghu, E.; Nan, Y.; Han, Y.; Xu, J.; et al. Chinese guidelines on the management of ascites in cirrhosis: Chinese Society of Hepatology, Chinese Medical Association. Hepatol. Int. 2024, 18, 1071–1089. [Google Scholar] [CrossRef]
- Mandorfer, M.; Aigner, E.; Cejna, M.; Ferlitsch, A.; Datz, C.; Gräter, T.; Graziadei, I.; Gschwantler, M.; Hametner-Schreil, S.; Hofer, H.; et al. Austrian consensus on the diagnosis and management of portal hypertension in advanced chronic liver disease (Billroth IV). Wien. Klin. Wochenschr. 2023, 135 (Suppl. S3), 493–523. [Google Scholar] [CrossRef]
- Sarin, S.K.; Choudhury, A.; Sharma, M.K.; Maiwall, R.; Al Mahtab, M.; Rahman, S.; Saigal, S.; Saraf, N.; Soin, A.S.; Devarbhavi, H.; et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver (APASL): An update. Hepatol. Int. 2019, 13, 353–390. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Cirrhosis in over 16s: Assessment and Management. (NICE Guideline NG50): 2023. Available online: https://www.nice.org.uk/guidance/ng50 (accessed on 22 April 2025).
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Ide, T.; Ito, H.; Kihara, Y.; Kinugawa, K.; Kinugawa, S.; Makaya, M.; Murohara, T.; Node, K.; Saito, Y.; et al. JCS/JHFS 2021 Guideline Focused Update on Diagnosis and Treatment of Acute and Chronic Heart Failure. Circ. J. 2021, 85, 2252–2291. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.-J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 American College of Cardiology/American Heart Association/Heart Failure Society of America Guideline for the Management of Heart Failure: Executive Summary. J. Card. Fail. 2022, 28, 810–830. [Google Scholar] [CrossRef]
- Kittleson, M.M.; Breathett, K.; Ziaeian, B.; Aguilar, D.; Blumer, V.; Bozkurt, B.; Diekemper, R.L.; Dorsch, M.P.; Heidenreich, P.A.; Jurgens, C.Y.; et al. 2024 Update to the 2020 ACC/AHA Clinical Performance and Quality Measures for Adults with Heart Failure: A Report of the American Heart Association/American College of Cardiology Joint Committee on Performance Measures. Circ. Cardiovasc. Qual. Outcomes 2024, 17, e000132. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Kostopoulou, M.; Andersen, J.; Aringer, M.; Arnaud, L.; Bae, S.-C.; Boletis, J.; Bruce, I.N.; Cervera, R.; Doria, A.; et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann. Rheum. Dis. 2024, 83, 15–29. [Google Scholar] [CrossRef]
- Gordon, C.; Amissah-Arthur, M.-B.; Gayed, M.; Brown, S.; Bruce, I.N.; D’cRuz, D.; Empson, B.; Griffiths, B.; Jayne, D.; Khamashta, M.; et al. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology 2018, 57, e1–e45. [Google Scholar] [CrossRef]
- Keeling, S.O.; Alabdurubalnabi, Z.; Avina-Zubieta, A.; Barr, S.; Bergeron, L.; Bernatsky, S.; Bourre-Tessier, J.; Clarke, A.; Baril-Dionne, A.; Dutz, J.; et al. Canadian Rheumatology Association Recommendations for the Assessment and Monitoring of Systemic Lupus Erythematosus. J. Rheumatol. 2018, 45, 1426–1439. [Google Scholar] [CrossRef]
- Mok, C.C.; Hamijoyo, L.; Kasitanon, N.; Chen, D.Y.; Chen, S.; Yamaoka, K.; Oku, K.; Li, M.T.; Zamora, L.; Bae, S.-C.; et al. The Asia-Pacific League of Associations for Rheumatology consensus statements on the management of systemic lupus erythematosus. Lancet Rheumatol. 2021, 3, e517–e531. [Google Scholar] [CrossRef]
- Tuersun, R.; Abudouwayiti, A.; Li, Y.X.; Pan, Y.; Aimaier, S.; Wen, Z.-Y.; Gao, W.-T.; Ma, L.-J.; Mahemuti, A.; Zheng, Y.-Y. Serum CA125: A prognostic biomarker for mortality in chronic heart failure. BMC Cardiovasc. Disord. 2025, 25, 227. [Google Scholar] [CrossRef]
- Miñana, G.; de la Espriella, R.; Palau, P.; Llácer, P.; Núñez, E.; Santas, E.; Valero, E.; Lorenzo, M.; Núñez, G.; Bodí, V.; et al. Carbohydrate antigen 125 and risk of heart failure readmissions in patients with heart failure and preserved ejection fraction. Sci. Rep. 2022, 12, 1344. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Muñoz, E.; Pineda, M.A.G.; Parra, C.A.; Castillo, C.A.V.; Marrugo, V.O.; Jassir, J.B.; Nieto, J.F.P.; Parra-Medina, R.; Rojas-Villarraga, A. Is there any relationship between massive ascites and elevated CA-125 in systemic lupus erythematosus? Case report and review of the literature. Mod. Rheumatol. Case Rep. 2021, 5, 292–299. [Google Scholar] [CrossRef]
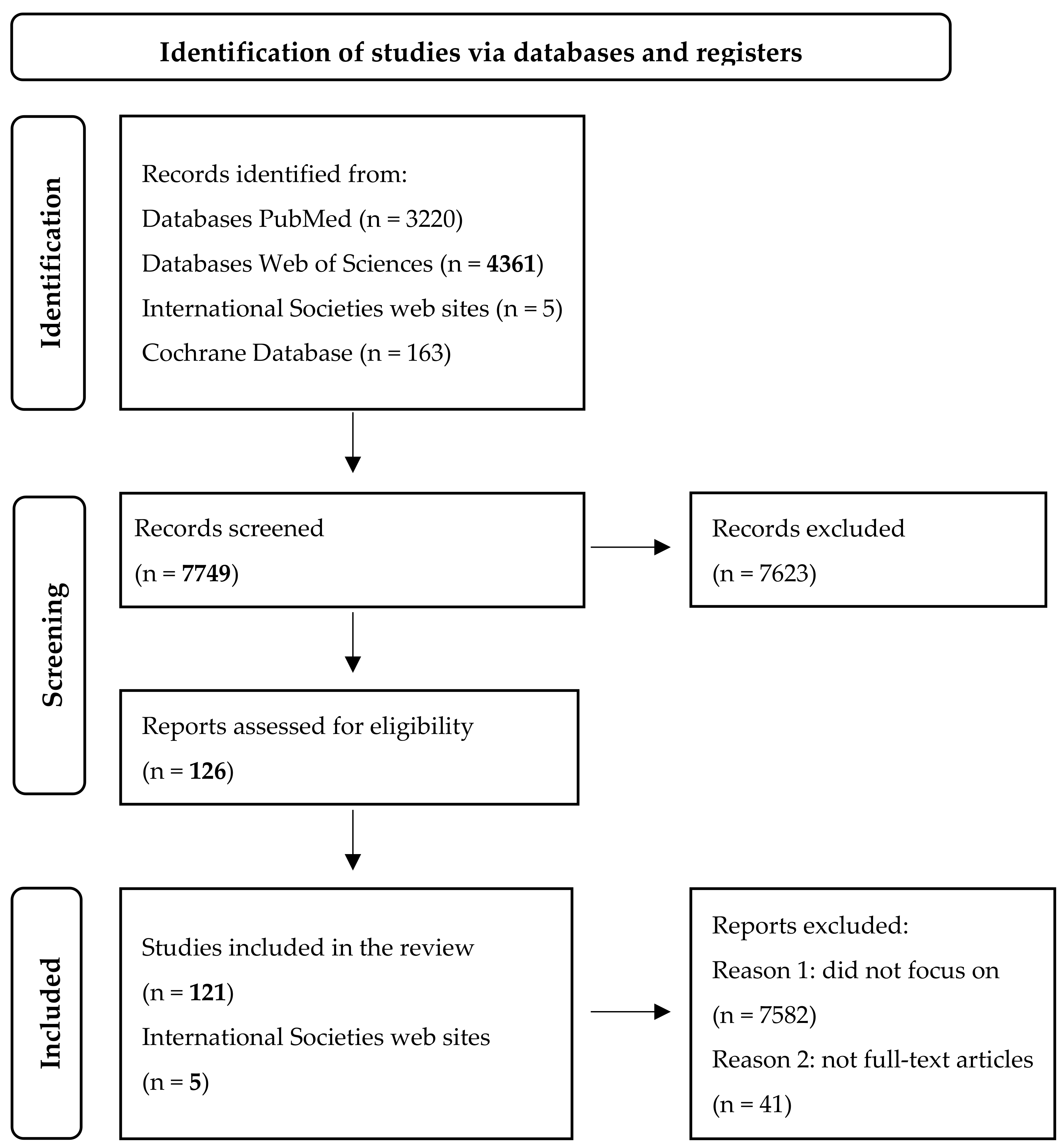
| Author (Year) | Study Design | Population | Condition | CA125 Context | Key Findings |
|---|---|---|---|---|---|
| Núñez et al. (2021) [22] | Review | Acute heart failure patients | Heart failure | Serum CA125 < 23 U/mL | Monitoring and treatment guidance |
| Zuckerman et al. (1999) [23] | Observational | Patients with cirrhotic ascites | Liver cirrhosis | Serum CA125: mean 321 U/mL | Higher ascitic CA125 than serum; related to fluid overload |
| Cheng et al. (2015) [24] | Prospective | Patients with Budd-Chiari syndrome | Budd–Chiari syndrome | Serum CA125 associated with ascites volume | Correlated with prognosis and recurrence risk |
| Edula et al. (2018) [25] | Retrospective | Cirrhosis with ascites | Liver cirrhosis | Serum CA125 linked to fluid volume | Correlated with a degree of decompensation |
| Oliveira Júnior et al. (2021) [26] | Observational | Patients on peritoneal dialysis | CKD/Peritoneal dialysis | CA125 in dialysate | Marker for peritoneal inflammation and fibrosis risk |
| Trapé et al. (2024) [9] | Review | Various non-malignant conditions | Endometriosis, gynecologic disease, effusions, cardiovascular disease | Serum CA125 > 10× normal in some benign states | Marked elevation is possible even in benign gynecologic cases |
| Zhong et al. (2024) [27] | Retrospective | Patients with systemic lupus erythematosus | SLE | Associated with pleurisy and ascites | Reflects serosal involvement in active disease |
| Hung et al. (2012) [28] | Prospective | Women with HFpEF | Heart failure with preserved EF | Linked to left atrial volume | Predictor of hospitalization and congestion severity |
| Guidelines | Year | References |
|---|---|---|
| EASL | 2018 | [109] |
| AASLD | 2021 | [110] |
| CMA | 2024 | [111] |
| Austrian Consensus | 2023 | [112] |
| APASL | 2019 | [113] |
| NICE | 2023 | [114] |
| ESC | 2021 | [115] |
| ESC | 2023 | [116] |
| JCS/JHFS | 2021 | [117] |
| ACC/AHA | 2022 | [118] |
| ACC/AHA | 2024 | [119] |
| EULAR | 2023 | [120] |
| BSR | 2018 | [121] |
| CRA | 2018 | [122] |
| Asia-Pacific | 2021 | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bălăceanu, L.A.; Grigore, C.; Dina, I.; Gurău, C.-D.; Mihai, M.M.; Bălăceanu-Gurău, B. CA125 as a Potential Biomarker in Non-Malignant Serous Effusions: Diagnostic and Prognostic Considerations. J. Clin. Med. 2025, 14, 4152. https://doi.org/10.3390/jcm14124152
Bălăceanu LA, Grigore C, Dina I, Gurău C-D, Mihai MM, Bălăceanu-Gurău B. CA125 as a Potential Biomarker in Non-Malignant Serous Effusions: Diagnostic and Prognostic Considerations. Journal of Clinical Medicine. 2025; 14(12):4152. https://doi.org/10.3390/jcm14124152
Chicago/Turabian StyleBălăceanu, Lavinia Alice, Cristiana Grigore, Ion Dina, Cristian-Dorin Gurău, Mara Mădălina Mihai, and Beatrice Bălăceanu-Gurău. 2025. "CA125 as a Potential Biomarker in Non-Malignant Serous Effusions: Diagnostic and Prognostic Considerations" Journal of Clinical Medicine 14, no. 12: 4152. https://doi.org/10.3390/jcm14124152
APA StyleBălăceanu, L. A., Grigore, C., Dina, I., Gurău, C.-D., Mihai, M. M., & Bălăceanu-Gurău, B. (2025). CA125 as a Potential Biomarker in Non-Malignant Serous Effusions: Diagnostic and Prognostic Considerations. Journal of Clinical Medicine, 14(12), 4152. https://doi.org/10.3390/jcm14124152








