Impact of Polyvascular Disease on Long-Term Prognosis of Patients with Acute Coronary Syndrome—A Retrospective Cohort Study in Italy
Abstract
1. Introduction
2. Material and Methods
2.1. Outcomes
2.2. Statistical Analysis
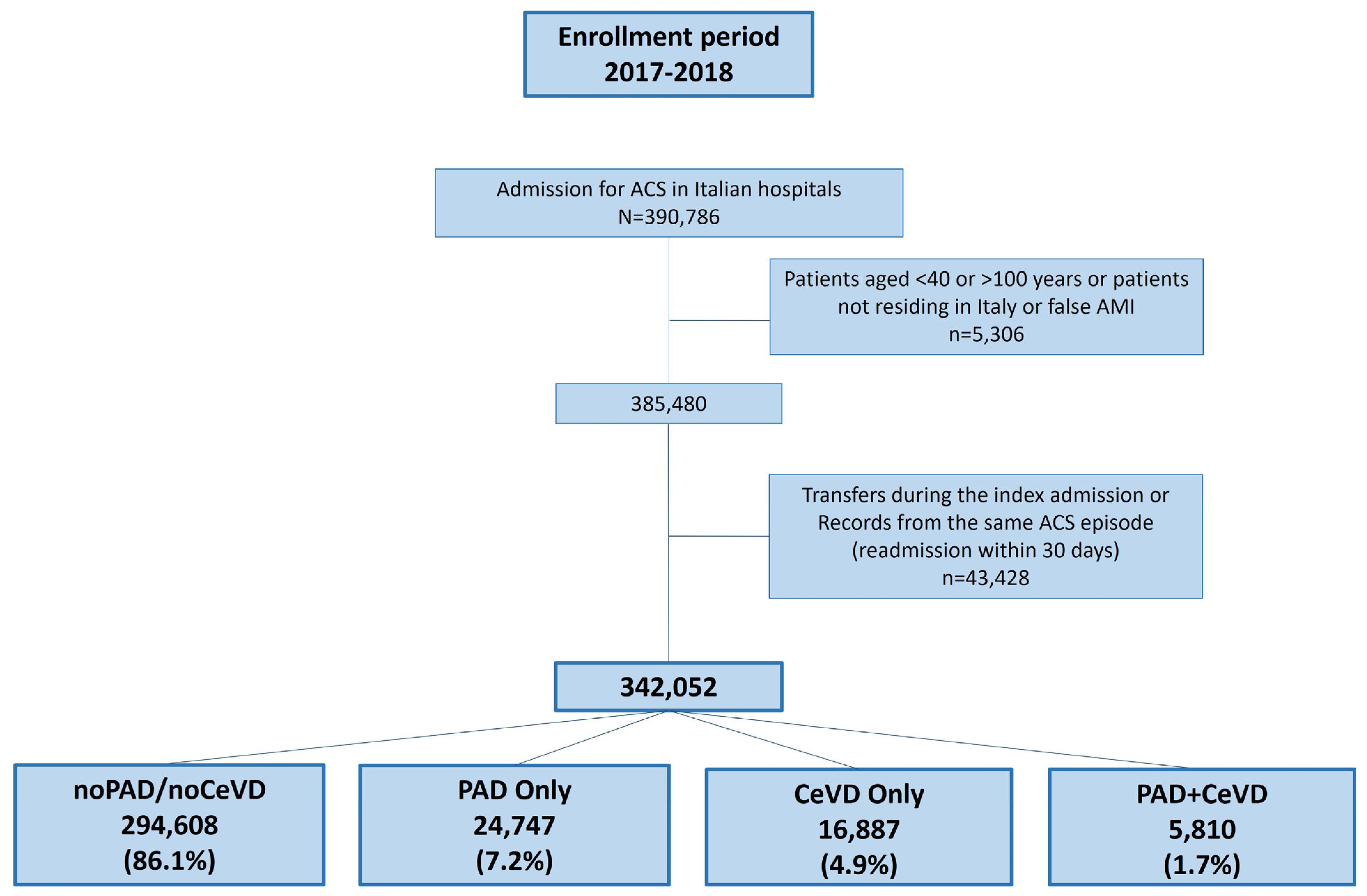
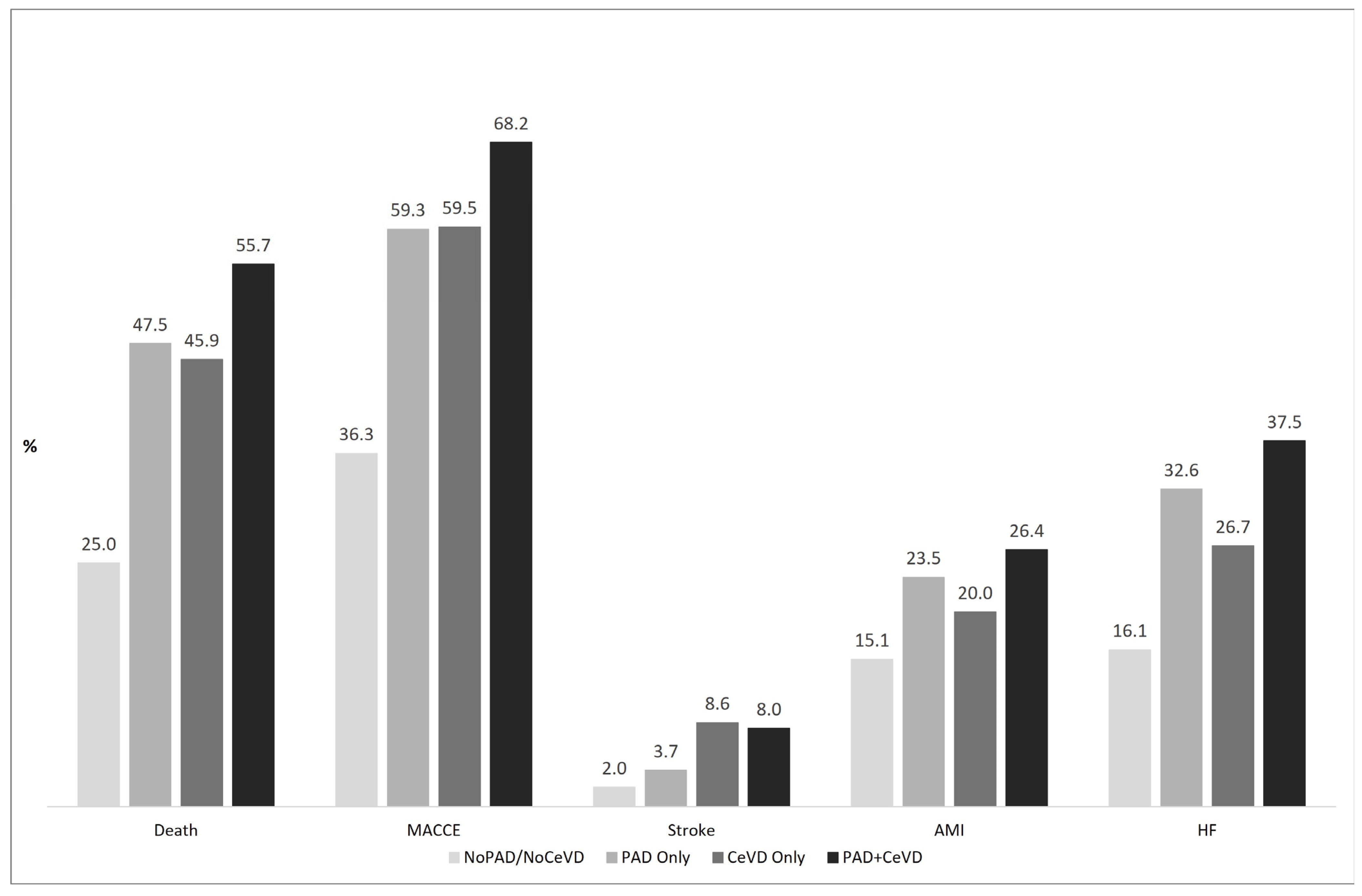
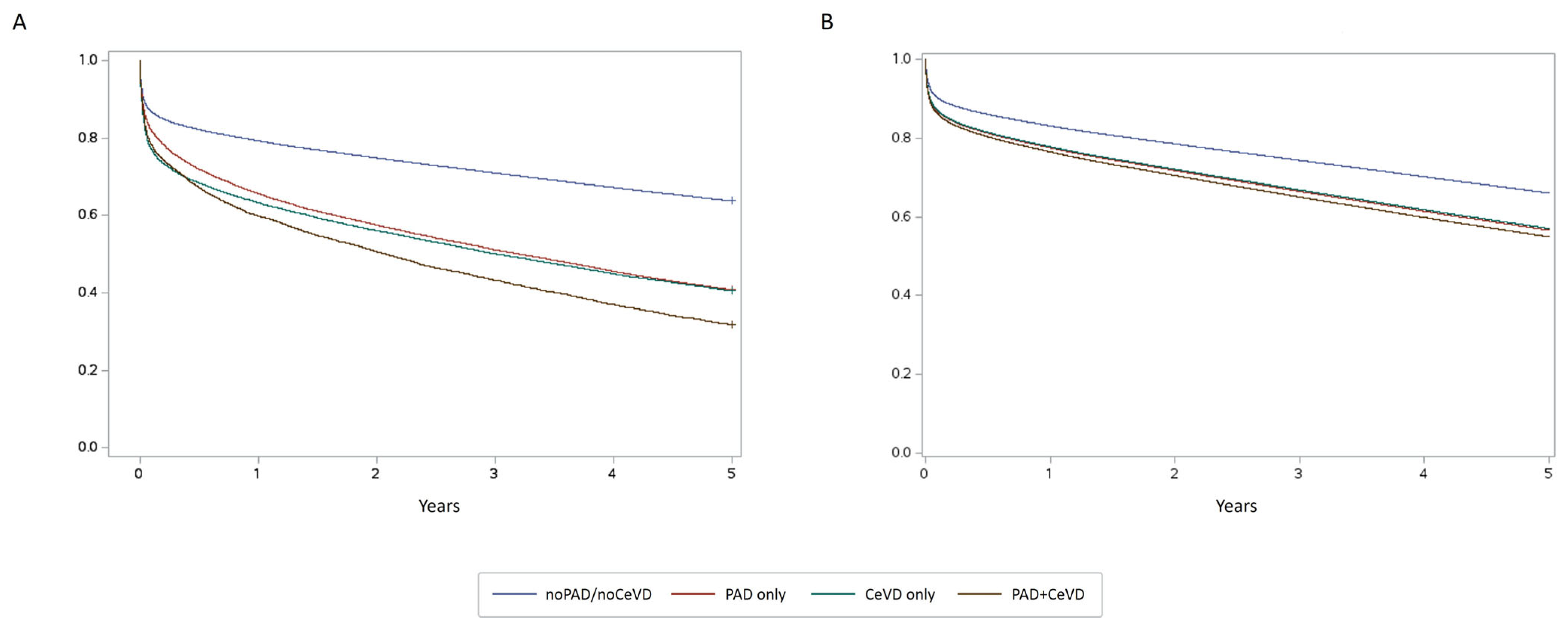
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.; Aboyans, V.; Fowkes, F.J.; McDermott, M.M.; Sampson, U.K.; Criqui, M.H. Peripheral artery disease: Epidemiology and global perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170. [Google Scholar] [CrossRef]
- Alberts, M.J.; Bhatt, D.L.; Mas, J.L.; Ohman, E.M.; Hirsch, A.T.; Röther, J.; Salette, G.; Goto, S.; Smith, S.C., Jr.; Liau, C.S.; et al. Three-year follow-up and event rates in the international REduction of Atherothrombosis for Continued Health Registry. Eur. Heart J. 2009, 30, 2318–2326. [Google Scholar] [CrossRef]
- Kobo, O.; Contractor, T.; Mohamed, M.O.; Parwani, P.; Paul, T.K.; Ghosh, R.K.; Alraes, M.C.; Patel, B.; Osman, M.; Ludwig, J.; et al. Impact of pre-existent vascular and poly-vascular disease on acute myocardial infarction management and outcomes: An analysis of 2 million patients from the National Inpatient Sample. Int. J. Cardiol. 2021, 327, 1–8. [Google Scholar] [CrossRef]
- Kalbaugh, C.A.; Kucharska-Newton, A.; Wruck, L.; Lund, J.L.; Selvin, E.; Matsushita, K.; Bengtson, L.G.S.; Heiss, G.; Loehr, L. Peripheral Artery Disease Prevalence and Incidence Estimated from Both Outpatient and Inpatient Settings Among Medicare Fee-for-Service Beneficiaries in the Atherosclerosis Risk in Communities (ARIC) Study. J. Am. Heart Assoc. 2017, 6, e003796. [Google Scholar] [CrossRef]
- Ovbiagele, B.; Goldstein, L.B.; Higashida, R.T.; Howard, V.J.; Johnston, S.C.; Khavjou, O.A.; Lackland, D.T.; Lichtman, J.H.; Mohl, S.; Sacco, R.L.; et al. Forecasting the future of stroke in the United States: A policy statement from the American Heart Association and American Stroke Association. Stroke 2013, 44, 2361–2375, Erratum in Stroke 2015, 46, e179. [Google Scholar] [CrossRef]
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Wafa, H.A.; Wolfe, C.D.A.; Emmett, E.; Roth, G.A.; Johnson, C.O.; Wang, Y. Burden of Stroke in Europe. Thirty-Year Projections of Incidence, Prevalence, Deaths, and Disability-Adjusted Life Years. Stroke 2020, 51, 2418–2427. [Google Scholar] [CrossRef]
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; Hess, C.N.; Hicks, C.W.; Kwan, T.W.; McDermott, M.M.; Misra, S.; Ujueta, F. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e171–e191. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the global burden of cardiovascular disease, part 1: The epidemiology and risk factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Makhamreh, H.; Qoussoos, T.; Alawwa, I.; Alsmady, M.; Salah, Z.A.; Shakhatreh, A.; Alhazaymeh, L.; Jabber, M. Prevalence of previously unrecognized peripheral arterial disease in patients undergoing coronary angiography. Medicine 2018, 97, e11519. [Google Scholar] [CrossRef] [PubMed]
- Bashar, H.; Matetić, A.; Curzen, N.; Mamas, M.A. Impact of extracardiac vascular disease on outcomes of 1.4 million patients undergoing percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2022, 100, 737–746. [Google Scholar] [CrossRef]
- Kobo, O.; Saada, M.; Von Birgelen, C.; Tonino, P.A.; Íñiguez-Romo, A.; Fröbert, O.; Halabi, M.; Oemrawsingh, R.M.; Polad, J.; IJsselmuiden, A.J.; et al. Impact of multisite artery disease on clinical outcomes after percutaneous coronary intervention: An analysis from the e-Ultimaster registry. Eur. Heart J. Qual. Care Clin. Outcomes 2023, 9, 417–426. [Google Scholar] [CrossRef]
- Garg, S.; Chichareon, P.; Kogame, N.; Takahashi, K.; Modolo, R.; Chang, C.C.; Tomaniak, M.; Fath-Ordoubadi, F.; Anderson, R.; Oldroyd, K.G.; et al. Impact of established cardiovascular disease on outcomes in the randomized global leaders trial. Catheter. Cardiovasc. Interv. 2020, 96, 1369–1378. [Google Scholar] [CrossRef]
- Wang, R.; Garg, S.; Gao, C.; Kawashima, H.; Ono, M.; Hara, H.; Van Geuns, R.J.; Morice, M.C.; Davierwala, P.M.; Kappetein, A.P.; et al. Impact of established cardiovascular disease on 10-year death after coronary revascularization for complex coronary artery disease. Clin. Res. Cardiol. 2021, 110, 1680–1691. [Google Scholar] [CrossRef]
- Adam, L.; Strickler, E.; Borozadi, M.K.; Bein, S.; Bano, A.; Muka, T.; Drexel, H.; Dopheide, J.F. Prognostic Role of Polyvascular Involvement in Patients with Symptomatic Peripheral Artery Disease. J. Clin. Med. 2023, 12, 3410. [Google Scholar] [CrossRef]
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002, 324, 71–86. [Google Scholar] [CrossRef]
- Diehm, C.; Lange, S.; Darius, H.; Pittrow, D.; Von Stritzky, B.; Tepohl, G.; Haberl, R.L.; Allenberg, J.R.; Dasch, B.; Trampisch, H.J. Association of low ankle brachial index with high mortality in primary care. Eur. Heart J. 2006, 27, 1743–1749. [Google Scholar] [CrossRef]
- Alkhalil, M.; Kuzemczak, M.; Whitehead, N.; Kavvouras, C.; Džavík, V. Meta-Analysis of Intensive Lipid-Lowering Therapy in Patients with Polyvascular Disease. J. Am. Heart Assoc. 2021, 10, e017948. [Google Scholar] [CrossRef] [PubMed]
- Jukema, J.W.; Szarek, M.; Zijlstra, L.E.; de Silva, H.A.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; et al. Alirocumab in Patients With Polyvascular Disease and Recent Acute Coronary Syndrome ODYSSEY OUTCOMES Trial. J. Am. Coll. Cardiol. 2019, 74, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Oyama, K.; Giugliano, R.P.; Tang, M.; Bonaca, M.P.; Saver, J.L.; Murphy, S.A.; Ruzza, A.; Keech, A.C.; Sever, P.S.; Sabatine, M.S.; et al. Effect of evolocumab on acute arterial events across all vascular territories: Results from the FOURIER trial. Eur. Heart J. 2021, 42, 4821–4829. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef]
- Connolly, S.J.; Eikelboom, J.W.; Bosch, J.; Dagenais, G.; Dyal, L.; Lanas, F.; Metsarinne, K.; O’Donnell, M.; Dans, A.L.; Ha, J.W.; et al. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: An international, randomised, double-blind, placebo-controlled trial. Lancet 2018, 391, 205–218. [Google Scholar] [CrossRef]
- Anand, S.S.; Bosch, J.; Eikelboom, J.W.; Connolly, S.J.; Diaz, R.; Widimsky, P.; Aboyans, V.; Alings, M.; Kakkar, A.K.; Keltai, K.; et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: An international, randomised, double-blind, placebo-controlled trial. Lancet 2018, 391, 219–229. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for themanagement of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Aboyans, V.; Bauersachs, R.; Mazzolai, L.; Brodmann, M.; Palomares, J.F.R.; Debus, S.; Collet, J.P.; Drexel, H.; Espinola-Klein, C.; Lewis, B.S.; et al. Antithrombotic therapies in aortic and peripheral arterial diseases in 2021: A consensus document from the ESC working group on aorta and peripheral vascular diseases, the ESC working group on thrombosis, and the ESC working group on cardiovascular pharmacotherapy. Eur. Heart J. 2021, 42, 4013–4024. [Google Scholar]
- Byrne, R.A.; Rossello, X.; Coughlan, J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Visseren, F.L.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar]
- Ambrosetti, M.; Faggiano, P.; Greco, C.; Mureddu, G.F.; Temporelli, P.L.; Pedretti, R.F.E. Referral from vascular surgery to cardiovascular rehabilitation and related outcomes in patients with peripheral arterial disease:the THINKPAD-RELOADED survey. Monaldi Arch. Chest Dis. 2019, 89, 1101. [Google Scholar] [CrossRef]
- Taylor, R.S.; Dalal, H.M.; McDonagh, S.T.J. The role of cardiac rehabilitation in improving cardiovascular outcomes. Nat. Rev. Cardiol. 2022, 19, 180–194. [Google Scholar] [CrossRef]
| PVD Categories | TOT (n = 342,052) | |||||
|---|---|---|---|---|---|---|
| noPAD/noCeVD (n = 294,608) | PAD Only (n = 24,747) | CeVD Only (n = 16,887) | PAD+CeVD (n = 5810) | p-Value | ||
| Age | 69.3 ± 12.3 | 72.5 ± 10.4 | 75.3 ± 10.4 | 74.5 ± 8.9 | <0.0001 | 70 ± 12.1 |
| Female gender | 93,866 (31.9) | 6909 (27.9) | 6194 (36.7) | 1655 (28.5) | <0.0001 | 108,624 (31.8) |
| Diabetes | 21,211 (7.2) | 7262 (29.3) | 3291 (19.5) | 2281 (39.3) | <0.0001 | 34,045 (10.0) |
| Obesity | 3611 (1.2) | 856 (3.5) | 323 (1.9) | 175 (3.0) | <0.0001 | 4965 (1.5) |
| Hypertension | 39,895 (13.5) | 8660 (35.0) | 5996 (35.5) | 2955 (50.9) | <0.0001 | 57,506 (16.8) |
| COPD | 18,797 (6.4) | 3549 (14.3) | 2069 (12.3) | 1133 (19.5) | <0.0001 | 25,548 (7.5) |
| Previous AMI | 38,734 (13.1) | 6446 (26.0) | 3273 (19.4) | 1751 (30.1) | <0.0001 | 50,204 (14.7) |
| Previous other chronic coronary syndromes | 46,045 (15.6) | 9446 (38.2) | 4718 (27.9) | 2709 (46.6) | <0.0001 | 62,918 (18.4) |
| Previous CABG | 17,383 (5.9) | 3438 (13.9) | 1763 (10.4) | 1004 (17.3) | <0.0001 | 23,588 (6.9) |
| Previous PCI | 56,406 (19.1) | 7747 (31.3) | 3706 (21.9) | 1916 (33.0) | <0.0001 | 69,775 (20.4) |
| Previous cardiac surgery other than CABG | 4090 (1.4) | 808 (3.3) | 376 (2.2) | 180 (3.1) | <0.0001 | 5454 (1.6) |
| Heart failure | 48,350 (16.4) | 7890 (31.9) | 4597 (27.2) | 2090 (36.0) | <0.0001 | 62,927 (18.4) |
| Rheumatic heart disease | 4930 (1.7) | 730 (2.9) | 541 (3.2) | 206 (3.5) | <0.0001 | 6407 (1.9) |
| Cardiomyopathy | 6786 (2.3) | 1180 (4.8) | 578 (3.4) | 309 (5.3) | <0.0001 | 8853 (2.6) |
| Arrhythmias | 58,335 (19.8) | 6598 (26.7) | 5461 (32.3) | 1848 (31.8) | <0.0001 | 72,242 (21.1) |
| Other chronic heart diseases | 9812 (3.3) | 1878 (7.6) | 1068 (6.3) | 506 (8.7) | <0.0001 | 13,264 (3.9) |
| Endocarditis and acute myocarditis | 3857 (1.3) | 704 (2.8) | 329 (1.9) | 153 (2.6) | <0.0001 | 5043 (1.5) |
| Anaemia | 15,754 (5.3) | 3397 (13.7) | 1696 (10.0) | 942 (16.2) | <0.0001 | 21,789 (6.4) |
| Malignant neoplasms | 17,589 (6.0) | 2202 (8.9) | 1348 (8.0) | 493 (8.5) | <0.0001 | 21,632 (6.3) |
| Chronic Kidney Diseases | 26,083 (8.9) | 6705 (27.1) | 3013 (17.8) | 1820 (31.3) | <0.0001 | 37,621 (11.0) |
| Coagulation disorders | 350 (0.1) | 57 (0.2) | 49 (0.3) | 16 (0.3) | <0.0001 | 472 (0.1) |
| Other chronic diseases (liver, pancreas, bowel) | 385 (0.1) | 72 (0.3) | 46 (0.3) | 24 (0.4) | <0.0001 | 527 (0.2) |
| Diagnosis in index admission | ||||||
| AMI | 172,658 (58.6) | 14,286 (57.7) | 10,774 (63.8) | 3551 (61.1) | <0.0001 | 201,269 (58.8) |
| Angina pectoris | 61,664 (20.9) | 4416 (17.8) | 2594 (15.4) | 906 (15.6) | 69,580 (20.3) | |
| Other acute and subacute forms of ischemic heart disease | 60,286 (20.5) | 6045 (24.4) | 3519 (20.8) | 1353 (23.3) | 71,203 (20.8) | |
| HR | 95% CI | p-Value | ||
|---|---|---|---|---|
| PAD only | 1.375 | 1.351 | 1.400 | <0.0001 |
| CeVD only | 1.357 | 1.329 | 1.386 | <0.0001 |
| PAD+CeVD | 1.449 | 1.402 | 1.496 | <0.0001 |
| Female gender | 0.952 | 0.941 | 0.963 | <0.0001 |
| Age | 1.043 | 1.042 | 1.043 | <0.0001 |
| Malignant neoplasms | 1.397 | 1.371 | 1.422 | <0.0001 |
| Diabetes | 1.197 | 1.177 | 1.217 | <0.0001 |
| Obesity | 1.107 | 1.063 | 1.153 | <0.0001 |
| Anaemia | 1.274 | 1.251 | 1.296 | <0.0001 |
| Previous AMI | 0.98 | 0.964 | 0.995 | 0.0118 |
| Previous other chronic coronary syndromes | 0.919 | 0.904 | 0.934 | <0.0001 |
| Heart failure | 1.557 | 1.537 | 1.577 | <0.0001 |
| Rheumatic heart disease | 1.153 | 1.118 | 1.189 | <0.0001 |
| Cardiomyopathy | 1.11 | 1.079 | 1.141 | <0.0001 |
| Arrhythmias | 1.255 | 1.24 | 1.27 | <0.0001 |
| COPD | 1.2 | 1.179 | 1.22 | <0.0001 |
| Chronic Kidney Diseases | 1.37 | 1.35 | 1.39 | <0.0001 |
| Other chronic diseases (liver, pancreas, bowel) | 1.306 | 1.258 | 1.357 | <0.0001 |
| Previous PCI | 1.343 | 1.322 | 1.364 | <0.0001 |
| Endocarditis and acute myocarditis | 1.242 | 1.111 | 1.387 | 0.0001 |
| Diagnosis in index admission: Other ischemic heart disease | Ref. | |||
| Angina pectoris | 1.542 | 1.509 | 1.575 | <0.0001 |
| AMI | 2.753 | 2.702 | 2.804 | <0.0001 |
| HR/SHR | 95% CI | ||
|---|---|---|---|
| All-Cause Death | |||
| PAD only | 1.476 | 1.446 | 1.506 |
| CeVD only | 1.295 | 1.265 | 1.326 |
| PAD+CeVD | 1.518 | 1.464 | 1.573 |
| Rehospitalization for HF | |||
| PAD only | 1.321 | 1.285 | 1.358 |
| CeVD only | 1.156 | 1.117 | 1.196 |
| PAD+CeVD | 1.336 | 1.27 | 1.405 |
| Rehospitalization for Ischaemic Stroke | |||
| PAD only | 1.541 | 1.427 | 1.665 |
| CeVD only | 3.836 | 3.601 | 4.085 |
| PAD+CeVD | 3.217 | 2.897 | 3.574 |
| Rehospitalization for AMI | |||
| PAD only | 1.274 | 1.237 | 1.312 |
| CeVD only | 1.184 | 1.141 | 1.227 |
| PAD+CeVD | 1.321 | 1.250 | 1.395 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mureddu, G.F.; Rosato, S.; D’Errigo, P.; Faggiano, P.; Ciccarelli, P.; Badoni, G.; Ambrosetti, M.; Fattirolli, F.; Baglio, G. Impact of Polyvascular Disease on Long-Term Prognosis of Patients with Acute Coronary Syndrome—A Retrospective Cohort Study in Italy. J. Clin. Med. 2025, 14, 4158. https://doi.org/10.3390/jcm14124158
Mureddu GF, Rosato S, D’Errigo P, Faggiano P, Ciccarelli P, Badoni G, Ambrosetti M, Fattirolli F, Baglio G. Impact of Polyvascular Disease on Long-Term Prognosis of Patients with Acute Coronary Syndrome—A Retrospective Cohort Study in Italy. Journal of Clinical Medicine. 2025; 14(12):4158. https://doi.org/10.3390/jcm14124158
Chicago/Turabian StyleMureddu, Gian Francesco, Stefano Rosato, Paola D’Errigo, Pompilio Faggiano, Paola Ciccarelli, Gabriella Badoni, Marco Ambrosetti, Francesco Fattirolli, and Giovanni Baglio. 2025. "Impact of Polyvascular Disease on Long-Term Prognosis of Patients with Acute Coronary Syndrome—A Retrospective Cohort Study in Italy" Journal of Clinical Medicine 14, no. 12: 4158. https://doi.org/10.3390/jcm14124158
APA StyleMureddu, G. F., Rosato, S., D’Errigo, P., Faggiano, P., Ciccarelli, P., Badoni, G., Ambrosetti, M., Fattirolli, F., & Baglio, G. (2025). Impact of Polyvascular Disease on Long-Term Prognosis of Patients with Acute Coronary Syndrome—A Retrospective Cohort Study in Italy. Journal of Clinical Medicine, 14(12), 4158. https://doi.org/10.3390/jcm14124158






