Emerging Age-Specific Therapeutic Approaches for Dry Eye Disease
Abstract
1. Introduction
2. Methods
3. Epidemiology
4. Symptoms
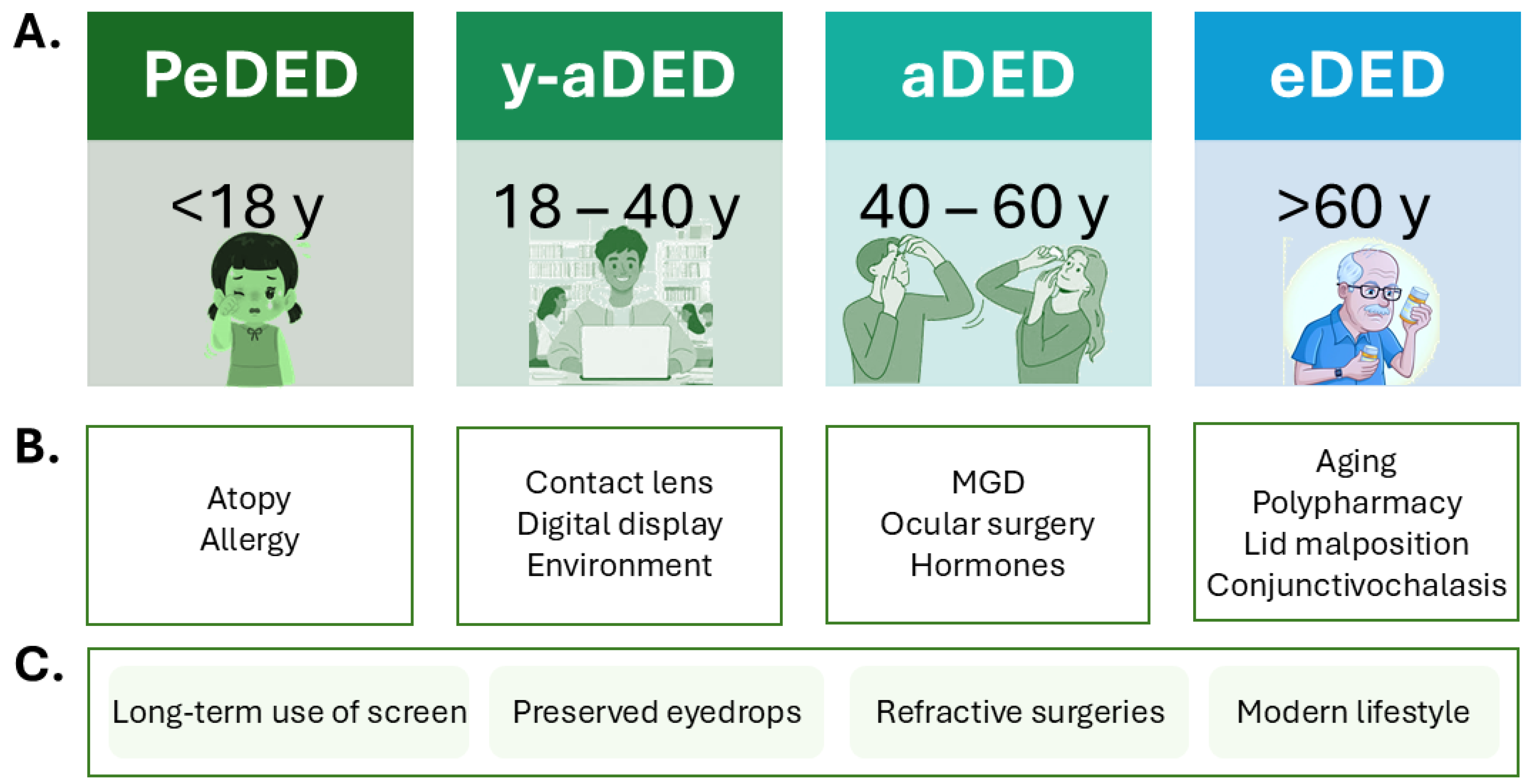
5. Risk Factors
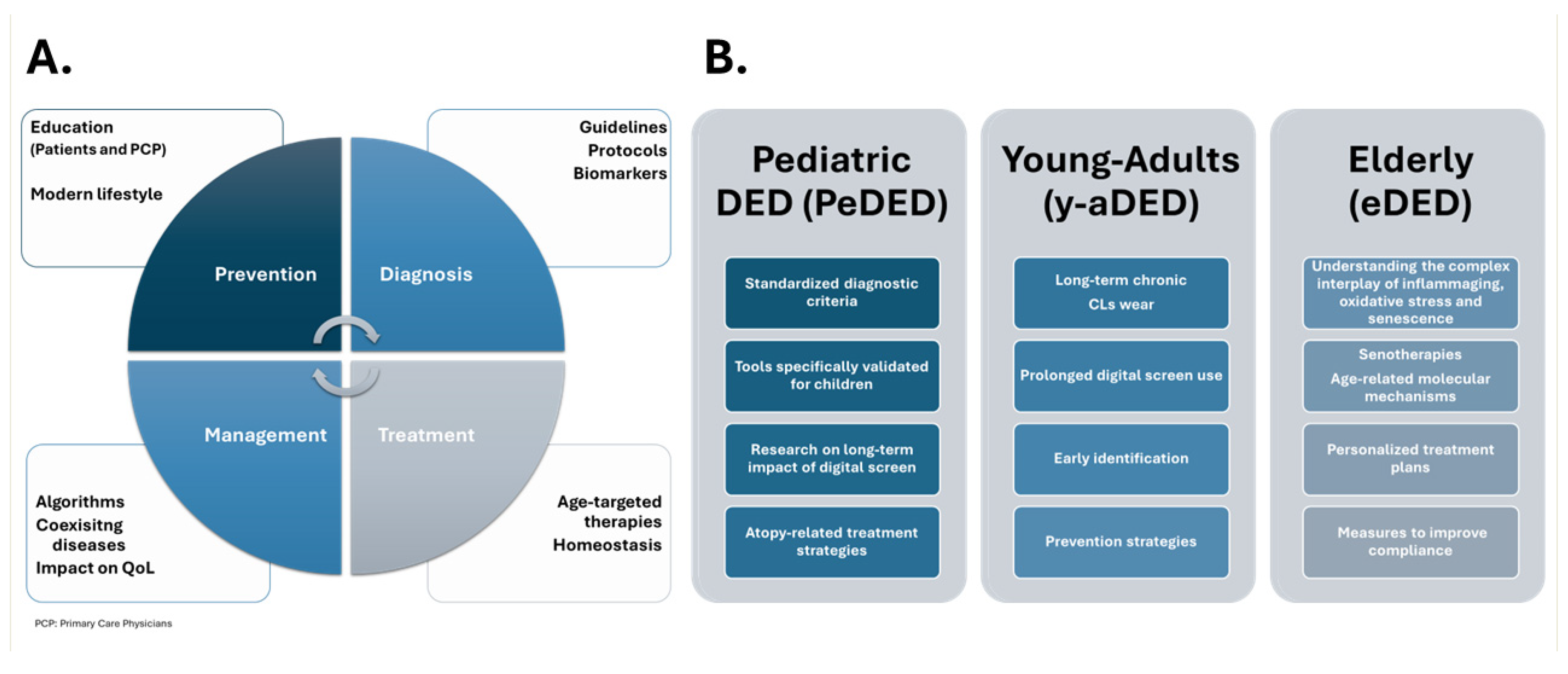
5.1. Pediatric Population
5.2. Risk Factors in Young Adults
5.3. Risk Factors in Elderly
6. Diagnosis
7. Pathophysiology
8. Coexisting Diseases
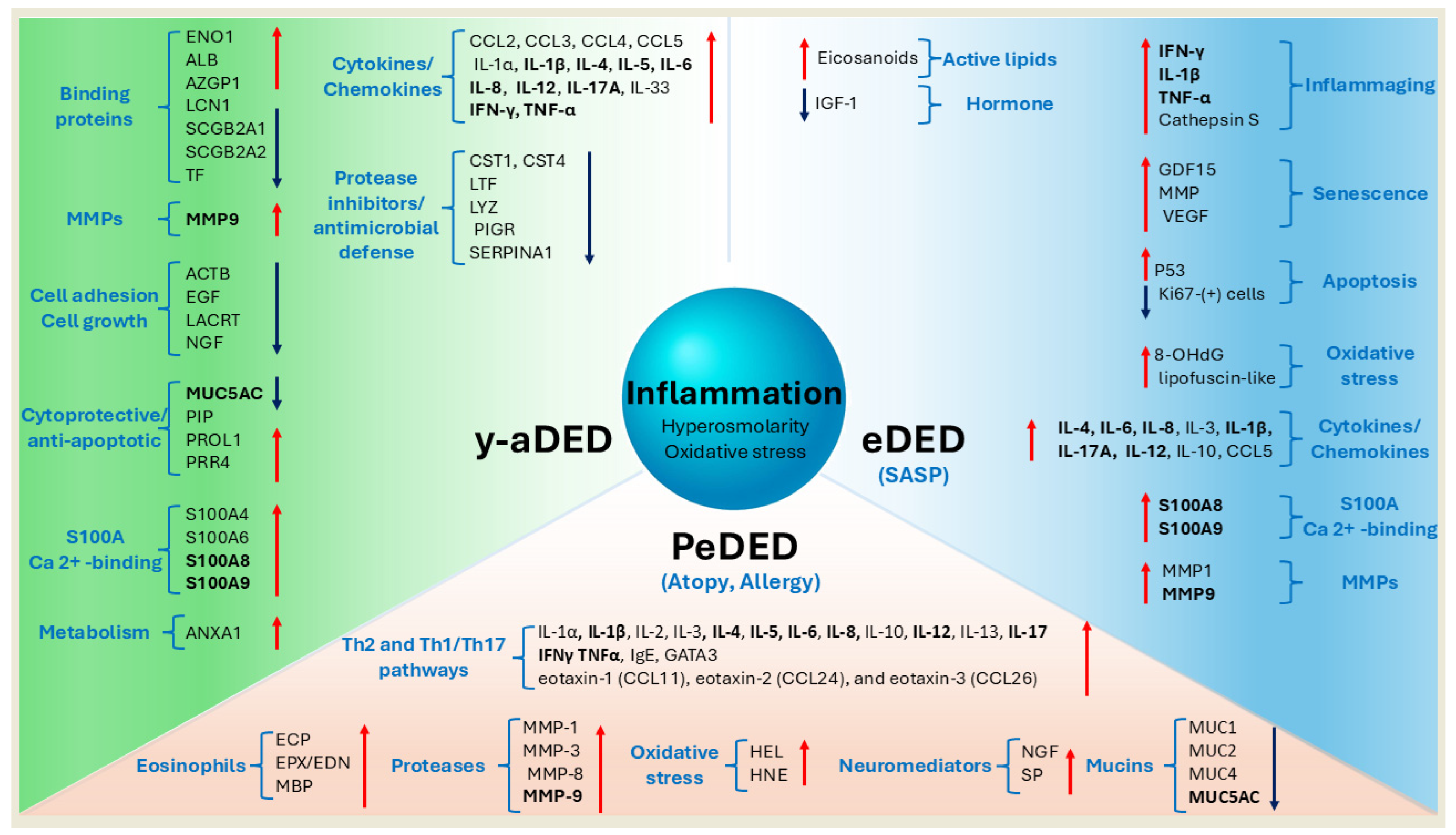
9. Molecular Mechanisms and Biomarkers
10. Treatments
10.1. Children
10.2. Young Adults
10.3. Elderly
11. Unmet Clinical Needs and Future Directions
12. Discussion
13. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Messmer, E.M.; Aragona, P.; Geerling, G.; Akova, Y.A.; Benítez-Del-Castillo, J.; Boboridis, K.G.; Merayo-Lloves, J.; Rolando, M.; Labetoulle, M. Revisiting the vicious circle of dry eye disease: A focus on the pathophysiology of meibomian gland dysfunction. Br. J. Ophthalmol. 2016, 100, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, K.; Singh, S.; Singh, K.; Kumar, S.; Dwivedi, K. Prevalence of dry eye, its categorization (Dry Eye Workshop II), and pathological correlation: A tertiary care study. Indian J. Ophthalmol. 2023, 71, 1454–1458. [Google Scholar] [CrossRef] [PubMed]
- Schaumberg, D.A.; Sullivan, D.A.; Buring, J.E.; Dana, M.R. Prevalence of dry eye syndrome among US women. Am. J. Ophthalmol. 2003, 136, 318–326. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Moon, J.H.; Kim, K.W.; Moon, N.J. Smartphone use is a risk factor for pediatric dry eye disease according to region and age: A case control study Pediatrics and Strabismus. BMC Ophthalmol. 2016, 16, 188. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Zheng, Y.; Deng, L.; Huang, X. Prevalence of dry eye disease in the elderly: A protocol of systematic review and meta-analysis. Medicine 2020, 99, e22234. [Google Scholar] [CrossRef]
- Wang, M.T.M.; Muntz, A.; Lim, J.; Kim, J.S.; Lacerda, L.; Arora, A.; Craig, J.P. Ageing and the natural history of dry eye disease: A prospective registry-based cross-sectional study. Ocul. Surf. 2020, 18, 736–741. [Google Scholar] [CrossRef]
- Zou, Y.; Li, D.; Gianni, V.; Congdon, N.; Piyasena, P.; Prakalapakorn, S.G.; Zhang, R.; Zhao, Z.; Chan, V.F.; Yu, M. Prevalence of dry eye disease among children: A systematic review and meta-analysis. BMJ Open Ophthalmol. 2025, 10, e002014. [Google Scholar] [CrossRef]
- Barabino, S. Is dry eye disease the same in young and old patients? A narrative review of the literature. BMC Ophthalmol. 2022, 22, 85. [Google Scholar] [CrossRef]
- Britten-Jones, A.C.; Wang, M.T.M.; Samuels, I.; Jennings, C.; Stapleton, F.; Craig, J.P. Epidemiology and Risk Factors of Dry Eye Disease: Considerations for Clinical Management. Medicina 2024, 60, 1458. [Google Scholar] [CrossRef] [PubMed]
- Yuanita, Y.; Ismail, I.S. The Analysis Study of Interventions of Dry Eye: A Comprehensive Systematic Review. Indones. J. Gen. Med. 2024, 5, 78–101. [Google Scholar] [CrossRef]
- Tellefsen Nøland, S.; Badian, R.A.; Utheim, T.P.; Utheim, Ø.A.; Stojanovic, A.; Tashbayev, B.; Raeder, S.; Dartt, D.A.; Chen, X. Sex and age differences in symptoms and signs of dry eye disease in a Norwegian cohort of patients. Ocul. Surf. 2021, 19, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Velez, F.G.; Lau, C.; Wolffsohn, J.S. Dry eye disease in the young: A narrative review. Ocul. Surf. 2024, 31, 11–20. [Google Scholar] [CrossRef]
- Kitazawa, K.; Inomata, T.; Shih, K.; Hughes, J.W.B.; Bozza, N.; Tomioka, Y.; Numa, K.; Yokoi, N.; Campisi, J.; Dana, R.; et al. Impact of aging on the pathophysiology of dry eye disease: A systematic review and meta-analysis. Ocul. Surf. 2022, 25, 108–118. [Google Scholar] [CrossRef]
- McCann, P.; Abraham, A.G.; Mukhopadhyay, A.; Panagiotopoulou, K.; Chen, H.; Rittiphairoj, T.; Gregory, D.G.; Hauswirth, S.G.; Ifantides, C.; Qureshi, R.; et al. Prevalence and Incidence of Dry Eye and Meibomian Gland Dysfunction in the United States: A Systematic Review and Meta-analysis. JAMA Ophthalmol. 2022, 140, 1181–1192. [Google Scholar] [CrossRef]
- Mohamed, Z.; Alrasheed, S.; Abdu, M.; Allinjawi, K. Dry Eye Disease Prevalence and Associated Risk Factors Among the Middle East Population: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e70522. [Google Scholar] [CrossRef]
- Micera, A.; Di Zazzo, A.; Esposito, G.; Longo, R.; Foulsham, W.; Sacco, R.; Sgrulletta, R.; Bonini, S. Age-related changes to human tear composition. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2024–2031. [Google Scholar] [CrossRef]
- Donthineni, P.R.; Das, A.V.; Basu, S. Dry eye disease in children and adolescents in India. Ocul. Surf. 2020, 18, 777–782. [Google Scholar] [CrossRef]
- Girolomoni, G.; Busà, V.M. Flare management in atopic dermatitis: From definition to treatment. Ther. Adv. Chronic Dis. 2022, 13, 20406223211066728. [Google Scholar] [CrossRef]
- Courtin, R.; Pereira, B.; Naughton, G.; Chamoux, A.; Chiambaretta, F.; Lanhers, C.; Dutheil, F. Prevalence of dry eye disease in visual display terminal workers: A systematic review and meta-analysis. BMJ Open 2016, 6, e009675. [Google Scholar] [CrossRef] [PubMed]
- Fjærvoll, H.; Fjærvoll, K.; Magno, M.; Moschowits, E.; Vehof, J.; Dartt, D.A.; Utheim, T.P. The association between visual display terminal use and dry eye: A review. Acta Ophthalmol. 2022, 100, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Schaumberg, D.A.; Dogru, M.; Uchino, Y.; Fukagawa, K.; Shimmura, S.; Satoh, T.; Takebayashi, T.; Tsubota, K. Prevalence of Dry Eye Disease among Japanese Visual Display Terminal Users. Ophthalmology 2008, 115, 1982–1988. [Google Scholar] [CrossRef]
- Benítez-del-Castillo, J.M.; Burgos-Blasco, B. Prevalence of dry eye disease in Spain: A population-based survey (PrevEOS). Ocul. Surf. 2025, 36, 126–133. [Google Scholar] [CrossRef]
- Bikbov, M.M.; Kazakbaeva, G.M.; Rakhimova, E.M.; Rusakova, I.A.; Fakhretdinova, A.A.; Tuliakova, A.M.; Panda-Jonas, S.; Gilmanshin, T.R.; Zainullin, R.M.; Bolshakova, N.I.; et al. The prevalence of dry eye in a very old population. Acta Ophthalmol. 2022, 100, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wei, J.; Zhou, J.; Zou, W. Prevalence and Incidence of Dry Eye Disease in Asia: A Systematic Review and Meta-Analysis. Ophthalmic Res. 2022, 65, 647–658. [Google Scholar] [CrossRef]
- Song, P.; Xia, W.; Wang, M.; Chang, X.; Wang, J.; Jin, S.; Wang, J.; Wei, W.; Rudan, I. Variations of dry eye disease prevalence by age, sex and geographic characteristics in China: A systematic review and meta-analysis. J. Glob. Health 2018, 8, 020503. [Google Scholar] [CrossRef]
- Chen, H.; McCann, P.; Lien, T.; Xiao, M.; Abraham, A.G.; Gregory, D.G.; Hauswirth, S.G.; Qureshi, R.; Liu, S.H.; Saldanha, I.J.; et al. Prevalence of dry eye and Meibomian gland dysfunction in Central and South America: A systematic review and meta-analysis. BMC Ophthalmol. 2024, 24, 50. [Google Scholar] [CrossRef]
- Selegatto, I.B.; de Castro, R.S.; de Castro, J.S.; de Vasconcelos, J.P.C.; Arieta, C.E.L.; de Carvalho, K.M.; Miranda, E.C.M.; Alves, M. Prevalence and Risk Factors of self-reported dry eye in Brazil using a short symptom questionnaire. Sci. Rep. 2018, 8, 2076. [Google Scholar] [CrossRef]
- Martinez, J.D.; Galor, A.; Ramos-Betancourt, N.; Lisker-Cervantes, A.; Beltrán, F.; Ozorno-Zárate, J.; Sánchez-Huerta, V.; Torres-Vera, M.A.; Hernández-Quintela, E. Frequency and risk factors associated with dry eye in patients attending a tertiary care ophthalmology center in Mexico City. Clin. Ophthalmol. 2016, 10, 1335. [Google Scholar]
- Kaido, M.; Toda, I.; Oobayashi, T.; Kawashima, M.; Katada, Y.; Tsubota, K. Reducing short-wavelength blue light in dry eye patients with unstable tear film improves performance on tests of visual acuity. PLoS ONE 2016, 11, e0152936. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, C.; Amato, R.; Pizzo, A.; Pezzino, S.; Rusciano, D. Age-related differential efficacy on dry eye of lactobionic acid-based eye drops. Paripex Indian J. Res. 2018, 7, 41–43. [Google Scholar]
- Tong, L.; Lim, L.; Tan, D.; Heng, W.J.; Lim, J.; Chan, C.; Arundhati, A.; Tan, A. Assessment and Management of Dry Eye Disease and Meibomian Gland Dysfunction: Providing a Singapore Framework. Asia-Pac. J. Ophthalmol. 2021, 10, 530–541. [Google Scholar] [CrossRef]
- Han, S.B.; Yang, H.K.; Hyon, J.Y.; Hwang, J.M. Children with dry eye type conditions may report less severe symptoms than adult patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Diel, R.J.; Kroeger, Z.A.; Levitt, R.C.; Sarantopoulos, C.; Sered, H.; Martinez-Barrizonte, J.; Galor, A. Botulinum Toxin A for the Treatment of Photophobia and Dry Eye. Ophthalmology 2018, 125, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Schein, O.D.; Hochberg, M.C.; Munoz, B.; Tielsch, J.M.; Bandeen-Roche, K.; Provost, T.; Anhalt, G.J.; West, S. Dry Eye and Dry Mouth in the Elderly A Population-Based Assessment. Arch Intern Med. 1999, 159, 1359–1363. [Google Scholar] [CrossRef]
- Stern, M.E. Dry eye: Is this a disease or a natural consequence of aging? Arch. Soc. Esp. Oftalmol. 2005, 80, 129–131. [Google Scholar]
- De Paiva, C.S. Effects of aging in dry eye. Int. Ophthalmol. Clin. 2017, 57, 47–64. [Google Scholar] [CrossRef]
- Roszkowska, A.M.; Colosi, P.; Ferreri, F.M.B.; Galasso, S. Age-related modifications of corneal sensitivity. Ophthalmologica 2004, 218, 350–355. [Google Scholar] [CrossRef]
- Villani, E.; Nucci, P.; Benitez-del-Castillo, J.M.; Dahlmann-Noor, A.; Lagrèze, W.A.; Bremond-Gignac, D. Expert consensus on pediatric dry eye: Insights from a European Delphi study. Ocul. Surf. 2025, 37, 189–197. [Google Scholar] [CrossRef]
- Dogru, M.; Gunay, M.; Celik, G.; Aktas, A. Evaluation of the tear film instability in children with allergic diseases. Cutan. Ocul. Toxicol. 2016, 35, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Jain, S. Ocular pharmacology of tear film, dry eye, and allergic conjunctivitis. Handb. Exp. Pharmacol. 2017, 242, 97–118. [Google Scholar] [PubMed]
- Akil, H.; Celik, F.; Ulas, F.; Kara, I.S. Dry eye syndrome and allergic conjunctivitis in the pediatric population. Middle East. Afr. J. Ophthalmol. 2015, 22, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Sabu, S.; Gupta, N.; Raj, N.; Panigrahi, A.; Lomi, N.; Vanathi, M.; Vashist, P.; Sen, S.; Tandon, R. Ocular surface characteristics in pediatric vernal keratoconjunctivitis: A clinico-cytological study. J. AAPOS 2022, 26, e1–e240. [Google Scholar] [CrossRef]
- Lin, A.; Ahmad, S.; Amescua, G.; Cheung, A.Y.; Choi, D.S.; Jhanji, V.; Mian, S.I.; Rhee, M.K.; Viriya, E.T.; Mah, F.S.; et al. Blepharitis Preferred Practice Pattern®. Ophthalmology 2024, 131, P50–P86. [Google Scholar] [CrossRef]
- Valeille, A.; Ouilhon, C.; Subtil, F.; Hacard, F.; Jaulent, C.; Bérard, F.; Nicolas, J.F.; Fauquert, J.L.; Nosbaum, A. Comprehensive Ophthalmological Evaluation in Atopic Dermatitis. Dermatology 2024, 240, 434–442. [Google Scholar] [CrossRef]
- Bayat, A.H.; Aydemir, E.; Aydemir, G.A.; Gencer, H. Assessment of Tear Film Anomalies in Childhood Obesity. Klin. Monbl Augenheilkd. 2022, 239, 331–337. [Google Scholar] [CrossRef]
- Kaur, K.; Gurnani, B.; Nayak, S.; Deori, N.; Kaur, S.; Jethani, J.; Singh, D.; Agarkar, S.; Hussaindeen, J.R.; Sukhija, J.; et al. Digital Eye Strain—A Comprehensive Review. Ophthalmol. Ther. 2022, 11, 1655–1680. [Google Scholar] [CrossRef]
- Yahalomi, T.; Achiron, A.; Arnon, R.; Stanescu, N.; Pikkel, J. Dry Eye Disease following LASIK, PRK, and LASEK: An Observational Cross-Sectional Study. J. Clin. Med. 2023, 12, 3761. [Google Scholar] [CrossRef]
- Lin, F.; Cai, Y.; Fei, X.; Wang, Y.; Zhou, M.; Liu, Y. Prevalence of dry eye disease among Chinese high school students during the COVID-19 outbreak. BMC Ophthalmol. 2022, 22, 190. [Google Scholar] [CrossRef]
- García-Ayuso, D.; Di Pierdomenico, J.; Moya-Rodríguez, E.; Valiente-Soriano, F.J.; Galindo-Romero, C.; Sobrado-Calvo, P. Assessment of dry eye symptoms among university students during the COVID-19 pandemic. Clin. Exp. Optom. 2022, 105, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Aberame, A.R.; Bhandary, S.V.; Rao, L.G.; Gupta, C. Assessment of prevalence of dry eye among medical students using ocular surface disease index questionnaire—Is COVID-19 to be really blamed? Indian J. Ophthalmol. 2023, 71, 1450–1453. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Mittal, R.; Kumar, N.; Galor, A. The environment and dry eye—Manifestations, mechanisms, and more. Front. Toxicol. 2023, 5, 1173683. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.A.; da Costa, A.X.; Del Duca, E.; Doll, T.; Grupcheva, C.N.; Lazreg, S.; Liu, S.H.; McGee, S.R.; Murthy, R.; Narang, P.; et al. TFOS Lifestyle: Impact of cosmetics on the ocular surface. Ocul. Surf. 2023, 29, 77–130. [Google Scholar]
- Galor, A.; Britten-Jones, A.C.; Feng, Y.; Ferrari, G.; Goldblum, D.; Gupta, P.K.; Merayo-Lloves, J.; Na, K.S.; Naroo, S.A.; Nichols, K.K.; et al. TFOS Lifestyle: Impact of lifestyle challenges on the ocular surface. Ocul. Surf. 2023, 28, 262–303. [Google Scholar] [CrossRef]
- Fraunfelder, F.T.; Sciubba, J.J.; Mathers, W.D. The role of medications in causing dry eye. J. Ophthalmol. 2012, 2012, 285851. [Google Scholar] [CrossRef]
- Walimbe, T.; Chelerkar, V.; Bhagat, P.; Joshi, A.; Raut, A. Effect of benzalkonium chloride-free latanoprost ophthalmic solution on ocular surface in patients with glaucoma. Clin. Ophthalmol. 2016, 10, 821. [Google Scholar]
- Ozek, D.; Karaca, E.E.; Evren Kemer, O. The effect of conjunctivochalasis detected by anterior segment optical coherence tomography on tear function in an elderly population. Ther. Adv. Ophthalmol. 2020, 12, 2515841420930876. [Google Scholar] [CrossRef]
- Alghamdi, Y.A.; Mercado, C.; McClellan, A.L.; Batawi, H.; Karp, C.L.; Galor, A. Epidemiology of meibomian gland dysfunction in an elderly population. Cornea 2016, 35, 731–735. [Google Scholar] [CrossRef]
- Damasceno, R.W.; Osaki, M.H.; Dantas, P.E.C.; Belfort, R. Involutional entropion and ectropion of the lower eyelid: Prevalence and associated risk factors in the elderly population. Ophthalmic Plast. Reconstr. Surg. 2011, 27, 317–320. [Google Scholar] [CrossRef]
- Achtsidis, V.; Eleftheriadou, I.; Kozanidou, E.; Voumvourakis, K.I.; Stamboulis, E.; Theodosiadis, P.G.; Tentolouris, N. Dry eye syndrome in subjects with diabetes and association with neuropathy. Diabetes Care 2014, 37, e210–e211. [Google Scholar] [CrossRef] [PubMed]
- Manaviat, M.R.; Rashidi, M.; Afkhami-Ardekani, M.; Shoja, M.R. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol. 2008, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Mikalauskiene, L.; Grzybowski, A.; Zemaitiene, R. Ocular surface changes associated with ophthalmic surgery. J. Clin. Med. 2021, 10, 1642. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Rojas-Carabali, W.; Uribe-Reina, P.; Muñoz-Ortiz, J.; Terreros-Dorado, J.P.; Ruiz-Botero, M.E.; Torres-Arias, N.; Reyes-Guanes, J.; Rodriguez Zarante, A.; Arteaga-Rivera, J.Y.; Mosos, C.; et al. High prevalence of abnormal ocular surface tests in a healthy pediatric population. Clin. Ophthalmol. 2020, 14, 3427–3438. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, E.; Soares, I.; Brardo, F.M.; Nunes, A.F. Clinical validation of the Standardized Patient Evaluation of Eye Dryness Questionnaire in European Portuguese in a non-clinical sample. Int. Ophthalmol. 2025, 45, 64. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar]
- Weng, H.Y.; Ho, W.T.; Chiu, C.Y.; Tsai, T.Y.; Chang, S.W. Characteristics of tear film lipid layer in young dry eye patients. J. Formos. Med. Assoc. 2021, 120, 1478–1484. [Google Scholar] [CrossRef]
- Belmonte, C.; Nichols, J.J.; Cox, S.M.; Brock, J.A.; Begley, C.G.; Bereiter, D.A.; Dartt, D.A.; Galor, A.; Hamrah, P.; Ivanusic, J.J.; et al. TFOS DEWS II pain and sensation report. Ocul. Surf. 2017, 15, 404–437. [Google Scholar] [CrossRef]
- Sullivan, B.D.; Evans, J.E.; Dana, M.R.; Sullivan, D.A. Influence of aging on the polar and neutral lipid profiles in human meibomian gland secretions. Arch. Ophthalmol. 2006, 124, 1286–1292. [Google Scholar] [CrossRef]
- Kelagere, Y.; Scholand, K.K.; DeJong, E.N.; Boyd, A.I.; Yu, Z.; Astley, R.A.; Callegan, M.C.; Bowdish, D.M.; Makarenkova, H.P.; de Paiva, C.S. TNF is a critical cytokine in age-related dry eye disease. Ocul. Surf. 2023, 30, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Coursey, T.G.; Tukler Henriksson, J.; Barbosa, F.L.; De Paiva, C.S.; Pflugfelder, S.C. Interferon-γ-Induced Unfolded Protein Response in Conjunctival Goblet Cells as a Cause of Mucin Deficiency in Sjögren Syndrome. Am. J. Pathol. 2016, 186, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.G.; Yu, Z.; Hernandez, H.; Trujillo-Vargas, C.M.; Lee, A.; Mauk, K.E.; Cai, J.; Alves, M.R.; de Paiva, C.S. Modulation of Oxidative Stress and Inflammation in the Aged Lacrimal Gland. Am. J. Pathol. 2021, 191, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Hua, X.; Li, J.; Chi, W.; Zhang, Z.; Lu, F.; Zhang, L.; Pflugfelder, S.C.; Li, D.Q. Oxidative Stress Markers Induced by Hyperosmolarity in Primary Human Corneal Epithelial Cells. PLoS ONE 2015, 10, e0126561. [Google Scholar] [CrossRef]
- Nien, C.J.; Paugh, J.R.; Massei, S.; Wahlert, A.J.; Kao, W.W.; Jester, J.V. Age-related changes in the meibomian gland. Exp. Eye Res. 2009, 89, 1021–1027. [Google Scholar] [CrossRef]
- Shi, V.Y.; Chamberlain, W.; Siegfried, E.; Kraff-Cooper, C.; Beckman, K.; Lio, P.; Paller, A.S.; Simpson, E. Practical management of ocular surface disease in patients with atopic dermatitis, with a focus on conjunctivitis: A review. J. Am. Acad. Dermatol. 2023, 89, 309–315. [Google Scholar] [CrossRef]
- Yilmaz, U.; Gökler, M.E.; Unsal, A. Dry eye disease and depression-anxiety-stress: A hospital-based case control study in Turkey. Pak. J. Med. Sci. 2015, 31, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, S.A.; Alomran, A.A.; Abusharha, A.; Fagehi, R.; Al-Johani, N.J.; El-Hiti, G.A.; Masmali, A.M. An assessment of the ocular tear film in patients with thyroid disorders. Clin. Ophthalmol. 2019, 13, 1019–1026. [Google Scholar] [CrossRef]
- Wang, M.T.M.; Thomson, W.M.; Craig, J.P. Association between symptoms of xerostomia and dry eye in older people. Contact Lens Anterior Eye 2020, 43, 99–102. [Google Scholar] [CrossRef]
- Wei, Y.; Asbell, P.A. The core mechanism of dry eye disease is inflammation. Eye Contact Lens 2014, 40, 248–256. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Bian, F.; De Paiva, C.S. Matrix metalloproteinase-9 in the pathophysiology and diagnosis of dry eye syndrome. Met. Med. 2017, 4, 37–46. [Google Scholar] [CrossRef]
- Soria, J.; Acera, A.; Merayo-LLoves, J.; Durán, J.A.; González, N.; Rodriguez, S.; Bistolas, N.; Schumacher, S.; Bier, F.F.; Peter, H.; et al. Tear proteome analysis in ocular surface diseases using label-free LC-MS/MS and multiplexed-microarray biomarker validation. Sci. Rep. 2017, 7, 17478. [Google Scholar] [CrossRef] [PubMed]
- Messmer, E.M.; von Lindenfels, V.; Garbe, A.; Kampik, A. Matrix Metalloproteinase 9 Testing in Dry Eye Disease Using a Commercially Available Point-of-Care Immunoassay. Ophthalmology 2016, 123, 2300–2308. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (Inflammaging) and its potential contribution to age-associated diseases. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, A.; Micera, A.; Coassin, M.; Varacalli, G.; Foulsham, W.; De Piano, M.; Bonini, S. Inflammaging at ocular surface: Clinical and biomolecular analyses in healthy volunteers. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1769–1775. [Google Scholar] [CrossRef]
- Yu, Z.; Li, J.; Govindarajan, G.; Hamm-Alvarez, S.F.; Alam, J.; Li, D.Q.; de Paiva, C.S. Cathepsin S is a novel target for age-related dry eye. Exp. Eye Res. 2022, 214, 108895. [Google Scholar] [CrossRef]
- Bentley, D.; Fisher, B.A.; Barone, F.; Kolb, F.A.; Attley, G. A randomized, double-blind, placebo-controlled, parallel group study on the effects of a cathepsin S inhibitor in primary Sjögren’s syndrome. Rheumatology 2023, 62, 3644–3653. [Google Scholar] [CrossRef]
- Aljohani, S.; Jazzar, A. Tear Cytokine Levels in Sicca Syndrome-Related Dry Eye: A Meta-Analysis. Diagnostics 2023, 13, 2184. [Google Scholar] [CrossRef]
- Tong, L.; Lan, W.; Lim, R.R.; Chaurasia, S.S. S100A proteins as molecular targets in the ocular surface inflammatory diseases. Ocul. Surf. 2014, 12, 23–31. [Google Scholar] [CrossRef]
- Willcox, M.D.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef]
- Yamada, K.; Takaki, S.; Komuro, N.; Suzuki, K.; Citterio, D. An antibody-free microfluidic paper-based analytical device for the determination of tear fluid lactoferrin by fluorescence sensitization of Tb3+. Analyst 2014, 139, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Sonobe, H.; Ogawa, Y.; Yamada, K.; Shimizu, E.; Uchino, Y.; Kamoi, M.; Saijo, Y.; Yamane, M.; Citterio, D.; Suzuki, K.; et al. A novel and innovative paper-based analytical device for assessing tear lactoferrin of dry eye patients. Ocul. Surf. 2019, 17, 160–166. [Google Scholar] [CrossRef]
- Martínez-Carrasco, R.; Sharma, A. Ocular surface glycocalyx in health and disease. Front. Cell Dev. Biol. 2025, 13, 1561324. [Google Scholar] [CrossRef] [PubMed]
- Enríquez-de-Salamanca, A.; Castellanos, E.; Stern, M.E.; Fernández, I.; Carreño, E.; García-Vázquez, C.; Herreras, J.M.; Calonge, M. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol. Vis. 2010, 16, 862. [Google Scholar] [PubMed]
- Leonardi, A.; Bogacka, E.; Fauquert, J.L.; Kowalski, M.L.; Groblewska, A.; Jedrzejczak-Czechowicz, M.; Doan, S.; Marmouz, F.; Demoly, P.; Delgado, L. Ocular allergy: Recognizing and diagnosing hypersensitivity disorders of the ocular surface. Allergy Eur. J. Allergy Clin. Immunol. 2012, 67, 1327–1337. [Google Scholar] [CrossRef]
- Leonardi, A.; Modugno, R.L.; Salami, E. Allergy and Dry Eye Disease. Ocul. Immunol. Inflamm. 2021, 29, 1168–1176. [Google Scholar] [CrossRef]
- Suárez-Cortés, T.; Merino-Inda, N.; Benitez-del-Castillo, J.M. Tear and ocular surface disease biomarkers: A diagnostic and clinical perspective for ocular allergies and dry eye disease. Exp. Eye Res. 2022, 221, 109121. [Google Scholar] [CrossRef]
- Portal, C.; Gouyer, V.; Gottrand, F.; Desseyn, J.L. Ocular mucins in dry eye disease. Exp. Eye Res. 2019, 186, 107724. [Google Scholar] [CrossRef]
- Zemba, M.; Ionescu, M.; Pîrvulescu, R.A.; Dumitrescu, O.M.; Daniel-Constantin, B.; Radu, M.; Stamate, A.C.; Istrate, S. Biomarkers of ocular allergy and dry eye disease. Rom. J. Ophthalmol. 2023, 67, 250. [Google Scholar] [CrossRef]
- Nättinen, J.; Jylhä, A.; Aapola, U.; Mäkinen, P.; Beuerman, R.; Pietilä, J.; Vaajanen, A.; Uusitalo, H. Age-associated changes in human tear proteome. Clin. Proteom. 2019, 16, 11. [Google Scholar] [CrossRef]
- Gunay, M.; Celik, G.; Yildiz, E.; Bardak, H.; Koc, N.; Kirmizibekmez, H.; Gunay, B.O.; Yesiltepe Mutlu, R.G. Ocular Surface Characteristics in Diabetic Children. Curr. Eye Res. 2016, 41, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Çalık Başaran, N.; Kırağı, D.; Tan, Ç.; Özışık, L.; Çağdaş Ayvaz, N.D.; Kocabeyoğlu, S.; Öz, Ş.G.; İrkeç, M.; Tezcan, F.İ. Ocular Changes and Tear Cytokines in Individuals with Low Serum Vitamin D Levels: A Cross-Sectional, Controlled Study. Ocul. Immunol. Inflamm. 2024, 32, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, L.; Jian Hu, R.; Yu, P.P.; Jin, X. The influence of overnight orthokeratology on ocular surface and dry eye-related cytokines IL-17A, IL-6, and PGE2 in children. Contact Lens Anterior Eye 2021, 44, 81–88. [Google Scholar] [CrossRef]
- Bilgic, A.A.; Kocabeyoglu, S.; Dikmetas, O.; Tan, C.; Karakaya, J.; Irkec, M. Influence of video display terminal use and meibomian gland dysfunction on the ocular surface and tear neuromediators. Int. Ophthalmol. 2023, 43, 1537–1544. [Google Scholar] [CrossRef]
- Casemore, R.K.; Wolffsohn, J.S.; Utheim, T.P.; Reppe, S.; Aass, H.C.D.; Dutta, D. A prospective, longitudinal study to assess progression of ocular surface signs, tear cytokines and protein profiles in young adults. Ocul. Surf. 2025, 37, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Ambaw, Y.A.; Chao, C.; Ji, S.; Raida, M.; Torta, F.; Wenk, M.R.; Tong, L. Tear eicosanoids in healthy people and ocular surface disease. Sci. Rep. 2018, 8, 11296. [Google Scholar] [CrossRef]
- Tong, L.; Wong, T.Y.; Cheng, Y. Level of tear cytokines in population-level participants and correlation with clinical features. Cytokine 2018, 110, 452–458. [Google Scholar] [CrossRef]
- Patel, R.; Zhu, M.; Robertson, D.M. Shifting the IGF-axis: An age-related decline in human tear IGF-1 correlates with clinical signs of dry eye. Growth Horm. IGF Res. 2018, 40, 69–73. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar]
- Lazreg, S.; Hosny, M.; Ahad, M.A.; Sinjab, M.M.; Awwad, S.T.; Rousseau, A.; Messaoud, R. Dry Eye Disease in the Middle East and Northern Africa: A Position Paper on the Current State and Unmet Needs. Clin. Ophthalmol. 2024, 18, 679–698. [Google Scholar] [CrossRef]
- Buzzonetti, L.; Petroni, S.; Federici, M. Effectiveness of hyaluronic acid and arnica extract ophthalmic solution in reducing dry eye symptoms in pediatric population. Eur. J. Ophthalmol. 2023, 33, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.B.; Abd El-Hamid, B.N.; Fathalla, D.; Fouad, E.A. Current trends in pharmaceutical treatment of dry eye disease: A review. Eur. J. Pharm. Sci. 2022, 175, 106206. [Google Scholar] [CrossRef] [PubMed]
- Choy, B.N.K.; Zhu, M.M.; Pang, J.C.S.; Chan, J.C.H.; Ng, A.L.K.; Fan, M.C.Y.; Iu, L.P.L.; Kwan, J.S.K.; Lai, J.S.M.; Chiu, P.K.C. Factors Associated with Poor Eye Drop Administration Technique and the Role of Patient Education among Hong Kong Elderly Population. J. Ophthalmol. 2019, 2019, 5962065. [Google Scholar] [CrossRef]
- Abdelrahman Ahmed, R.; Gamal El-Sayed, N.; Gomaa Mohammed, S. Dry Eyes Related Quality of Life in Elderly Patients with Meibomian Gland Dysfunction at Zagazig University Hospitals. Egypt. J. Health Care 2024, 15, 2148–2164. [Google Scholar] [CrossRef]
- Narang, P.; Donthineni, P.R.; D’Souza, S.; Basu, S. Evaporative dry eye disease due to meibomian gland dysfunction: Preferred practice pattern guidelines for diagnosis and treatment. Indian J. Ophthalmol. 2023, 71, 1348–1356. [Google Scholar] [CrossRef]
- Teo, C.H.Y.; Ong, H.S.; Liu, Y.C.; Tong, L. Meibomian gland dysfunction is the primary determinant of dry eye symptoms: Analysis of 2346 patients. Ocul. Surf. 2020, 18, 604–612. [Google Scholar] [CrossRef]
- Kathuria, A.; Shamloo, K.; Jhanji, V.; Sharma, A. Categorization of marketed artificial tear formulations based on their ingredients: A rational approach for their use. J. Clin. Med. 2021, 10, 1289. [Google Scholar] [CrossRef]
- Rana, H.S.; Akella, S.S.; Clabeaux, C.E.; Skurski, Z.P.; Aakalu, V.K. Ocular surface disease in thyroid eye disease: A narrative review. Ocul. Surf. 2022, 24, 67–73. [Google Scholar] [CrossRef]
- Ziaragkali, S.; Kotsalidou, A.; Trakos, N. Dry eye disease in routine rheumatology practice. Mediterr. J. Rheumatol. 2018, 29, 127–139. [Google Scholar] [CrossRef]
- Mohamed, M.; Abu-Steit, M.; Shalaby, A.; Sherif, A. Evaluation of dry eye syndrome after LASIK and surface ablation: A comparative study. J. Egypt. Ophthalmol. Soc. 2015, 108, 221. [Google Scholar] [CrossRef]
- Fauquert, J.L. Diagnosing and managing allergic conjunctivitis in childhood: The allergist’s perspective. Pediatr. Allergy Immunol. 2019, 30, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Saldanha, I.J.; Abraham, A.G.; Rittiphairoj, T.; Hauswirth, S.; Gregory, D.; Ifantides, C.; Li, T. Topical corticosteroids for dry eye. In Cochrane Database of Systematic Reviews; Cochrane Library: London, UK, 2022. [Google Scholar] [CrossRef]
- Labetoulle, M.; Benitez-Del-castillo, J.M.; Barabino, S.; Vanrell, R.H.; Daull, P.; Garrigue, J.S.; Rolando, M. Artificial Tears: Biological Role of Their Ingredients in the Management of Dry Eye Disease. Int. J. Mol. Sci. 2022, 23, 2434. [Google Scholar] [CrossRef]
- Safarzadeh, M.; Azizzadeh, P.; Akbarshahi, P. Comparación de la eficacia clínica de los colirios con conservantes y los colirios con contenido de hidroxipropil metilcelulosa-dextran sin conservantes. J. Optom. 2017, 10, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Rong, B.; Tu, P.; Tang, Y.; Song, W.; Toyos, R.; Toyos, M.; Yan, X. Analysis of Cytokine Levels in Tears and Clinical Correlations After Intense Pulsed Light Treating Meibomian Gland Dysfunction. Am. J. Ophthalmol. 2017, 183, 81–90. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, C.; Jiang, J.; Ouyang, J.; Zhang, D.; Chen, T.; Chu, Y.; Hu, K. The efficacy of vitamin D supplementation in dry eye disease: A systematic review and meta-analysis. Contact Lens Anterior Eye 2024, 47, 102169. [Google Scholar] [CrossRef] [PubMed]
- Vagge, A.; Senni, C.; Bernabei, F.; Pellegrini, M.; Scorcia, V.; Traverso, C.E.; Giannaccare, G. Therapeutic effects of lactoferrin in ocular diseases: From dry eye disease to infections. Int. J. Mol. Sci. 2020, 21, 6668. [Google Scholar] [CrossRef]
- Galletti, J.G.; Scholand, K.K.; Trujillo-Vargas, C.M.; Haap, W.; Santos-Ferreira, T.; Ullmer, C.; Yu, Z.; de Paiva, C.S. Effects of Cathepsin S Inhibition in the Age-Related Dry Eye Phenotype. Investig. Ophthalmol Vis Sci. 2023, 64, 7. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, Y.; Du, Y.; Wang, Z.; Cheng, L.; Du, Z. Comprehensive dry eye therapy: Overcoming ocular surface barrier and combating inflammation, oxidation, and mitochondrial damage. J. Nanobiotechnol. 2024, 22, 233. [Google Scholar] [CrossRef]
- Dunn, J.D.; Karpecki, P.M.; Meske, M.E.; Reissman, D. Evolving knowledge of the unmet needs in dry eye disease. Am. J. Manag. Care 2021, 27, S23–S32. [Google Scholar]
- Li, B.; Zhang, F. Research advances in myopic children with dry eye. Chin. J. Ophthalmol. 2024, 60, 193–199. [Google Scholar]

| Region/Country (Years) | Prevalence (%) | Reference |
|---|---|---|
| Global population | 5–50 | Stapleton et al. 2017 [5] |
| 5.5–65.4% (population-based studies) | ||
| Global children (<18 y) | 5.5–23.1 | Stapleton et al. 2024 [14] |
| 23.7 | Zou et al. 2025 [9] | |
| Global ≥40 y | 10–20 | Britten-Jones et al. 2024 [11] |
| Global ≥50 y | 5–30% | Stapleton et al. 2017 [5] |
| Global ≥60 y | 9.2 | Kitazawa et al. 2022 [15] |
| Global VDT users | ||
| 22–60 y | 49.5 | Courtin et al. 2016 [21] |
| 20–58 y | 26–70 | Fjaervoll et al. 2022 [22] |
| Japan 20–60 y | 10.1 (male) | Uchino et al. 2008 [23] |
| 21.5 (female) | ||
| United States | 8.1 | McCann et al. 2022 [16] |
| >18 y | 3.5 | |
| >68 y | 7.8 | |
| Europe | ||
| Spain | 16.6 (WHS criteria) | Benítez-Del-Castillo and Burgos-Blasco 2025 [24] |
| 22.5 (BES criteria) | ||
| 18–29 y | 5.7 | |
| 30–39 y | 4.7 | |
| 40–49 y | 9.8 | |
| 50–59 y | 11.6 | |
| 60–69 y | 17.1 | |
| 70–79 y | 21.8 | |
| ≥80 y | 26.4 | |
| Norway | Tellefsen et al. 2021 [13] | |
| 20–39 y | 24.1 | |
| 40–59 y | 39.4 | |
| ≥60 y | 36.5 | |
| Rusia | Bikbov et al. 2022 [25] | |
| ≥84.5 y | 35.8 | |
| Asia | Cai et al. 2022 [26] | |
| <20 y | 11.9 | |
| 20–29 y | 7.5 | |
| 30–39 y | 7.7 | |
| 40–49 y | 11.5 | |
| 50–59 y | 13.3 | |
| 60–69 y | 22.6 | |
| ≥70 y | 28.9 | |
| India | Donthineni et al. 2020 [19] | |
| Total (<21 y) | 0.4 | |
| Infancy (<1 y) | 0.05 | |
| Toddlerhood (1–2 y) | 0.01 | |
| Early childhood (3–5 y) | 0.02 | |
| Middle childhood (6–11 y) | 0.10 | |
| Early adolescence (12–18 y) | 0.15 | |
| Late adolescence (19–21 y) | 0.15 | |
| China | Song et al. 2018 [27] | |
| 5–89 y | 13.6 (by symptoms and signs) | |
| 31.4 (by symptoms) | ||
| Central and South America | Chen et al. 2024 [28] | |
| Brazil | Castro et al. 2018 [29] | |
| ≥18 y | 13.0 | |
| 18–39 y | 9.9 | |
| 40–60 y | 13.2 | |
| >60 y | 21.1 | |
| Mexico | Martinez et al. 2016 [30] | |
| ≥50 y | 41.0 | |
| 46–55 y | 36.0 | |
| 66–75 y | 18.0 | |
| 76–85 y | 38.0 |
| Ocular/Systemic Disease | Children (<18 y) | Young Adults (18–40 y) | Adults (40–60 y) | Elderly (>60 y) |
|---|---|---|---|---|
| Atopic dermatitis | ||||
| Ocular allergy (AC, AKC) | ||||
| Stevens–Johnson syndrome | ||||
| Vitamin A deficiency | ||||
| Ocular allergy (VKC) | ||||
| Acne (early and late adolescence) | ||||
| Meibomian gland dysfunction | ||||
| Mental health disorders | ||||
| Altered sleep conditions | ||||
| Thyroid disorders | ||||
| Glaucoma | ||||
| Sjögren’s syndrome | ||||
| Diabetes mellitus | ||||
| Conjunctivochalasis | ||||
| Age-related macular degeneration | ||||
| Lupus erythematosus | ||||
| Rheumatoid arthritis | ||||
| Xerostomia |
| Recommended and/or Available Treatments | Description | References |
|---|---|---|
| Children (<18 years) | ||
| Lifestyle modifications |
| Stapleton et al. 2024 [14] |
| Blinking exercises |
| |
| Management of lid diseases (anterior blepharitis and MGD) |
| Villani et al. 2025 [40] |
| Warm compress therapy |
| Lazreg et al. 2024 [110] |
| Unpreserved lubricants or artificial tears |
| Buzzonetti et al. 2023 [111] |
| Management of ocular allergy |
| Stapleton et al. 2024 [14] Villani 2025 et al. [40] |
| Topical macrolides |
| Villani et al. 2025 [40] |
| Oral macrolides |
| |
| Topical steroids |
| |
| Topical 0.2% HA Topical 0.2% HA with 0.1% arnica extract |
| Buzzonetti et al. 2023 [111] |
| Vitamin A supplementation |
| Stapleton et al. 2024 [14] |
| Management of severe underlying conditions |
| Donthineni et al. 2020 [19] |
| Tear replacement, tear stimulation, or tear conservation approaches |
| Stapleton et al. 2024 [14] |
| Elderly (over 65 years) | ||
| Conventional/Available Treatments | ||
| Artificial tears/lubricants |
| Mohamed et al. 2022 [112] Ozek et al. 2020 [58] |
| Eye drops containing HA and LA |
| Gagliano et al. 2018 [32] Barabino 2022 [10] |
| Management of lid diseases | MGD | de Paiva 2017 [38] |
| New Possible Treatments (based on new molecules/targets and inflammaging): | ||
| TNF modulation |
| Kelagere et al. 2023 [71] |
| CTSS modulation |
| Yu et al. 2022 [86] |
| Challenges in Treatment Administration in the Elderly | ||
| Patient education on the correct technique for administering eye drops |
| Choy et al. 2019 [113] Abdelrahman et al. 2024 [114] |
| Treatments Generally Applicable to Adult Age Groups (typically >18 y) | ||
| Patient education |
| Mohamed et al. 2022 [112] |
| Environmental and lifestyle modifications |
| Lazreg et al. 2024 [110] |
| MGD treatment |
| Narang et al. 2023 [115] Jones et al. 2017 [109] |
| Artificial tears/lubricants |
| Teo et al. 2020 [116] Kathuria et al. 2021 [117] Lazreg et al. 2024 [110] Mohamed et al. 2022 [112] |
| Topical cyclosporine |
| Jones et al. 2017 [109] Lazreg et al. 2024 [110] Wei and Asbell 2014 [80] |
| Topical or oral azithromycin |
| Jones et al. 2017 [109] |
| Oral omega-3 EFA supplementation |
| |
| Newer therapies for EDE due to MGD (non-pharmacological) |
| Narang et al. 2023 [115] |
| Management of underlying systemic diseases |
| Mohamed et al. 2022 [112] Rana et al. 2022 [118] Ziaragkali et al. 2018 [119] |
| Management of ocular comorbidities |
| Stapleton et al. 2024 [14] Mohamed et al. 2022 [112] Shi et al. 2023 [76] Mohamed et al. 2015 [120] Jones et al. 2017 [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez-Cortés, T.; Herrera, I. Emerging Age-Specific Therapeutic Approaches for Dry Eye Disease. J. Clin. Med. 2025, 14, 4147. https://doi.org/10.3390/jcm14124147
Suárez-Cortés T, Herrera I. Emerging Age-Specific Therapeutic Approaches for Dry Eye Disease. Journal of Clinical Medicine. 2025; 14(12):4147. https://doi.org/10.3390/jcm14124147
Chicago/Turabian StyleSuárez-Cortés, Tatiana, and Itxaso Herrera. 2025. "Emerging Age-Specific Therapeutic Approaches for Dry Eye Disease" Journal of Clinical Medicine 14, no. 12: 4147. https://doi.org/10.3390/jcm14124147
APA StyleSuárez-Cortés, T., & Herrera, I. (2025). Emerging Age-Specific Therapeutic Approaches for Dry Eye Disease. Journal of Clinical Medicine, 14(12), 4147. https://doi.org/10.3390/jcm14124147







