Ureteric Complications and Urinary Tract Reconstruction Techniques in Renal Transplantation: A Surgical Essay
Abstract
1. Introduction
2. Ureteric Complications After Renal Transplantation
2.1. Ureteric Ischemia and Its Pathogenic Role
2.2. Epidemiology and Risk Stratification
2.3. Ureteric Leaks (Urinary Fistulas)
2.4. Ureteric Strictures
2.5. Pre-Reconstruction Urological Assessment and Workup
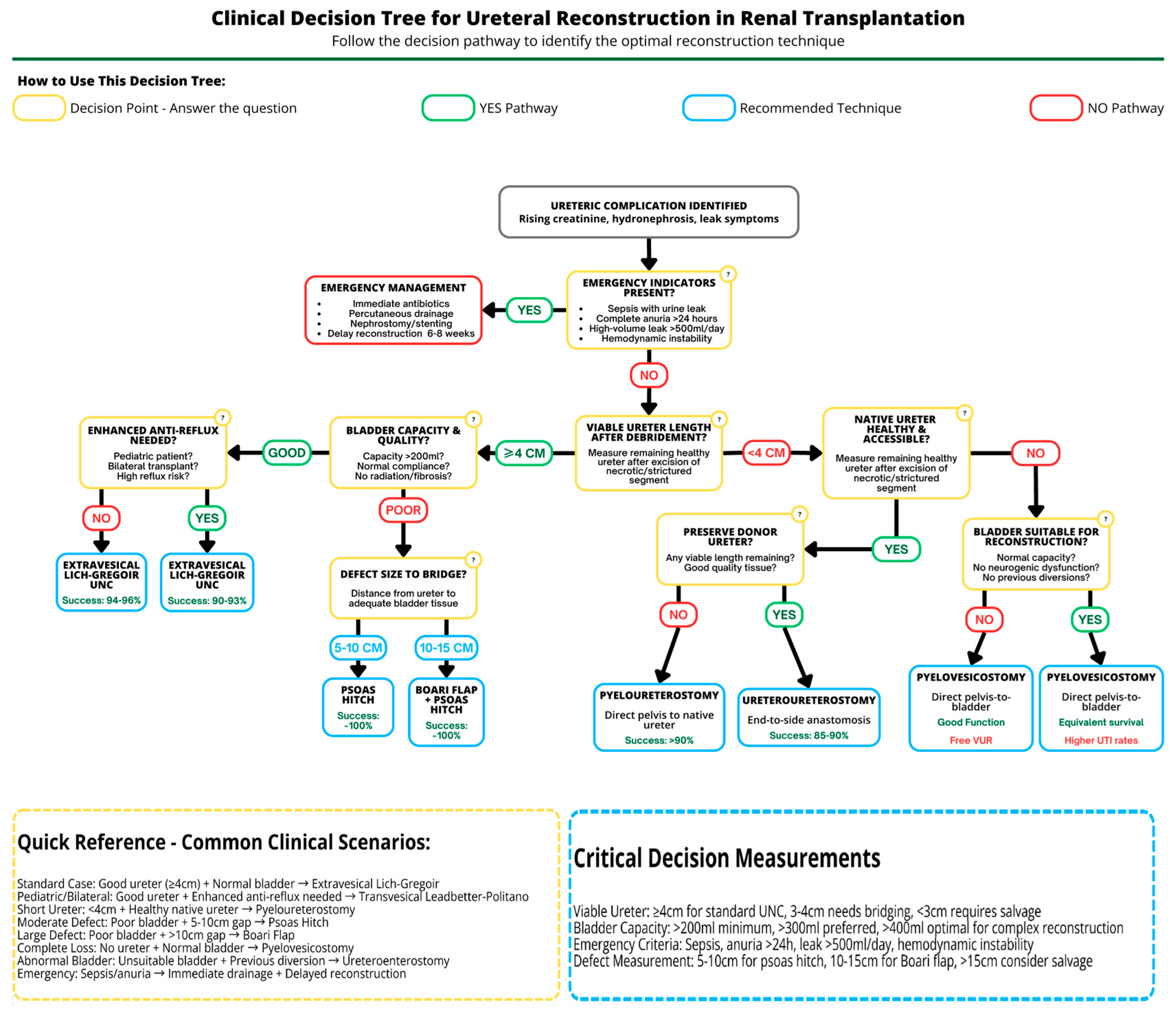
3. Recipient Bladder Anastomoses
3.1. Extravesical Lich–Gregoir Ureteroneocystostomy
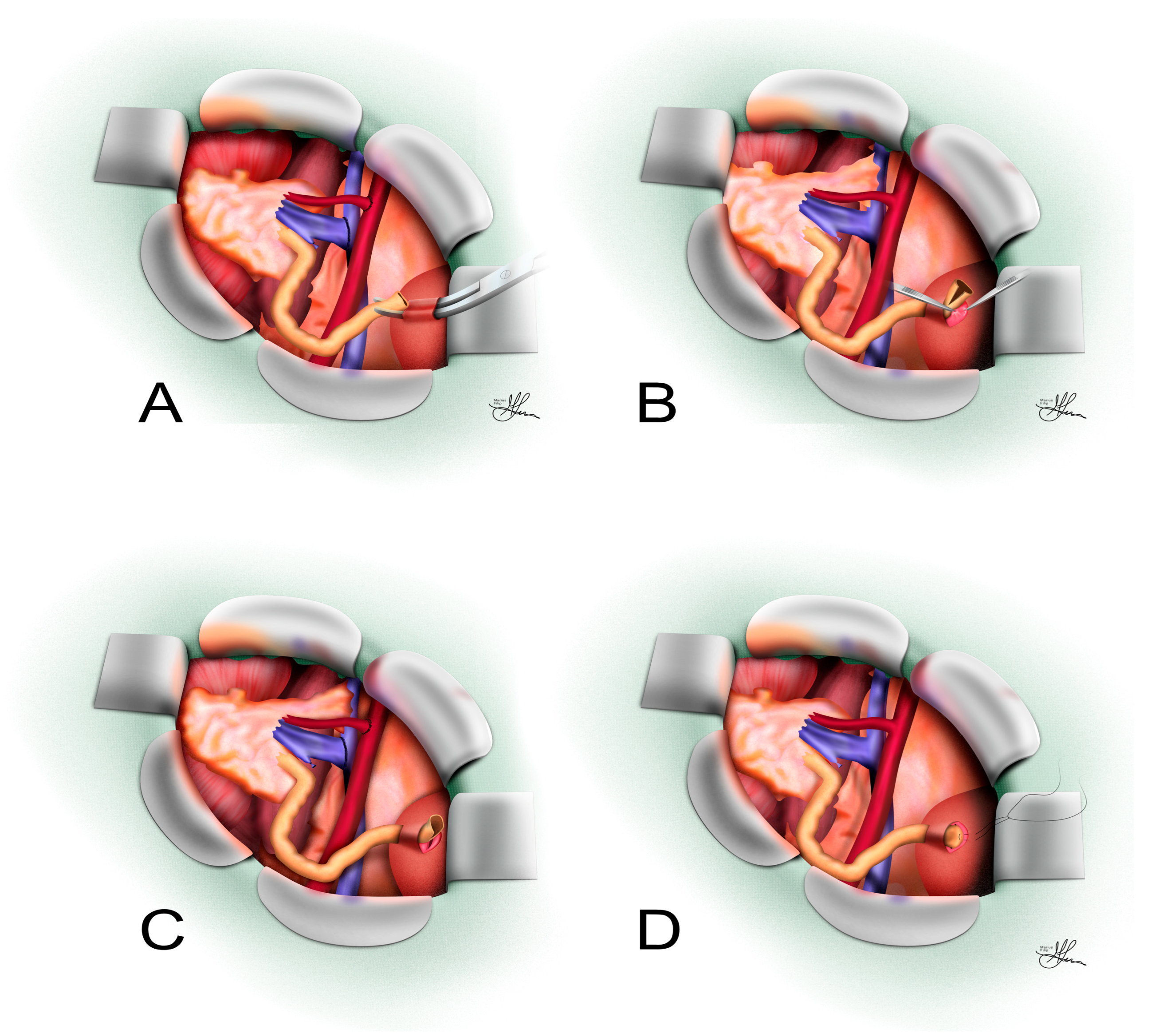
3.1.1. Surgical Technique
3.1.2. Indications and Outcomes
3.2. Transvesical Leadbetter–Politano Ureteroneocystostomy
3.2.1. Surgical Technique
3.2.2. Indications and Outcomes
3.3. Alternative Ureteroneocystostomy Techniques
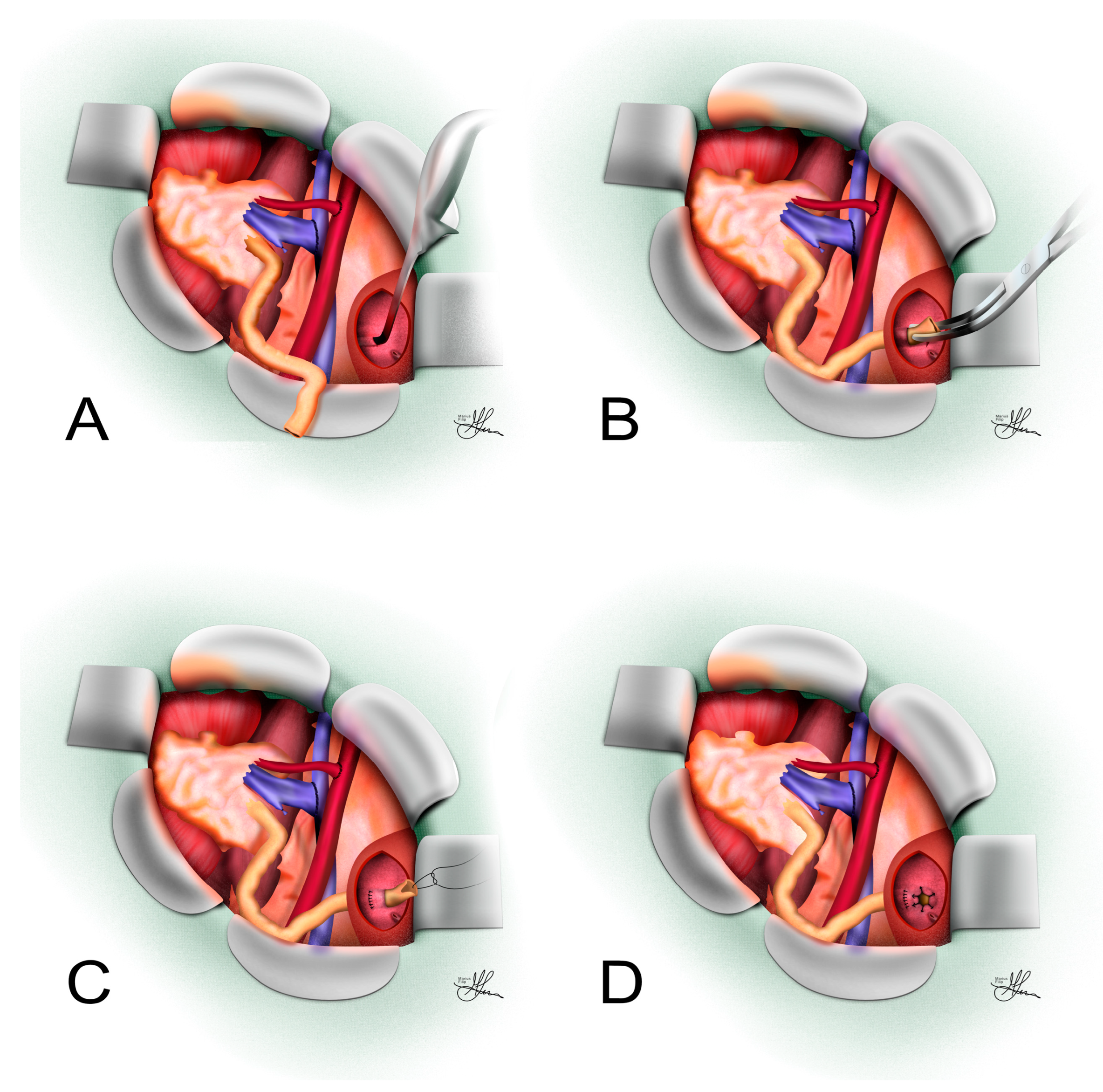
3.3.1. Psoas Hitch
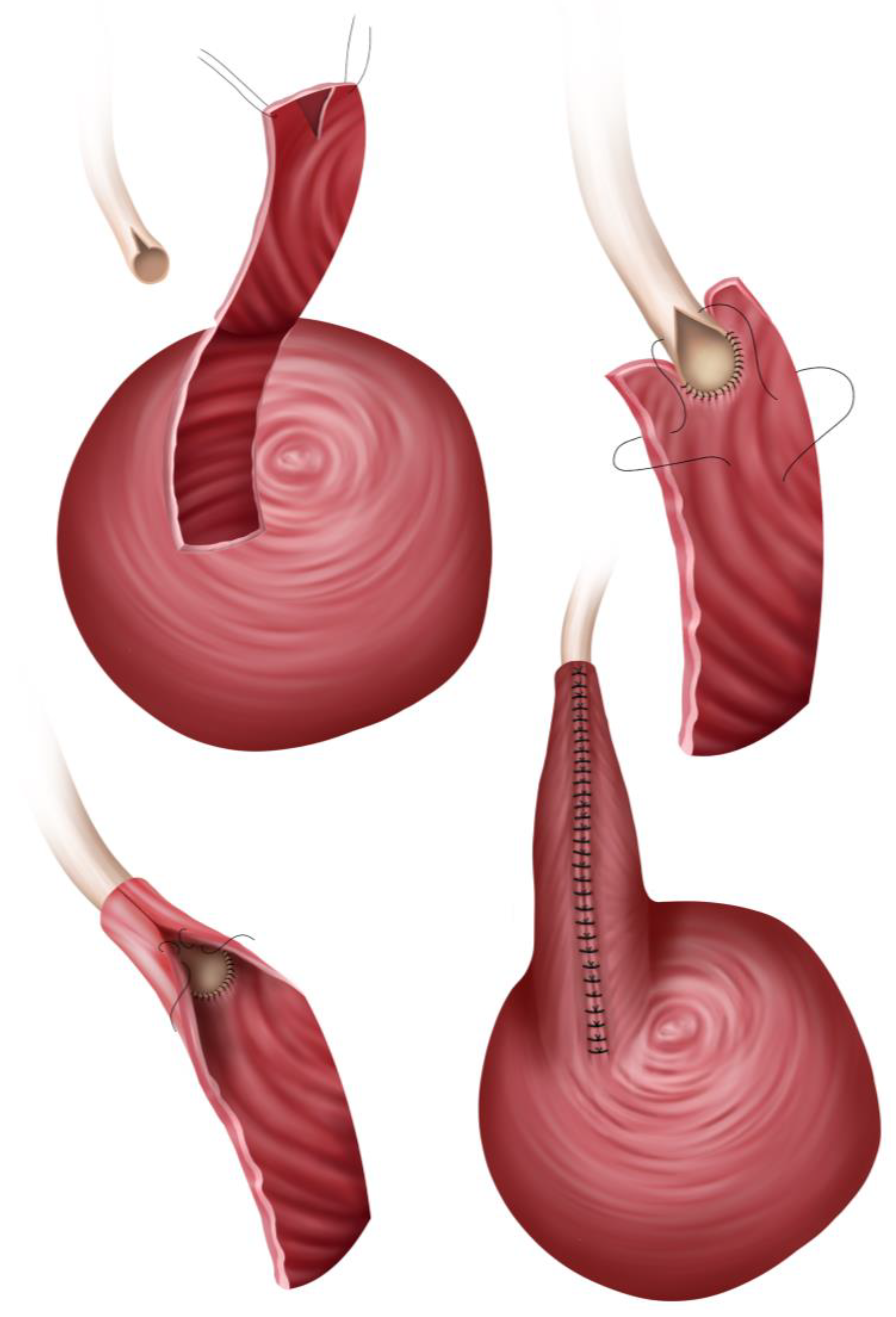
3.3.2. Boari Flap
- The Übelhör modification involves making a slightly oblique incision on the affected side of the bladder, which is then extended anteriorly and distally to the contralateral side. This creates a wide rhombic flap with a broad base that can be rotated towards the psoas muscle [41].
3.3.3. Indications and Outcomes
- Extensive distal ureteric necrosis: If the entire lower half of the donor ureter is necrotic, simply reimplanting it into the bladder may not be possible due to the insufficient length of viable ureter left. Thus, a Boari flap may be used, as it can reach upward to a high ureteric stump or renal pelvis, bringing up to 15 cm-long defects [44,48].
- Failed prior reimplants: In a patient who already had one or more attempts at UNC that have failed (e.g., persistent leak or stricture) and the donor ureter is now shortened, a Boari flap offers an alternative to using the native ureter [48].
- Complex ureteric injuries: Rarely, intraoperative damage to the donor ureter or recipient bladder might necessitate using such a flap in the repair, i.e., to reestablish continuity of the urinary tract in the recipient.
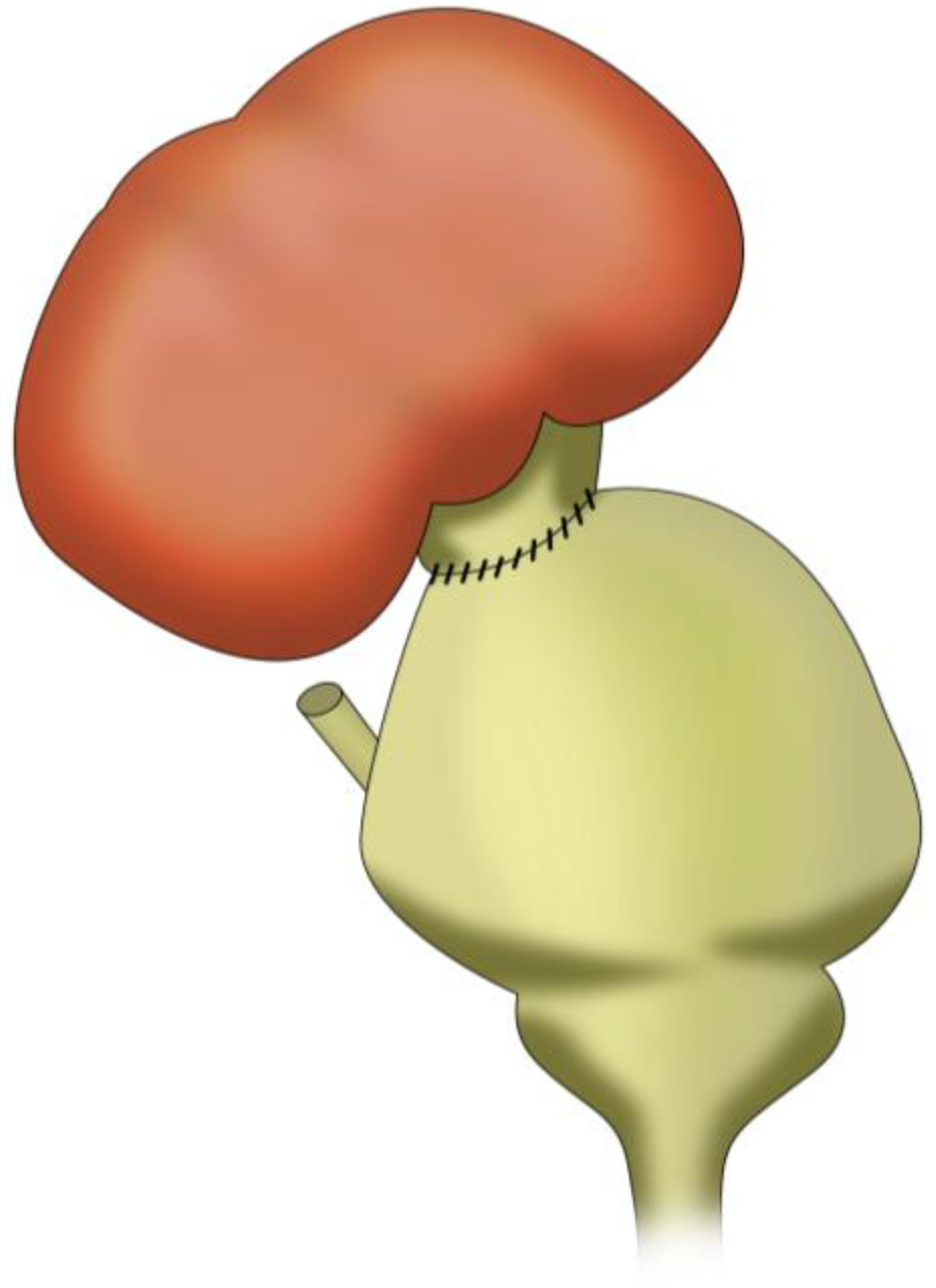
3.4. Pyelovesicostomy
3.4.1. Surgical Technique
3.4.2. Indications and Outcomes
4. Native Upper Urinary Tract Anastomoses
4.1. Pyelopyelostomy
- In an ESRD patient with maintained diuresis who still has a functioning or semi-functioning ipsilateral native kidney and ureter and the bladder cannot be used, i.e., is difficult to identify because of pelvic scarring or shows insufficient distension for UNC, a surgeon might directly connect the transplant pelvis to the native pelvis or an upper ureteric segment, especially if the distal graft ureter vascularization seems compromised [24,29,54,55,56].
- In re-transplant scenarios where a patient has a previous transplant kidney in place, a pyelopyelostomy could theoretically connect a new graft’s pelvis to the old transplant’s collecting system (though this would be highly unusual and technically very challenging).
4.2. Pyeloureterostomy
- Difficult access to the bladder: For example, if the recipient has severe pelvic adhesions, a defunctionalized bladder, or other anatomic issues (like a recent bladder augmentation or urinary diversion) that make a direct bladder implant problematic.
- Doubtful graft ureter viability: If the transplant ureter looks poorly perfused or is damaged (e.g., limited length, signs of ischemia), the surgeon may elect to do a primary pyeloureterostomy to avoid using the compromised ureter, i.e., proactively preventing leaks/strictures.
- Secondary (salvage) anastomosis: If a UNC was performed initially, but the patient develops a ureteric complication, a pyeloureterostomy can be employed as a rescue procedure (often preferred instead of a second UNC) [8]. Many centers consider it the preferred salvage technique for distal ureteric complications [2,8].
4.3. Ureteroureterostomy
- The donor ureter is still viable in part, but the very distal end or implantation site is problematic (so one can join the donor ureter to the native ureter a little higher up instead of to the bladder).
- The native ureter is easily accessible and healthy, making a ureter-to-ureter anastomosis straightforward.
- As a salvage for a distal stricture, some have managed short strictures by excising the damaged segment and performing an end-to-end anastomosis between the remaining donor ureter and the native ureter. However, more commonly surgeons go up to the pelvis of the transplant because it is a larger target; thus, ureteroureterostomy per se is slightly less common than pyeloureterostomy [2,8,24].
5. The Use of Bowel in Urinary Reconstruction
- The classical approach: implant the transplant ureter into the native bladder portion (to take advantage of some anti-reflux capacity, both inherent and surgically constructed). Many sources suggest performing it this way [72].
- The alternative approach: implant directly into the bowel segment of the augmented bladder [52].
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kusuma Duarsa, G.W.; Gede Oka, A.A.; Santosa, K.B.; Yudiana, I.W.; Wisnu Tirtayasa, P.M.; Putra Pramana, I.B.; Kloping, Y.P. Successful Boari Flap Ureteroneocystostomy for Distal Ureteral Necrosis after Renal Transplantation. Urol. Case Rep. 2018, 23, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kono, Y.; Yagi, Y.; Ishizaka, K.; Suzuki, H. Posttransplant Urine Leakage with Extensive Ureteral Stricture Corrected by Pyelopyelostomy: A Challenging Case. JOJ Urol. Nephrol. 2019, 6, 555690. [Google Scholar] [CrossRef]
- Choate, H.R.; Mihalko, L.A.; Choate, B.T. Urologic Complications in Renal Transplants. Transl. Androl. Urol. 2019, 8, 14147. [Google Scholar] [CrossRef] [PubMed]
- Harza, M.; Baston, C.; Preda, A.; Olaru, V.; Ismail, G.; Domnisor, L.; Daia, D.; Mitroi, I.; Baston, M.O.; Sinescu, I. Impact of Ureteral Stenting on Urological Complications after Kidney Transplantation Surgery: A Single-Center Experience. Transplant. Proc. 2014, 46, 3459–3462. [Google Scholar] [CrossRef]
- Englesbe, M.J.; Dubay, D.A.; Gillespie, B.W.; Moyer, A.S.; Pelletier, S.J.; Sung, R.S.; Magee, J.C.; Punch, J.D.; Campbell, D.A.; Merion, R.M. Risk Factors for Urinary Complications after Renal Transplantation. Am. J. Transplant. 2007, 7, 1536–1541. [Google Scholar] [CrossRef]
- Baston, C.; Harza, M.; Preda, A.; Gener, I.; Manea, I.; Voinea, S.; Olaru, V.; Badescu, B.; Sinescu, I. Comparative Urologic Complications of Ureteroneocystostomy in Kidney Transplantation: Transvesical Leadbetter-Politano versus Extravesical Lich-Gregoir Technique. Transplant. Proc. 2014, 46, 176–179. [Google Scholar] [CrossRef]
- Kato, T.; Selvaggi, G.; Burke, G.; Ciancio, G.; Zilleruelo, G.; Hattori, M.; Gosalbez, R.; Tzakis, A. Partial Bladder Transplantation with En Bloc Kidney Transplant—The First Case Report of a ‘Bladder Patch Technique’ in a Human. Am. J. Transplant. 2008, 8, 1060–1063. [Google Scholar] [CrossRef]
- Neto, H.M.; Tedesco Silva Junior, H.; Pestana, J.M.; Foresto, R.D.; Aguiar, W.F. Urological Complications Associated With Pyeloureterostomy Without Ipsilateral Nephrectomy in Renal Transplant Recipients. Transpl. Int. 2022, 35, 10213. [Google Scholar] [CrossRef]
- Sui, W.; Lipsky, M.; Matulay, J.; Robins, D.; Onyeji, I.; James, M.; Theofanides, M.; Wenske, S. Timing and Predictors of Early Urologic and Infectious Complications After Renal Transplant: An Analysis of a New York Statewide Database. Exp. Clin. Transplant. 2017, 16, 665–670. [Google Scholar] [CrossRef]
- Karam, G.; Maillet, F.; Parant, S.; Soulillou, J.-P.; Giral-Classe, M. Ureteral Necrosis after Kidney Transplantation: Risk Factors and Impact on Graft and Patient Survival. Transplantation 2004, 78, 725–729. [Google Scholar] [CrossRef]
- Fonio, P.; Appendino, E.; Calandri, M.; Faletti, R.; Righi, D.; Gandini, G. Treatment of Urological Complications in More than 1,000 Kidney Transplantations: The Role of Interventional Radiology. Radiol. Med. 2015, 120, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Gunawansa, N.; Sharma, A.; Halawa, A. Post-Transplant Urinary Leak; the Perennial “Achilles Heel” in Renal Transplant Surgery. Trends Transpl. 2018, 11, 1–4. [Google Scholar] [CrossRef]
- Bezherano, I.; Kayler, L.K. Timing of Urinary Catheter Removal Following Kidney Transplantation: A Retrospective Study. Kidney Med. 2022, 4, 100484. [Google Scholar] [CrossRef] [PubMed]
- Schult, M.; Küster, J.; Kliem, V.; Brunkhorst, R.; Nashan, B.; Oldhafer, K.J.; Schlitt, H.J. Native Pyeloureterostomy after Kidney Transplantation: Experience in 48 Cases. Transpl. Int. 2000, 13, 340–343. [Google Scholar] [CrossRef]
- Peretti, N.; Saïd, M.H.; Bouvier, R.; Koch-Nogueira, P.C.; Thouvenot, D.; Martin, X.; Cochat, P. Cytomegalovirus Infection May Cause Ureteral Necrosis. Transplantation 2000, 69, 670–671. [Google Scholar] [CrossRef]
- Ducloux, D.; Bresson-Vautrin, C.; Chalopin, J.M. Is Cytomegalovirus a Cause of Ureteral Stricture in Renal Transplant Recipients? Transpl. Int. 1997, 10, 238–240. [Google Scholar] [CrossRef]
- Minkovich, M.; Famure, O.; Li, Y.; Ghanekar, A.; Selzner, M.; Kim, S.J.; Lee, J.Y. Ureteral Strictures Post-Kidney Transplantation: Trends, Impact on Patient Outcomes, and Clinical Management. Can. Urol. Assoc. J. 2021, 15, E524–E530. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, W.; Peng, Y.; Li, L.; Gao, X.; Liu, M.; Fang, Z.; Wang, Z.; Ming, S.; Dong, H.; et al. Endoscopic Balloon Dilatation in the Treatment of Benign Ureteral Strictures: A Meta-Analysis and Systematic Review. J. Endourol. 2019, 33, 255–262. [Google Scholar] [CrossRef]
- Bilotta, A.; Wiegand, L.R.; Heinsimer, K.R. Ureteral Reconstruction for Complex Strictures: A Review of the Current Literature. Int. Urol. Nephrol. 2021, 53, 2211–2219. [Google Scholar] [CrossRef]
- Chen, P.-D.; Lee, C.-Y.; Yang, C.-Y.; Tsai, M.-K. Successful Rescue of Transplant Ureteral Obstruction by Boari Flap. Transplantation 2014, 98, e75–e76. [Google Scholar] [CrossRef]
- Kroczak, T.; Koulack, J.; McGregor, T. Management of Complicated Ureteric Strictures After Renal Transplantation: Case Series of Pyelovesicostomy With Boari Flap. Transplant. Proc. 2015, 47, 1850–1853. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ameli-Renani, S.; Hakim, A.; Jeon, J.H.; Shrivastava, S.; Patel, U. Ureteral Obstruction Following Renal Transplantation: Causes, Diagnosis and Management. Br. J. Radiol. 2014, 87, 20140169. [Google Scholar] [CrossRef] [PubMed]
- Alberts, V.P.; Idu, M.M.; Legemate, D.A.; Laguna Pes, M.P.; Minnee, R.C. Ureterovesical Anastomotic Techniques for Kidney Transplantation: A Systematic Review and Meta-Analysis. Transpl. Int. 2014, 27, 593–605. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Friend, P.J. Chapter 11—Surgical Techniques of Kidney Transplantation. In Kidney Transplantation–Principles and Practice, 7th ed.; Morris, P.J., Knechtle, S.J., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2014; pp. 161–175. [Google Scholar] [CrossRef]
- Streeter, E.H.; Little, D.M.; Cranston, D.W.; Morris, P.J. The Urological Complications of Renal Transplantation: A Series of 1535 Patients. BJU Int. 2002, 90, 627–634. [Google Scholar] [CrossRef]
- Lich, R. Obstructive Diseases of the Urinary Tract in Children. J. Ark. Med. Soc. 1961, 58, 127–130. [Google Scholar]
- Woodruff, M.F.; Nolan, B.; Robson, J.S.; MacDonald, M.K. Renal Transplantation in Man. Experience in 35 Cases. Lancet 1969, 1, 6–12. [Google Scholar] [CrossRef]
- Konnak, J.W.; Herwig, K.R.; Turcotte, J.G. External Ureteroneocystostomy in Renal Transplantation. J. Urol. 1972, 108, 380–381. [Google Scholar] [CrossRef]
- Barry, J.M.; Morris, P.J. Surgical Techniques of Renal Transplantation. In Kidney Transplantation; Elsevier Inc.: Amsterdam, The Netherlands, 2008; pp. 158–171. [Google Scholar] [CrossRef]
- Thrasher, J.B.; Temple, D.R.; Spees, E.K. Extravesical versus Leadbetter-Politano Ureteroneocystostomy: A Comparison of Urological Complications in 320 Renal Transplants. J. Urol. 1990, 144, 1105–1109. [Google Scholar] [CrossRef]
- Butterworth, P.C.; Horsburgh, T.; Veitch, P.S.; Bell, P.R.; Nicholson, M.L. Urological Complications in Renal Transplantation: Impact of a Change of Technique. Br. J. Urol. 1997, 79, 499–502. [Google Scholar] [CrossRef]
- Tso, P.L.; Pearson, T.C. Kidney Transplantation Procedure and Surgical Technique. In Textbook of Organ Transplantation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 617–630. [Google Scholar] [CrossRef]
- Smith, J.A.; Howards, S.S.; Preminger, G.M. Hinman’s Atlas of Urologic Surgery, Expert Consult—Online and Print,3: Hinman’s Atlas of Urologic Surgery; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Izquierdo, L.; Peri, L.; Piqueras, M.; Revuelta, I.; Alvarez-Vijande, R.; Musquera, M.; Oppenheimer, F.; Alcaraz, A. Third and Fourth Kidney Transplant: Still a Reasonable Option. Transplant. Proc. 2010, 42, 2498–2502. [Google Scholar] [CrossRef]
- Delbridge, M.S.; Raftery, A.T. Renal Transplantation. Medicine 2007, 35, 479–482. [Google Scholar] [CrossRef]
- Arudchelvam, J. Surgical Aspects of Kidney Transplantation Kidney Transplantation; In Kidney Transplantation; Las Vegas, NV, USA. 2019. Available online: https://www.researchgate.net/publication/337010753_Surgical_Aspects_of_Kidney_Transplantation_Kidney_Transplantation (accessed on 1 June 2025).
- Mangus, R.S.; Haag, B.W. Stented versus Nonstented Extravesical Ureteroneocystostomy in Renal Transplantation: A Metaanalysis. Am. J. Transplant. 2004, 4, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Mure, P.-Y.; Mouriquand, P.D.E. Surgical Atlas The Cohen Procedure. BJU Int. 2004, 94, 679–698. [Google Scholar] [CrossRef] [PubMed]
- Merrill, J.P.; Murray, J.E.; Harrison, J.H.; Guild, W.R. Successful Homotransplantation of the Human Kidney between Identical Twins. J. Am. Med. Assoc. 1956, 160, 277–282. [Google Scholar] [CrossRef]
- Starzl, T.E. The Development of Clinical Renal Transplantation. Am. J. Kidney Dis. 1990, 16, 548–556. [Google Scholar] [CrossRef]
- Stein, R.; Rubenwolf, P.; Ziesel, C.; Kamal, M.M.; Thüroff, J.W. Psoas Hitch and Boari Flap Ureteroneocystostomy. BJU Int. 2013, 112, 137–155. [Google Scholar] [CrossRef]
- Law, Z.W.; Ong, C.C.P.; Yap, T.-L.; Loh, A.H.P.; Joseph, U.; Sim, S.W.; Ong, L.Y.; Low, Y.; Jacobsen, A.S.; Chen, Y. Extravesical vs. Intravesical Ureteric Reimplantation for Primary Vesicoureteral Reflux: A Systematic Review and Meta-Analysis. Front. Pediatr. 2022, 10, 935082. [Google Scholar] [CrossRef]
- Berli, J.U.; Montgomery, J.R.; Segev, D.L.; Ratner, L.E.; Maley, W.R.; Cooper, M.; Melancon, J.K.; Burdick, J.; Desai, N.M.; Dagher, N.N.; et al. Surgical Management of Early and Late Ureteral Complications after Renal Transplantation: Techniques and Outcomes. Clin. Transplant. 2015, 29, 26–33. [Google Scholar] [CrossRef]
- Cheng, N.; Ahmed, M.; Stifelman, M.D. Ureterolysis and Boari Flap. In Techniques of Robotic Urinary Tract Reconstruction: A Complete Approach; Stifelman, M.D., Zhao, L.C., Eun, D.D., Koh, C.J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 123–140. [Google Scholar] [CrossRef]
- Bansal, A.; Sinha, R.J.; Jhanwar, A.; Prakash, G.; Purkait, B.; Singh, V. Laparoscopic Ureteral Reimplantation with Boari Flap for the Management of Long- Segment Ureteral Defect: A Case Series with Review of the Literature. Turk. J. Urol. 2017, 43, 313–318. [Google Scholar] [CrossRef]
- Di Michele, S.; Bramante, S.; Rosati, M. A Systematic Review of Ureteral Reimplantation Techniques in Endometriosis: Laparoscopic Versus Robotic-Assisted Approach. J. Clin. Med. 2024, 13, 5677. [Google Scholar] [CrossRef]
- NARUS 2019: Boari Flap—Stepwise Approach to Surgical Management. Available online: https://www.urotoday.com/recent-abstracts/endourology-urolithiasis/minimally-invasive-procedures/110130-narus-2019-boari-flap-stepwise-approach-to-surgical-management.html (accessed on 22 March 2025).
- Burks, F.N.; Santucci, R.A. Management of Iatrogenic Ureteral Injury. Ther. Adv. Urol. 2014, 6, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Tonyali, S.; Haberal, H.B.; Bilen, C.Y.; Aki, F.T. Feasibility of Boari Bladder Flap Procedure in Patients With Heterotrophic Renal Transplant. Exp. Clin. Transplant. 2019, 17, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Alsaadi, S.; Toussi, H.; Alseiari, M.; Al Ahmed, M.; Zaman, M. POS-749 Boari Flap Ureteric Re-Implantation, a Salvage Procedure for Kidney Transplant Ureteral Stenosis. Kidney Int. Rep. 2022, 7 (Suppl. S2), S324. [Google Scholar] [CrossRef]
- Navratil, P.; Sahi, S.; Spacek, J.; Pacovsky, J.; Lesko, M.; Gunka, I.; Astapenko, D. Pyelovesicostomy as an Alternative Surgical Treatment for Complex Ureteral Lesions After Kidney Transplant. Exp. Clin. Transplant. 2023, 21, 712–716. [Google Scholar] [CrossRef]
- Tan, L.R.L.; Tiong, H.Y. Ureteric Implantation into the Bowel Portion of Augmented Bladders during Kidney Transplantation: A Review of Urological Complications and Outcomes. ANZ J. Surg. 2019, 89, 930–934. [Google Scholar] [CrossRef]
- Rajfer, J.; Koyle, M.A.; Ehrlich, R.M.; Smith, R.B. Pyelovesicostomy as a Form of Urinary Reconstruction in Renal Transplantation. J. Urol. 1986, 136, 372–375. [Google Scholar] [CrossRef]
- Corriere, J.N.; Perloff, L.J.; Barker, C.F.; Henderson, L.W.; Schoenberg, H.W.; Murphy, J.J. The Ureteropyelostomy in Human Renal Transplantation. J. Urol. 1973, 110, 24–26. [Google Scholar] [CrossRef]
- Witters, G.; Baert, L. Secondary Pyelo-Pyelic Anastomosis in Renaltransplant Patients. Urology 1990, 36, 183–185. [Google Scholar] [CrossRef]
- Wagner, M.; Dieckmann, K.P.; Klän, R.; Fielder, U.; Offermann, G. Rescue of Renal Transplants with Distal Ureteral Complications by Pyelo-Pyelostomy. J. Urol. 1994, 151, 578–581. [Google Scholar] [CrossRef]
- Timsit, M.-O.; Lalloué, F.; Bayramov, A.; Taylor, M.; Billaut, C.; Legendre, C.; Kreis, H.; Badet, L.; Méjean, A. Should Routine Pyeloureterostomy Be Advocated in Adult Kidney Transplantation? A Prospective Study of 283 Recipients. J. Urol. 2010, 184, 2043–2048. [Google Scholar] [CrossRef]
- Martin, X.; Aboutaieb, R.; Soliman, S.; el Essawy, A.; Dawahra, M.; Lefrancois, N. The Use of Long-Term Defunctionalized Bladder in Renal Transplantation: Is It Safe? Eur. Urol. 1999, 36, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, K.; Müller, M.K.; Schiesser, M.; Wildi, S.; Fehr, T.; Wüthrich, R.P.; Clavien, P.-A.; Weber, M. Treatment of Ureteral Complications after Kidney Transplantation with Native Ureteropyelostomy Reduces the Risk of Pyelonephritis. Clin. Transplant. 2011, 25, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Riediger, C.; Müller, M.W.; Bachmann, J.; Novotny, A.; Thorban, S.; Matevossian, E.; Friess, H.; Stangl, M. Native Ureteropyelostomy: An Effective Therapy for Urinary Tract Complications Following Kidney Transplantation. ANZ J. Surg. 2014, 84, 643–648. [Google Scholar] [CrossRef]
- Saidi, R.F.; Elias, N.; Hertl, M.; Kawai, T.; Cosimi, A.B.; Ko, D.S.C. Urinary Reconstruction after Kidney Transplantation: Pyeloureterostomy or Ureteroneocystostomy. J. Surg. Res. 2013, 181, 156–159. [Google Scholar] [CrossRef]
- Barry, J.M. Surgical Atlas Transureteroureterostomy. BJU Int. 2005, 96, 195–201. [Google Scholar] [CrossRef]
- Tyagi, V.; Jain, S.; Singh, M.; Pahwa, M.; Chadha, S.; Rasool, S. Native Ureteroureterostomy in Renal Allograft Recipient Surgery: A Single-Center 5-Year Experience. Indian J. Urol. 2019, 35, 218–221. [Google Scholar] [CrossRef]
- McDonald, J.C.; Rohr, M.S.; Frentz, G.D. External Ureteroneocystostomy and Ureteroureterostomy in Renal Transplantation. Ann. Surg. 1979, 190, 663–667. [Google Scholar] [CrossRef]
- Chaykovska, L.; Deger, S.; Wille, A.; Friedersdorff, F.; Kasper, A.; Dragun, D.; Liefeldt, L.; Miller, K.; Giessing, M.; Fuller, T.F. Kidney Transplantation Into Urinary Conduits With Ureteroureterostomy Between Transplant and Native Ureter: Single-Center Experience. Urology 2009, 73, 380–385. [Google Scholar] [CrossRef]
- Leão, C.S.; Foinquinos, R.A.; Leao, A.L.D.S.; Capela, I.C.K.O.; Mello, M.J.G. Ultrashort Anisoperistaltic End-to-Side Ureteroureterostomy in Renal Transplantation. Rev. Col. Bras. Cir. 2022, 49, e20223365. [Google Scholar] [CrossRef]
- Whelchel, D.J.; Cosimi, A.B.; Young, H.H.; Russell, P.S. Pyeloureterostomy Reconstruction in Human Renal Transplantation. Ann. Surg. 1975, 181, 61–66. [Google Scholar] [CrossRef]
- Bueno Jimenez, A.; Larreina, L.; Serradilla, J.; de Borja Nava, F.; Lobato, R.; Rivas, S.; Lopez-Pereira, P.; García, L.; Espinosa, L.; Martinez-Urrutia, M.J. Upside-down Kidney Placement: An Alternative in Pediatric Renal Transplantation. J. Pediatr. Surg. 2021, 56, 1417–1420. [Google Scholar] [CrossRef]
- Serrell, E.C.; Su, R.; O’Kelly, F.; Semanik, M.; Farhat, W.A. The Utility of Native Ureter in the Management of Ureteral Complications in Children after Renal Transplantation. Pediatr. Transplant. 2021, 25, e14051. [Google Scholar] [CrossRef] [PubMed]
- Penna, F.J.; Lorenzo, A.J.; Farhat, W.A.; Butt, H.; Koyle, M.A. Ureteroureterostomy: An Alternative to Ureteroneocystostomy in Select Cases of Pediatric Renal Transplantation. J. Urol. 2017, 197 Pt 2, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Tanna, R.J.; Powell, J.; Mambu, L.A. Ileal Conduit. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Wang, Z.Z.; Wang, C.; Cunningham, E.C.; Allen, R.D.M.; Sharland, A.F.; Bishop, G.A. Optimized Method for Ureteric Reconstruction in a Mouse Kidney Transplant Model. ANZ J. Surg. 2014, 84, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Naspro, R. Ileal Conduit as the Standard for Urinary Diversion After Radical Cystectomy for Bladder Cancer. Eur. Urol. Suppl. 2010, 9, 736–744. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Reinberg, Y.; Gonzalez, R.; Fryd, D.; Najarian, J.S. Outcome of Renal Transplantation after Urinary Diversion and Enterocystoplasty: A Retrospective, Controlled Study. J. Urol. 1990, 144, 1349–1351. [Google Scholar] [CrossRef]
- Surange, R.S.; Johnson, R.W.G.; Tavakoli, A.; Parrott, N.R.; Riad, H.N.; Campbell, B.A.; Augustine, T. Kidney Transplantation into an Ileal Conduit: A Single Center Experience of 59 Cases. J. Urol. 2003, 170, 1727–1730. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Tanabe, K.; Ota, T.; Ito, K.; Toma, H. Renal Transplantation into Augmented Bladders. Int. J. Urol. 1998, 5, 423–427. [Google Scholar] [CrossRef]
- Lopez Pereira, P.; Ortiz Rodriguez, R.; Fernandez Camblor, C.; Martínez Urrutia, M.J.; Lobato Romera, R.; Espinosa, L.; Jaureguizar Monereo, E. Renal Transplant Outcome in Children with an Augmented Bladder. Front. Pediatr. 2013, 1, 42. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novacescu, D.; Abol-Enein, H.; Latcu, S.; Zara, F.; Secasan, C.-C.; Barbos, V.; Pasecinic, V.; Musta, M.; Albarakaty, A.M.; Bakhsh, A.; et al. Ureteric Complications and Urinary Tract Reconstruction Techniques in Renal Transplantation: A Surgical Essay. J. Clin. Med. 2025, 14, 4129. https://doi.org/10.3390/jcm14124129
Novacescu D, Abol-Enein H, Latcu S, Zara F, Secasan C-C, Barbos V, Pasecinic V, Musta M, Albarakaty AM, Bakhsh A, et al. Ureteric Complications and Urinary Tract Reconstruction Techniques in Renal Transplantation: A Surgical Essay. Journal of Clinical Medicine. 2025; 14(12):4129. https://doi.org/10.3390/jcm14124129
Chicago/Turabian StyleNovacescu, Dorin, Hassan Abol-Enein, Silviu Latcu, Flavia Zara, Cosmin-Ciprian Secasan, Vlad Barbos, Victor Pasecinic, Mihael Musta, Ahmad Mohammed Albarakaty, Abdulaziz Bakhsh, and et al. 2025. "Ureteric Complications and Urinary Tract Reconstruction Techniques in Renal Transplantation: A Surgical Essay" Journal of Clinical Medicine 14, no. 12: 4129. https://doi.org/10.3390/jcm14124129
APA StyleNovacescu, D., Abol-Enein, H., Latcu, S., Zara, F., Secasan, C.-C., Barbos, V., Pasecinic, V., Musta, M., Albarakaty, A. M., Bakhsh, A., Ismail, H., & Cumpanas, A. A. (2025). Ureteric Complications and Urinary Tract Reconstruction Techniques in Renal Transplantation: A Surgical Essay. Journal of Clinical Medicine, 14(12), 4129. https://doi.org/10.3390/jcm14124129





