Arthroscopic Partial Trapeziectomy and Free Tendon Suspension and Interposition Combined with Internal Brace for Basal Joint Arthritis of Thumb
Abstract
1. Introduction
2. Materials and Methods
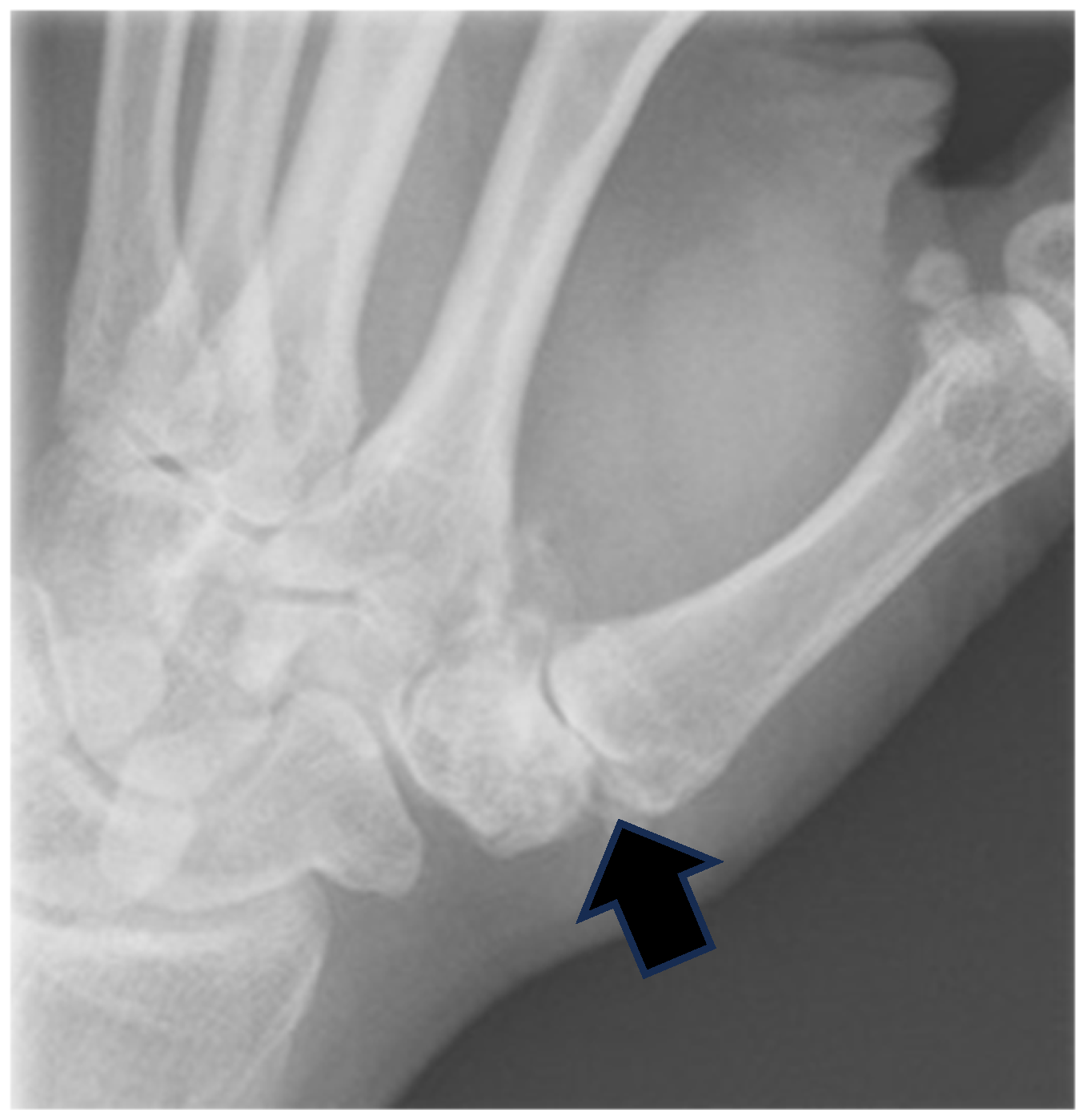
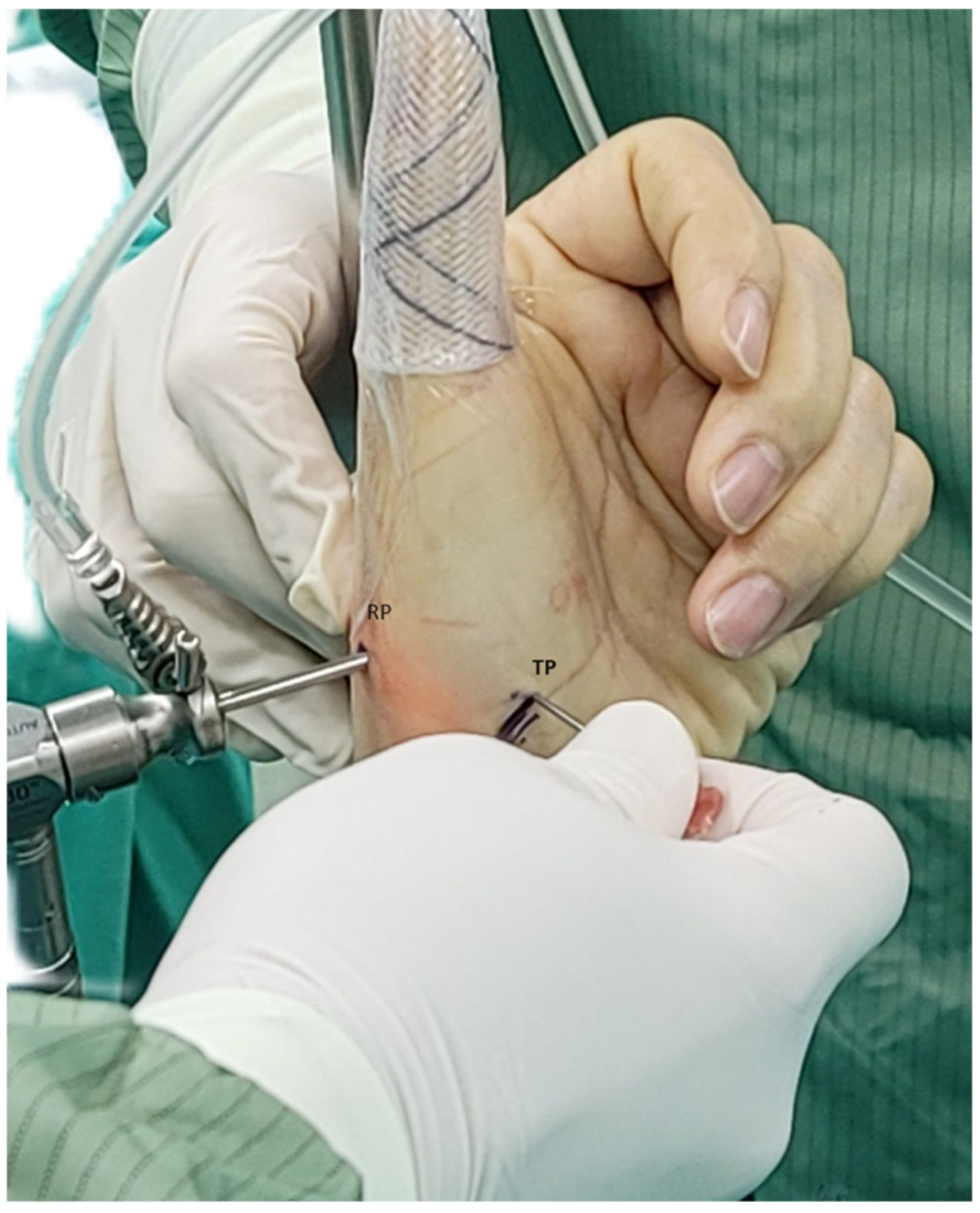
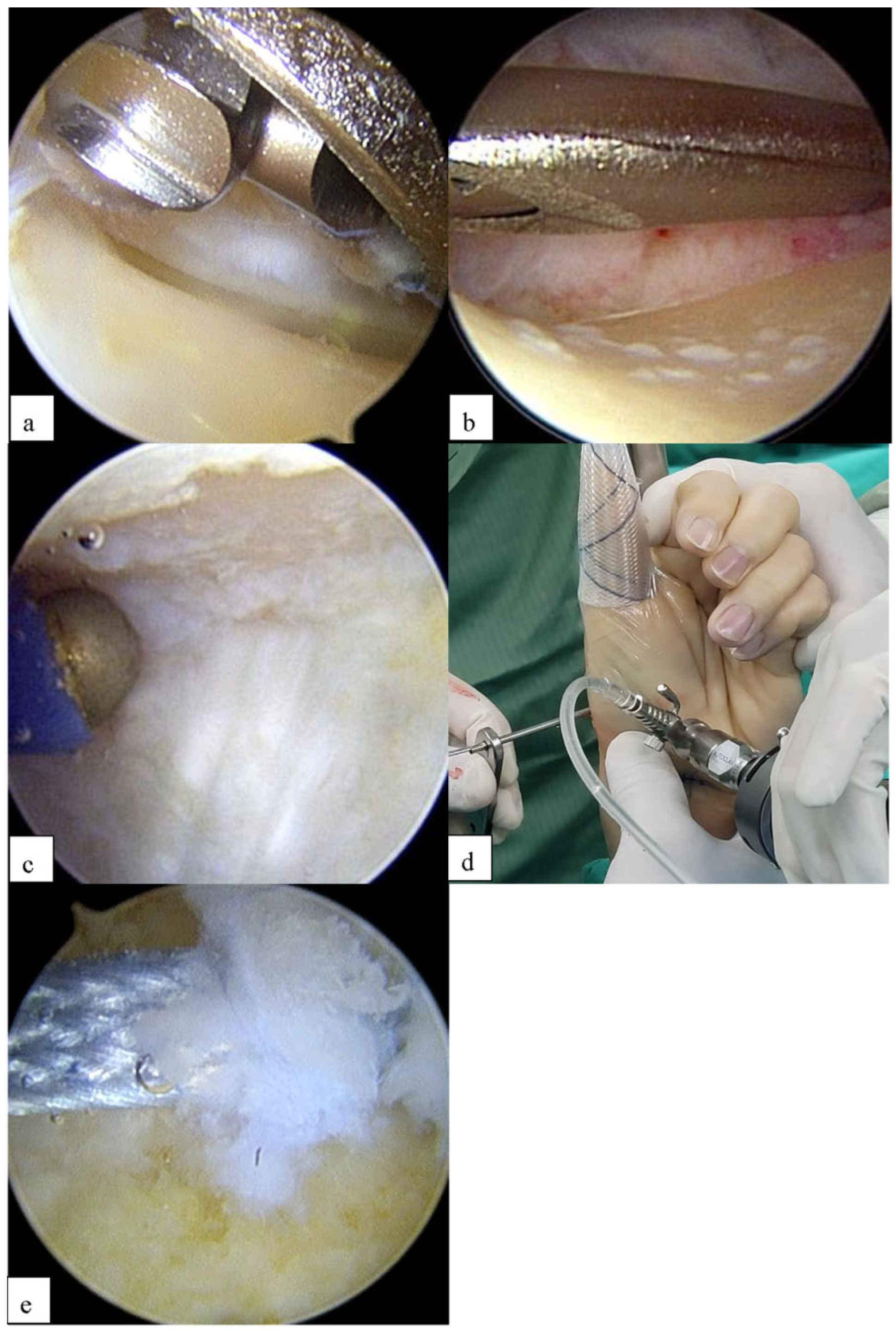
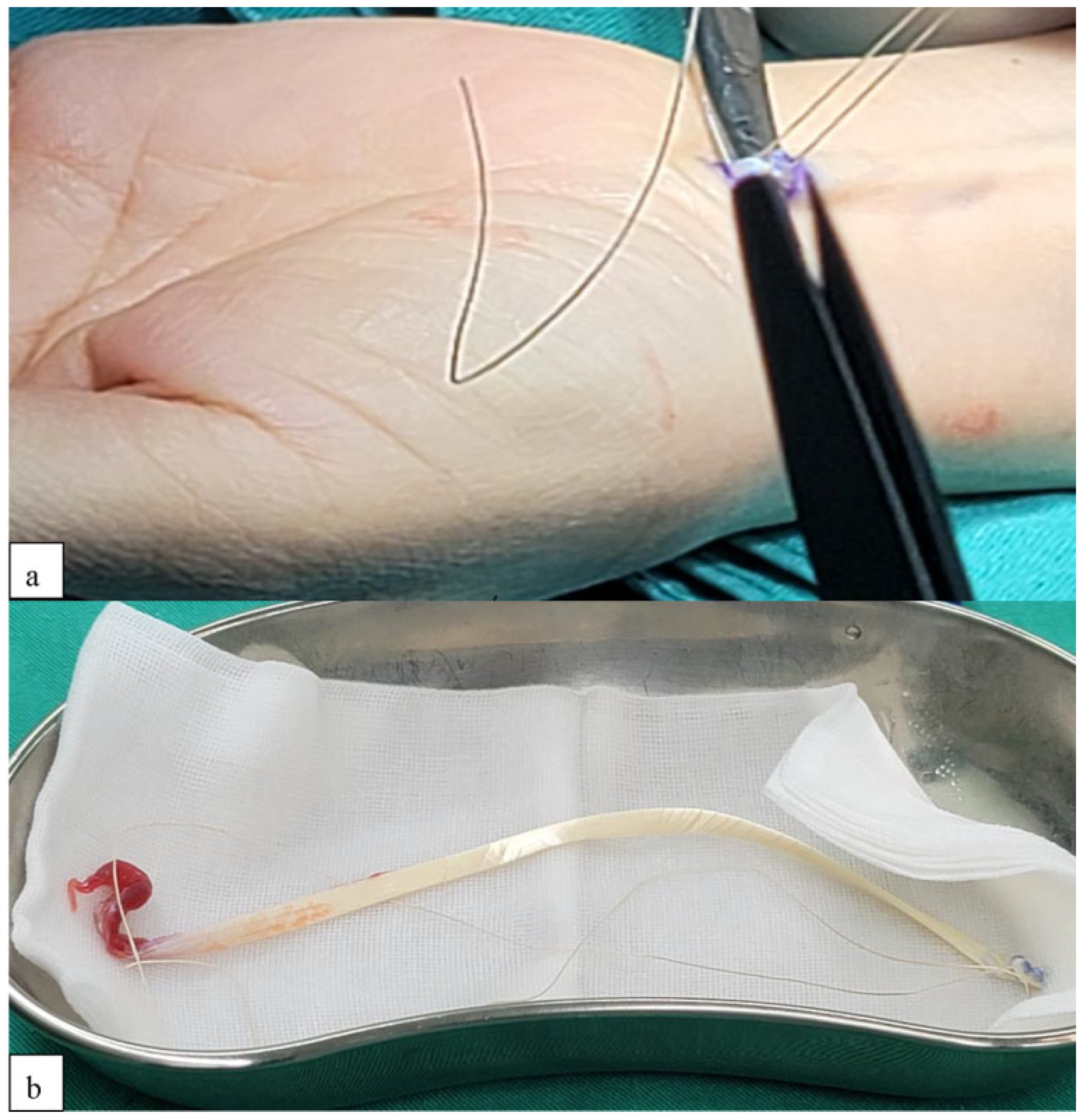
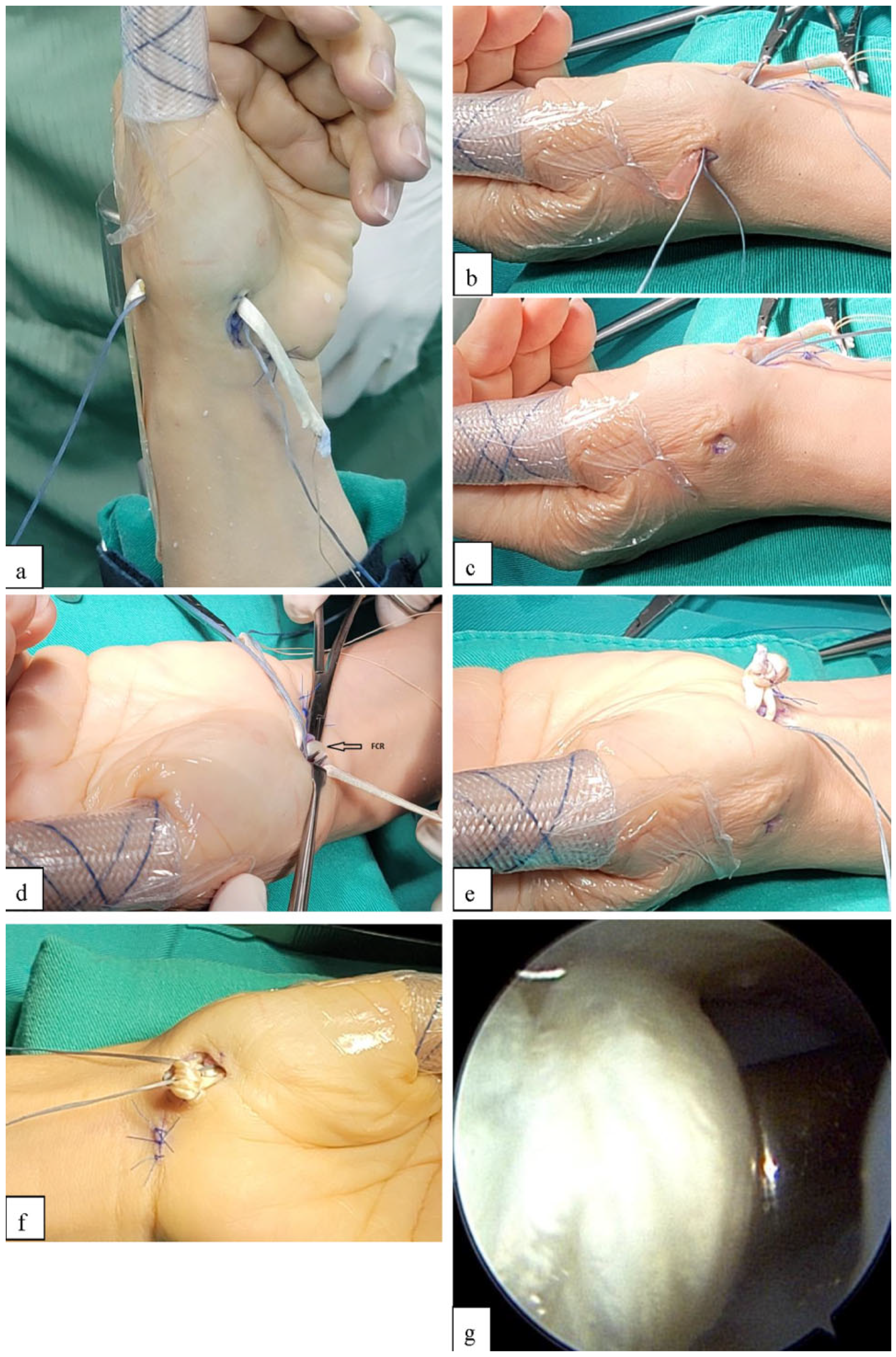
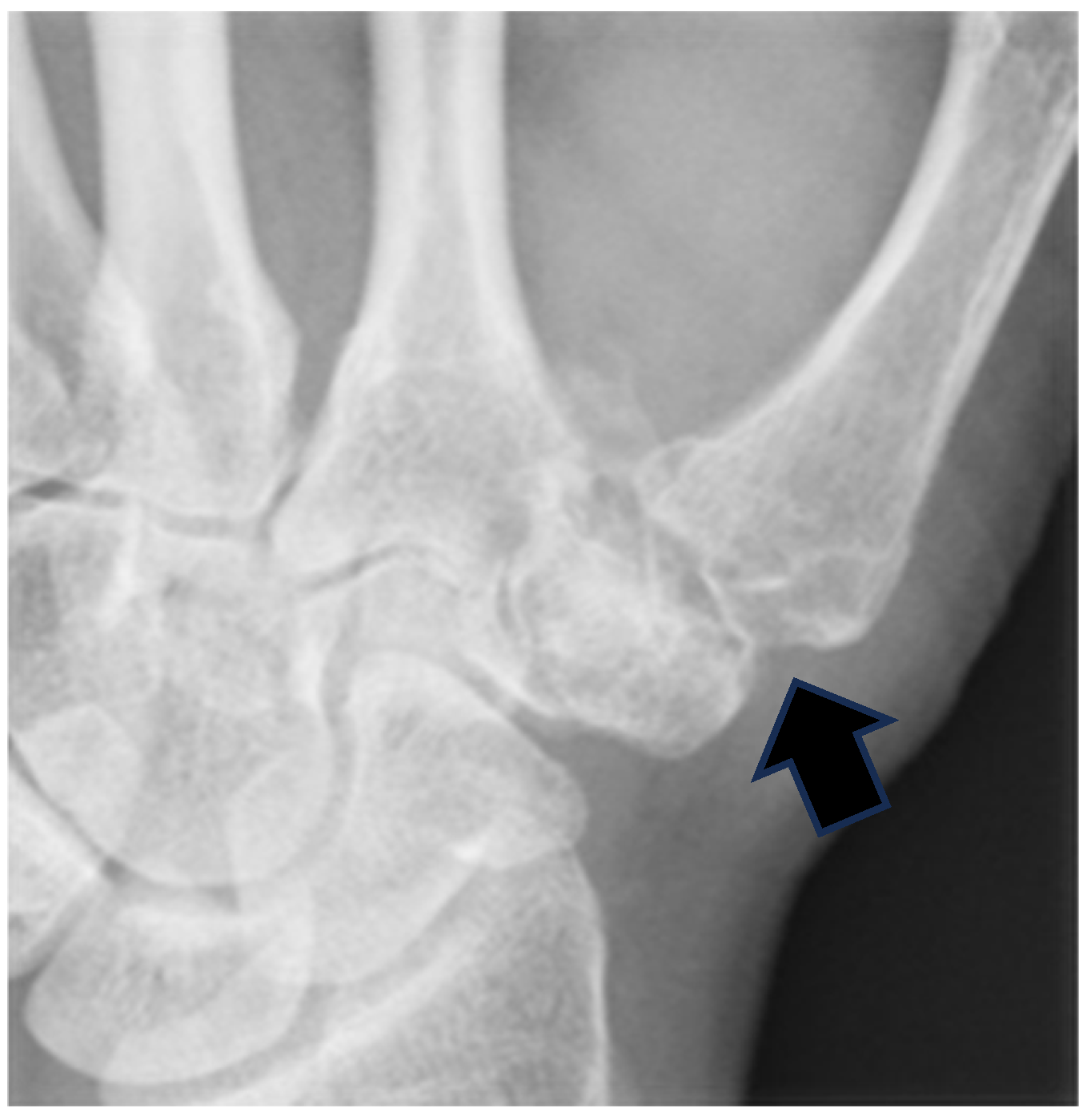
2.1. Surgical Technique
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APL | Abductor pollicis longus |
| CMC | Carpometacarpal |
| DASH | Disabilities of the Arm, Shoulder, and Hand |
| EPB | Extensor pollicis brevis |
| FCR | Flexor carpi radialis |
| LRTI | Ligament reconstruction and tendon interposition |
| PL | Palmar longus |
| ROM | Range of motion |
| RP | Radial portal |
| STT | Scaphotrapezial |
| TLA | Three-letter acronym |
| TP | Thenar portal |
References
- Buton, R.I.; Pellegrini, V.D., Jr. Surgical management of basal joint arthritis of the thumb. Part II. Ligament reconstruction with tendon interposition arthroplasty. J. Hand Surg. Am. 1986, 11, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Barron, O.A.; Eaton, R.G. Save the trapezium: Double interposition arthroplasty for the treatment of stage IV disease of the basal joint. J. Hand Surg. Am. 1998, 23, 196–204. [Google Scholar] [CrossRef]
- Menon, J. Partial trapeziectomy and interpositional arthroplasty for trapeziometacarpal osteoarthritis of the thumb. J. Hand Surg. Br. 1995, 20, 700–706. [Google Scholar] [CrossRef]
- Mo, J.H.; Gelberman, R.H. Ligament reconstruction with trapezium retention arthroplasty for carpometacarpal arthritis. J. Hand Surg. Am. 2004, 29, 240–246. [Google Scholar] [CrossRef]
- Berger, R.A. A technique for arthroscopic evaluation of the first carpometacarpal joint. J. Hand Surg. Am. 1997, 22, 1077–1080. [Google Scholar] [CrossRef]
- Eaton, R.G.; Glickel, S.Z. Trapeziometacarpal osteoarthritis. Staging as a rationale for treatment. Hand Clin. 1987, 3, 455–471. [Google Scholar] [CrossRef]
- Chu, P.J.; Lee, H.M.; Chung, L.J.; Shih, J.T. Electrothermal treatment of thumb basal joint instability. Arthroscopy 2009, 25, 290–295. [Google Scholar] [CrossRef]
- Aceituno-Gómez, J.; Avendaño-Coy, J.; Criado-Álvarez, J.J.; Ávila-Martín, G.; Marín-Guerrero, A.C.; Mohedano-Moriano, A.; Mohedano, A.; Viñuela, A. Correlation between three assessment pain tools in subacromial pain syndrome. Clin. Rehabil. 2020, 35, 114–118. [Google Scholar] [CrossRef]
- Atar, E.; Aşkın, A. Somatosensory dysfunction related neuropathic pain component affects disease activity, functional status and quality of life in ankylosing spondylitis. Int. J. Rheum. Dis. 2020, 23, 1656–1663. [Google Scholar] [CrossRef]
- Angst, F.; Drerup, S.; Werle, S.; Herren, D.B.; Simmen, B.R.; Goldhahn, J. Prediction of grip and key pinch strength in 978 healthy subjects. BMC Musculoskelet. Disorders. 2010, 11, 94. [Google Scholar] [CrossRef]
- Silva, G.S.d.; Lourenço, M.d.A.; Assis, M.R.d. Hand strength in patients with ra correlates strongly with function but not with activity of disease. Adv. Rheumatol. 2018, 58, 20. [Google Scholar] [CrossRef] [PubMed]
- McQuillan, T.J.; Kenney, D.; Crisco, J.J.; Weiss, A.; Ladd, A.L. Weaker functional pinch strength is associated with early thumb carpometacarpal osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Werle, S.; Goldhahn, J.; Drerup, S.; Simmen, B.R.; Sprott, H.; Herren, D.B. Age- and gender-specific normative data of grip and pinch strength in a healthy adult swiss population. J. Hand Surg. (Eur. Vol.) 2009, 34, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Badia, A. Trapeziometacarpal arthroscopy. A classification and treatment algorithm. Hand Clin. 2006, 22, 153–163. [Google Scholar] [CrossRef]
- Culp, R.W.; Rekant, M.S. The role of arthroscopy in evaluating and treating trapeziometacarpal disease. Hand Clin. 2001, 17, 315–319. [Google Scholar] [CrossRef]
- Edwards, S.G.; Osterman, A.L. Trapeziectomy with thermal capsular modification. In Wrist Arthroscopy; Geissler, W.B., Ed.; Springer: New York, NY, USA, 2005; pp. 170–181. [Google Scholar]
- Watanabe, M. Present status and future of arthroplasty. Geka Chiryo 1972, 26, 73–77. [Google Scholar]
- Chen, Y.C. Arthroscopy of the wrist and finger joints. Orthop. Clin. North Am. 1979, 10, 723–733. [Google Scholar]
- Ryu, J.; Fagan, R. Arthroscopic treatment of acute complete thumb metacarpophalangeal ulnar collateral ligament tears. J. Hand Surg. 1995, 20, 1037–1042. [Google Scholar] [CrossRef]
- Vaupel, G.L.; Andrews, J.R. Diagnostic and operative arthroscopy of the thumb metacarpophalangeal joint. A case report. Am. J. Sports Med. 1985, 13, 139–141. [Google Scholar] [CrossRef]
- Froimson, A.I. Tendon arthroplasty of the trapeziometacarpal joint. Clin. Orthop. Rel. Res. 1970, 70, 191–199. [Google Scholar] [CrossRef]
- Smeraglia, F.; Barrera-Ochoa, S.; Mendez-Sanchez, G.; Basso, M.A.; Balato, G.; Mir-Bullo, X. Partial trapeziectomy and pyrocarbon interpositional arthroplasty for trapeziometacarpal osteoarthritis: Minimum 8-year follow-up. J. Hand Surg. Eur. Vol. 2020, 45, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.A.; Mosher, J.F. Gortex arthroplasty for trapeziometacarpal joint. In Proceedings of the 65th Annual Meeting of the American Society for Surgery of the Hand, Toronto, ON, Canada, 24–27 September 1990. [Google Scholar]
- Greenberg, J.A. X-ray changes after Gortex interpositional arthroplasty. Evidence of particulate synovitis. In Proceedings of the Poster Exhibit, Annual Meeting of the American Society for Surgery of the Hand, Cincinnati, OH, Canada, 26–29 October 1994. [Google Scholar]
- North, E.R.; Eaton, R.G. Degenerative joint disease of the trapezium: A comparative radiographic and anatomic study. J. Hand Surg. Am. 1983, 8, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Menon, J.; Schoene, H.R.; Hohl, J.E. Trapeziometacarpal arthritis—Results of tendon interpositional arthroplasty. J. Hand Surg. Am. 1981, 6, 442–446. [Google Scholar] [CrossRef]
- Eaton, R.G.; Glickel, S.Z.; Littler, J.W. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J. Hand Surg. Am. 1985, 10, 645–654. [Google Scholar] [CrossRef]
- Menon, J. The Problem of trapeziometacarpal degenerative arthritis. Clin. Orthop. Rel. Res. 1983, 175, 155–165. [Google Scholar] [CrossRef]
- Froimson, A.I. Tendon interposition arthroplasty of carpometacarpal joint of the thumb. Hand Clin. 1987, 3, 489–505. [Google Scholar] [CrossRef]
- Mathoulin, C.; Moreel, P.; Costa, R.O.d.; Wilson, S. Abductor pollicis longus “hammock” ligamentoplasty for treatment of first carpometacarpal arthritis. J. Hand Surg. (Eur. Vol.) 2008, 33, 292–297. [Google Scholar] [CrossRef]
- Gibbons, C.E.R.; Gosal, H.S.; Choudri, A.H.; Magnussen, P.A. Trapeziectomy for basal thumb joint osteoarthritis: 3- to 19-year follow-up. Int. Orthop. 1999, 23, 216–218. [Google Scholar] [CrossRef]
- Raven, E.E.J.; Kerkhoffs, G.M.M.J.; Rutten, S.; Marsman, A.J.W.; Marti, R.K.; Albers, G.H.R. Long term results of surgical intervention for osteoarthritis of the trapeziometacarpal joint. Int. Orthop. 2006, 31, 547–554. [Google Scholar] [CrossRef]
- Vermeulen, G.M.; Slijper, H.P.; Feitz, R.; Hovius, S.E.R.; Moojen, T.M.; Selles, R.W. Surgical management of primary thumb carpometacarpal osteoarthritis: A systematic review. J. Hand Surg. 2011, 36, 157–169. [Google Scholar] [CrossRef]
- Kemper, R.; Wirth, J.; Baur, E. Arthroscopic synovectomy combined with autologous fat grafting in early stages of cmc osteoarthritis of the thumb. J. Wrist Surg. 2017, 07, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Carità, E.; Donadelli, A.; Laterza, M.; Rossettini, G.; Villafañe, J.H.; Perazzini, P. Assessing the predictive accuracy of the eaton–littler classification in thumb carpometacarpal osteoarthritis: A comparative analysis with the outerbridge classification in a cohort of 51 cases. Diagnostics 2024, 14, 1703. [Google Scholar] [CrossRef]
- Edwards, S.G.; Ramsey, P. Prospective outcomes of stage iii thumb carpometacarpal arthritis treated with arthroscopic hemitrapeziectomy and thermal capsular modification without interposition. J. Hand Surg. 2010, 35, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, S.C.; Bargon, C.A.; Mohamadi, A.; Chen, N.C.; Coert, J.H. A systematic review and meta-analysis of arthroscopic assisted techniques for thumb carpometacarpal joint osteoarthritis. J. Hand Surg. (Eur. Vol.) 2018, 43, 1098–1105. [Google Scholar] [CrossRef]
- Martín-Ferrero, M.Á. Ten-year long-term results of total joint arthroplasties with arpe® implant in the treatment of trapeziometacarpal osteoarthritis. J. Hand Surg. (Eur. Vol.) 2013, 39, 826–832. [Google Scholar] [CrossRef]
- Oh, W.; Chun, Y.; Koh, I.; Shin, J.; Choi, Y.; Kang, H.J. Tendon versus pyrocarbon interpositional arthroplasty in the treatment of trapeziometacarpal osteoarthritis. BioMed Res. Int. 2019, 2019, 7961507. [Google Scholar] [CrossRef]
- Ferrão, A.; Morais, B.S.d.; Marques, N.; Nóbrega, J.; Monteiro, J.E.d.S.; Jorge, J.T.; Jorge, J.T.; Teixeira, F. Trapeziectomy and suture-button suspensionplasty for basilar thumb arthritis: Is it enough to prevent first ray subsidence? J. Hand Microsurg. 2023, 15, 23–30. [Google Scholar] [CrossRef]
- Hansen, T.B.; Mosegaard, K.B.; Sørensen, O.G.; Mortensen, J.; Stilling, M. Bone mineral density of the trapezium in osteoarthritic trapeziometacarpal joints. J. Hand Surg. (Eur. Vol.) 2012, 38, 875–879. [Google Scholar] [CrossRef]
- Fulchignoni, C.; Morini, A.; Panzera, R.M.; Merendi, G.; Rocchi, L.F. brunelli ligamentoplasty as treatment in thumb basal joint arthritis: Up to 9 years follow-up. Tech. Hand Up. Extrem. Surg. 2020, 25, 77–83. [Google Scholar] [CrossRef]
- Yao, J. Suture-button suspensionplasty for the treatment of thumb carpometacarpal joint arthritis. Hand Clin. 2012, 28, 579–585. [Google Scholar] [CrossRef]
- Spiteri, M.; Giele, H. Systematic review of thumb carpometacarpal joint hemiresection interposition arthroplasty materials. Hand 2020, 17, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Maio, F.D.; Farsetti, P.; Potenza, V.; Petrungaro, L.; Marsiolo, M.; Caterini, A. Surgical treatment of primary trapezio-metacarpal osteoarthritis by trapeziectomy and ligament reconstruction without tendon interposition. long-term results of 50 cases. J. Orthop. Traumatol. 2019, 20, 25. [Google Scholar] [CrossRef]

| Eaton Stage | Total | p-Value | ||
|---|---|---|---|---|
| 3 | 2 | |||
| n | 23 | 37 | 60 | |
| Age | 60.17 ± 3.31 | 64.05 ± 4.22 | 62.57 ± 4.31 | <0.001 * |
| Sex (%) | 1.000 | |||
| Male | 3 (13.0%) | 5 (13.5%) | 8 (13.3%) | |
| Female | 20 (87.0%) | 32 (86.5%) | 52 (86.7%) | |
| PreOP VAS for thumb pain (rest) | 5.74 ± 0.45 | 5.65 ± 0.48 | 5.68 ± 0.47 | 0.472 |
| PreOP VAS for thumb pain (activity) | 7.39 ± 0.50 | 6.84 ± 0.50 | 7.05 ± 0.57 | <0.001 * |
| PreOP thumb ROM (°) | 45.43 ± 10.54 | 41.89 ± 11.69 | 43.25 ± 11.31 | 0.241 |
| PreOP pinch power (% of normal hand) | 44.78 ± 11.63 | 48.92 ± 7.65 | 47.33 ± 9.50 | 0.101 |
| Progress of pinch power (% of normal hand) | 38.04 ± 19.17 | 43.65 ± 7.61 | 41.50 ± 13.41 | 0.116 |
| Poor outcome (progress < 40%) (%) | 9 (39.1%) | 3 (8.1%) | 12 (20.0%) | 0.006 * |
| Collapse of basal joint (mm) | 2.35 ± 0.88 | 1.70 ± 0.57 | 1.95 ± 0.77 | 0.001 * |
| Follow-up time (M) | 27.83 ± 1.83 | 29.24 ± 3.38 | 28.70 ± 2.95 | 0.070 |
| Item | Eaton Stage | n | Pre-OP | Post-OP | Difference | Within-Group p-Value | Between-Group p-Value |
|---|---|---|---|---|---|---|---|
| VAS for thumb pain (rest) | 3 | 23 | 5.74 ± 0.45 | 1.35 ± 0.49 | −4.39 ± 0.50 | <0.001 * | 0.008 * |
| 2 | 37 | 5.65 ± 0.48 | 0.76 ± 0.72 | −4.89 ± 0.77 | <0.001 * | ||
| Total | 60 | 5.68 ± 0.47 | 0.98 ± 0.70 | −4.70 ± 0.72 | <0.001 * | ||
| VAS for thumb pain (activity) | 3 | 23 | 7.39 ± 0.50 | 1.74 ± 0.86 | −5.65 ± 1.03 | <0.001 * | 0.786 |
| 2 | 37 | 6.84 ± 0.50 | 1.11 ± 0.88 | −5.73 ± 1.10 | <0.001 * | ||
| Total | 60 | 7.05 ± 0.57 | 1.35 ± 0.92 | −5.70 ± 1.06 | <0.001 * | ||
| Thumb ROM (°) | 3 | 23 | 45.43 ± 10.54 | 53.26 ± 12.21 | 7.83 ± 6.18 | <0.001 * | 0.029 * |
| 2 | 37 | 41.89 ± 11.69 | 54.73 ± 7.99 | 12.84 ± 9.54 | <0.001 * | ||
| Total | 60 | 43.25 ± 11.31 | 54.17 ± 9.75 | 10.92 ± 8.71 | <0.001 * | ||
| Pinch power (% of normal hand) | 3 | 23 | 44.78 ± 11.63 | 82.83 ± 21.63 | 38.04 ± 19.17 | <0.001 * | 0.116 |
| 2 | 37 | 48.92 ± 7.65 | 92.57 ± 13.00 | 43.65 ± 7.61 | <0.001 * | ||
| Total | 60 | 47.33 ± 9.50 | 88.83 ± 17.33 | 41.50 ± 13.41 | <0.001 * |
| Crude | Adjusted | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age | 0.99 (0.86, 1.15) | 0.952 | 1.31 (0.87, 1.99) | 0.200 |
| Eaton stage (3 vs. 2) | 7.29 (1.67, 28.82) | 0.008 * | 10.28 (2.47, 26.6) | 0.012 * |
| PreOP VAS for thumb pain (activity) | 7.86 (2.20, 30.12) | 0.004 * | 11.48 (1.38, 40.23) | 0.038 * |
| PreOP thumb ROM (°) | 0.92 (0.86, 0.99) | 0.023 * | 0.69 (0.52, 0.93) | 0.015 * |
| PreOP pinch power (% of normal hand) | 0.97 (0.90, 1.04) | 0.341 | 1.10 (0.92, 1.33) | 0.303 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, K.-T.; Wang, J.-H.; Li, J.; Shih, J.-T. Arthroscopic Partial Trapeziectomy and Free Tendon Suspension and Interposition Combined with Internal Brace for Basal Joint Arthritis of Thumb. J. Clin. Med. 2025, 14, 4118. https://doi.org/10.3390/jcm14124118
Yeh K-T, Wang J-H, Li J, Shih J-T. Arthroscopic Partial Trapeziectomy and Free Tendon Suspension and Interposition Combined with Internal Brace for Basal Joint Arthritis of Thumb. Journal of Clinical Medicine. 2025; 14(12):4118. https://doi.org/10.3390/jcm14124118
Chicago/Turabian StyleYeh, Kuang-Ting, Jen-Hung Wang, Jochieh Li, and Jui-Tien Shih. 2025. "Arthroscopic Partial Trapeziectomy and Free Tendon Suspension and Interposition Combined with Internal Brace for Basal Joint Arthritis of Thumb" Journal of Clinical Medicine 14, no. 12: 4118. https://doi.org/10.3390/jcm14124118
APA StyleYeh, K.-T., Wang, J.-H., Li, J., & Shih, J.-T. (2025). Arthroscopic Partial Trapeziectomy and Free Tendon Suspension and Interposition Combined with Internal Brace for Basal Joint Arthritis of Thumb. Journal of Clinical Medicine, 14(12), 4118. https://doi.org/10.3390/jcm14124118









