The Role of Renal Denervation in HFpEF
Abstract
1. Introduction
2. Modalities of Renal Denervation and Their Role in Hypertension Management
3. Pathophysiology of HFpEF and the Impact of Renal Denervation
4. Clinical Studies on Renal Denervation in HFpEF
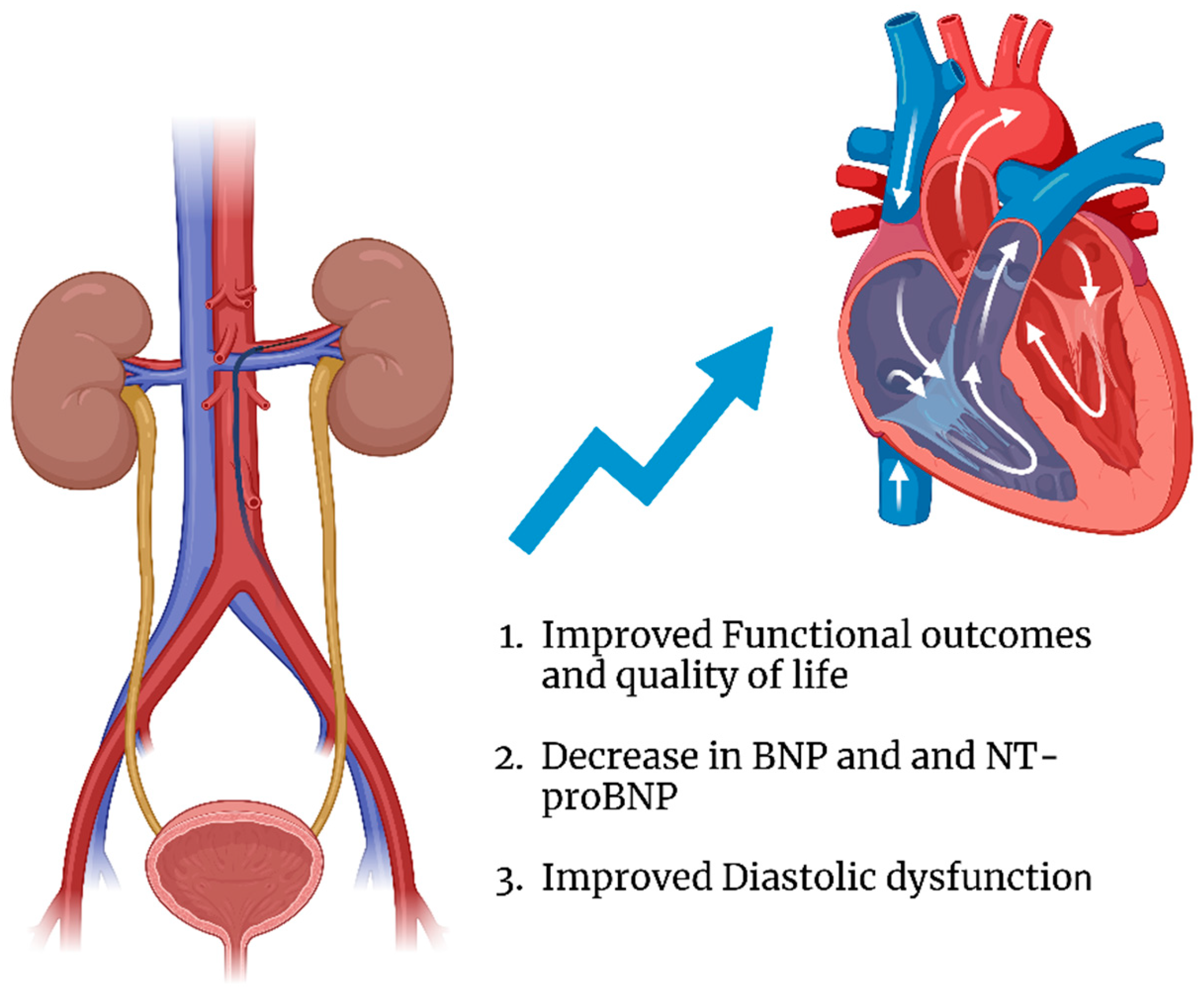
4.1. Functional Outcomes and Quality of Life
4.2. Cardiac Biomarkers
4.3. Structural Cardiac Changes
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aHT | Arterial Hypertension |
| AL | Afterload |
| AD | Aortic Distensibility |
| BNP | B-type Natriuretic Peptide |
| BPV | Blood Pressure Variability |
| CMR | Cardiac Magnetic Resonance |
| CPOI | Cardiac Power Output Index |
| DBP | Diastolic Blood Pressure |
| DOAJ | Directory of Open Access Journals |
| E/e′ | Ratio of Early Diastolic Transmitral Flow Velocity to Mitral Annular Early Diastolic Velocity |
| EF | Ejection Fraction |
| GLS | Global Longitudinal Strain |
| HFA-PEFF | Heart Failure Association Pre-test Assessment of HFpEF |
| HFpEF | Heart Failure with Preserved Ejection Fraction |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| HTN | Hypertension |
| KCCQ | Kansas City Cardiomyopathy Questionnaire |
| LAVI | Left Atrial Volume Index |
| LD | Linear Dichroism |
| LVEF | Left Ventricular Ejection Fraction |
| LVMI | Left Ventricular Mass Index |
| MLHFQ | Minnesota Living with Heart Failure Questionnaire |
| MDPI | Multidisciplinary Digital Publishing Institute |
| mos | Months |
| NT-proBNP | N-terminal pro-B-type Natriuretic Peptide |
| NYHA | New York Heart Association |
| OMT | Optimal Medical Therapy |
| PKG | Protein Kinase G |
| PWV | Pulse Wave Velocity |
| QOL | Quality of Life |
| RAAS | Renin–Angiotensin–Aldosterone System |
| RBF | Renal Blood Flow |
| RCT | Randomized Controlled Trial |
| RDN | Renal Denervation |
| RR | Relative Risk |
| SNS | Sympathetic Nervous System |
| SBP | Systolic Blood Pressure |
| SGLT2 | Sodium–Glucose Co-Transporter 2 |
| SVI | Stroke Volume Index |
| TGF-β | Transforming Growth Factor-beta |
| TLA | Three-Letter Acronym |
| VO2 peak | Peak Oxygen Consumption |
References
- Redfield, M.M.; Borlaug, B.A. Heart Failure with Preserved Ejection Fraction: A Review. JAMA 2023, 329, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Golla, M.S.G.; Shams, P. Heart Failure With Preserved Ejection Fraction (HFpEF). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK599960/ (accessed on 23 March 2025).
- Balestrieri, G.; Limonta, R.; Ponti, E.; Merlo, A.; Sciatti, E.; D’Isa, S.; Gori, M.; Casu, G.; Giannattasio, C.; Senni, M.; et al. The Therapy and Management of Heart Failure with Preserved Ejection Fraction: New Insights on Treatment. Card. Fail. Rev. 2024, 10, e05. [Google Scholar] [CrossRef] [PubMed]
- Oktay, A.A.; Rich, J.D.; Shah, S.J. The Emerging Epidemic of Heart Failure with Preserved Ejection Fraction. Curr. Heart Fail Rep. 2013, 10, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Vasan, R.S. Epidemiology of heart failure with preserved ejection fraction. Heart Fail Clin. 2014, 10, 377–388. [Google Scholar] [CrossRef]
- Andersson, C.; Vasan, R.S. Heart failure with preserved ejection fraction. Nat. Rev. Dis. Primers 2024, 10, 1–19. [Google Scholar] [CrossRef]
- Sharma, K.; Kass, D.A. Heart failure with preserved ejection fraction: Mechanisms, clinical features, and therapies. Circ. Res. 2014, 115, 79–96. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Sharma, K.; Shah, S.J.; Ho, J.E. Heart Failure with Preserved Ejection Fraction: JACC Scientific Statement. J. Am. Coll. Cardiol. 2023, 81, 1810–1834. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Davis, L.L.; Yancy, C.W.; Panjrath, G.S.; Dixon, D.L.; Amancherla, K.; Kittleson, M.M.; Deswal, A. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll Cardiol. 2023, 81, 1835–1878. [Google Scholar] [CrossRef]
- Probstfield, J.L.; Shah, S.J.; Heitner, J.F.; Solomon, S.D.; Sweitzer, N.K.; Boineau, R.; Rouleau, J.-L.; Claggett, B.; Assmann, S.F.; Diaz, R.; et al. Regional Variation in Patients and Outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) Trial. Circulation 2015, 131, 34–42. [Google Scholar] [CrossRef]
- Tang, F.; Liu, Q.; Zhou, S.; Huang, J.; Xiao, Y.; Han, H.; Fu, S. Nonpharmacological Approaches to Managing Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2024, 17, e011269. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Kim, L.K.; Swaminathan, R.V.; Feldman, D.N. Renal Denervation to Modify Hypertension and the Heart Failure State. Intervent. Cardiol. Clin. 2017, 6, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Ogoyama, Y.; Kario, K. Differences in the effectiveness and safety of different renal denervation devices. Hypertens. Res. 2024, 47, 2678–2684. [Google Scholar] [CrossRef] [PubMed]
- Rey-García, J.; Townsend, R.R. Renal Denervation: A Review. Am. J. Kidney Dis. 2022, 80, 527–535. [Google Scholar] [CrossRef]
- Renal Denervation for the Treatment of Hypertension: A Scientific Statement from the American Heart Association|Hypertension. Available online: https://www.ahajournals.org/doi/10.1161/HYP.0000000000000240 (accessed on 3 March 2025).
- Bhatt, D.L.; Kandzari, D.E.; O’Neill, W.W.; D’Agostino, R.; Flack, J.M.; Katzen, B.T.; Leon, M.B.; Liu, M.; Mauri, L.; Negoita, M.; et al. A Controlled Trial of Renal Denervation for Resistant Hypertension. N. Engl. J. Med. 2014, 370, 1393–1401. [Google Scholar] [CrossRef]
- Townsend, R.R.; Mahfoud, F.; Kandzari, D.E.; Kario, K.; Pocock, S.; Weber, M.A.; Ewen, S.; Tsioufis, K.; Tousoulis, D.; Sharp, A.S.P.; et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): A randomised, sham-controlled, proof-of-concept trial. Lancet 2017, 390, 2160–2170. [Google Scholar] [CrossRef]
- Kandzari, D.E.; Böhm, M.; Mahfoud, F.; Townsend, R.R.; Weber, M.A.; Pocock, S.; Tsioufis, K.; Tousoulis, D.; Choi, J.W.; East, C.; et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018, 391, 2346–2355. [Google Scholar] [CrossRef]
- Azizi, M.; Schmieder, R.E.; Mahfoud, F.; Weber, M.A.; Daemen, J.; Davies, J.; Basile, J.; Kirtane, A.J.; Wang, Y.; Lobo, M.D.; et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): A multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018, 391, 2335–2345. [Google Scholar] [CrossRef]
- Azizi, M.; Sanghvi, K.; Saxena, M.; Gosse, P.; Reilly, J.P.; Levy, T.; Rump, L.C.; Persu, A.; Basile, J.; Bloch, M.J.; et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): A randomised, multicentre, single-blind, sham-controlled trial. Lancet 2021, 397, 2476–2486. [Google Scholar] [CrossRef]
- Pathak, A.; Rudolph, U.M.; Saxena, M.; Zeller, T.; Müller-Ehmsen, J.; Lipsic, E.; Schmieder, R.E.; Sievert, H.; Halbach, M.; Sharif, F.; et al. Alcohol-mediated renal denervation in patients with hypertension in the absence of antihypertensive medications. EuroIntervention 2023, 19, 602–611. [Google Scholar] [CrossRef]
- Mufarrih, S.H.; Qureshi, N.Q.; Khan, M.S.; Kazimuddin, M.; Secemsky, E.; Bloch, M.J.; Giri, J.; Cohen, D.; Swaminathan, R.V.; Feldman, D.N.; et al. Randomized Trials of Renal Denervation for Uncontrolled Hypertension: An Updated Meta-Analysis. J. Am. Heart Assoc. 2024, 13, e034910. [Google Scholar] [CrossRef]
- Efficacy and Safety of Sympathetic Mapping and Ablation of Renal Nerves for the Treatment of Hypertension (SMART): 6-Month Follow-Up of a Randomised, Controlled Trial—eClinicalMedicine. Available online: https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(24)00205-0/fulltext (accessed on 3 March 2025).
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Krummerman, A.; Vijayaraman, P.; Rosengarten, M.; Suryadevara, V.; Lejem℡, T.; Ferrick, K.J. Heart Rate Variability and Diastolic Heart Failure. Pacing Clin. Electrophysiol. 2004, 27, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Mancia, G.; Esler, M. Central and peripheral sympathetic activation in heart failure. Cardiovasc. Res. 2022, 118, 1857–1871. [Google Scholar] [CrossRef]
- Kaye, D.M.; Nanayakkara, S.; Wang, B.; Shihata, W.; Marques, F.Z.; Esler, M.; Lambert, G.; Mariani, J. Characterization of Cardiac Sympathetic Nervous System and Inflammatory Activation in HFpEF Patients. JACC Basic Transl. Sci. 2022, 7, 116–127. [Google Scholar] [CrossRef]
- Wever-Pinzon, O.; Fang, J.C. Characterization of Sympathetic Innervation in Heart Failure with Preserved Ejection Fraction. J. Card. Fail. 2019, 25, 314–315. [Google Scholar] [CrossRef]
- Kitzman, D.W.; Little, W.C.; Brubaker, P.H.; Anderson, R.T.; Hundley, W.G.; Marburger, C.T.; Brosnihan, B.; Morgan, T.M.; Stewart, K.P. Pathophysiological Characterization of Isolated Diastolic Heart Failure in Comparison to Systolic Heart Failure. JAMA 2002, 288, 2144–2150. [Google Scholar] [CrossRef]
- Chang, J.W.-H.; Ramchandra, R. The sympathetic nervous system in heart failure with preserved ejection fraction. Heart Fail. Rev. 2025, 30, 209–218. [Google Scholar] [CrossRef]
- Silverman, D.N.; Plante, T.B.; Infeld, M.; Callas, P.W.; Juraschek, S.P.; Dougherty, G.B.; Meyer, M. Association of β-Blocker Use With Heart Failure Hospitalizations and Cardiovascular Disease Mortality Among Patients With Heart Failure With a Preserved Ejection Fraction: A Secondary Analysis of the TOPCAT Trial. JAMA Netw. Open 2019, 2, e1916598. [Google Scholar] [CrossRef]
- Edelmann, F.; Musial-Bright, L.; Gelbrich, G.; Trippel, T.; Radenovic, S.; Wachter, R.; Inkrot, S.; Loncar, G.; Tahirovic, E.; Celic, V.; et al. Tolerability and Feasibility of Beta-Blocker Titration in HFpEF Versus HFrEF: Insights From the CIBIS-ELD Trial. JACC Heart Fail. 2016, 4, 140–149. [Google Scholar] [CrossRef]
- Peikert, A.; Bart, B.A.; Vaduganathan, M.; Claggett, B.L.; Kulac, I.J.; Kosiborod, M.N.; Desai, A.S.; Jhund, P.S.; Lam, C.S.P.; Inzucchi, S.E.; et al. Contemporary Use and Implications of Beta-Blockers in Patients with HFmrEF or HFpEF. JACC Heart Fail. 2024, 12, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Kaddoura, R.; Madurasinghe, V.; Chapra, A.; Abushanab, D.; Al-Badriyeh, D.; Patel, A. Beta-blocker therapy in heart failure with preserved ejection fraction (B-HFpEF): A systematic review and meta-analysis. Curr. Probl. Cardiol. 2024, 49, 102376. [Google Scholar] [CrossRef] [PubMed]
- Smarz, K.; Tysarowski, M.; Zaborska, B.; Pilichowska-Paszkiet, E.; Sikora-Frac, M.; Budaj, A.; Jaxa-Chamiec, T. Chronotropic Incompetence Limits Aerobic Exercise Capacity in Patients Taking Beta-Blockers: Real-Life Observation of Consecutive Patients. Healthcare 2021, 9, 212. [Google Scholar] [CrossRef]
- Renal Denervation in the Setting of Heart Failure|Heart Failure Reviews. Available online: https://link.springer.com/article/10.1007/s10741-025-10489-z (accessed on 11 April 2025).
- Reddy, Y.N.V.; Rikhi, A.; Obokata, M.; Shah, S.J.; Lewis, G.D.; AbouEzzedine, O.F.; Dunlay, S.; McNulty, S.; Chakraborty, H.; Stevenson, L.W.; et al. Quality of life in heart failure with preserved ejection fraction: Importance of obesity, functional capacity, and physical inactivity. Eur. J. Heart Fail. 2020, 22, 1009–1018. [Google Scholar] [CrossRef]
- Napier, R.; McNulty, S.E.; Eton, D.T.; Redfield, M.M.; AbouEzzeddine, O.; Dunlay, S.M. Comparing Measures to Assess Health-Related Quality of Life in Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2018, 6, 552–560. [Google Scholar] [CrossRef]
- Patel, H.C.; Rosen, S.D.; Hayward, C.; Vassiliou, V.; Smith, G.C.; Wage, R.R.; Bailey, J.; Rajani, R.; Lindsay, A.C.; Pennell, D.J.; et al. Renal denervation in heart failure with preserved ejection fraction (RDT-PEF): A randomized controlled trial. Eur. J. Heart Fail. 2016, 18, 703–712. [Google Scholar] [CrossRef]
- Kresoja, K.-P.; Rommel, K.-P.; Fengler, K.; Von Roeder, M.; Besler, C.; Lücke, C.; Gutberlet, M.; Desch, S.; Thiele, H.; Böhm, M.; et al. Renal Sympathetic Denervation in Patients with Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2021, 14, E007421. [Google Scholar] [CrossRef]
- Rommel, K.-P.; Pagoulatou, S.; Kresoja, K.-P.; Rosch, S.; Schöber, A.R.; Von Roeder, M.; Thiele, H.; Fengler, K.; Stergiopulos, N.; Lurz, P. Modulation of Pulsatile Left Ventricular Afterload by Renal Denervation in Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2023, 16, E010543. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Alcidi, G.; Goffredo, G.; Correale, M.; Brunetti, N.D.; Iacoviello, M. Brain Natriuretic Peptide Biomarkers in Current Clinical and Therapeutic Scenarios of Heart Failure. J. Clin. Med. 2022, 11, 3192. [Google Scholar] [CrossRef]
- Utility and Validity of the HFA-PEFF and H2FPEF Scores in Patients with Symptomatic Atrial Fibrillation. Available online: https://pubmed.ncbi.nlm.nih.gov/38520461/ (accessed on 13 December 2024).
- Prognosis and NT-proBNP in Heart Failure Patients with Preserved Versus Reduced Ejection Fraction | Heart. Available online: https://heart.bmj.com/content/105/15/1182 (accessed on 13 December 2024).
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Plehn, A.; Atti, C.; Nussbaum, M.; Tongers, J.; Sedding, D.; Dutzmann, J. Left ventricular structure and function following renal sympathetic denervation in patients with HFpEF: An echocardiographic 9-year long-term follow-up. Front. Cardiovasc. Med. 2024, 11, 1408547. [Google Scholar] [CrossRef] [PubMed]
- Tsioufis, C.; Georgiopoulos, G.; Oikonomou, D.; Thomopoulos, C.; Katsiki, N.; Kasiakogias, A.; Chrysochoou, C.; Konstantinidis, D.; Kalos, T.; Tousoulis, D. Hypertension and heart failure with preserved ejection fraction: Connecting the dots. Curr. Vasc. Pharmacol. 2018, 16, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kassab, K.; Soni, R.; Kassier, A.; Fischell, T.A. The Potential Role of Renal Denervation in the Management of Heart Failure. J. Clin. Med. 2022, 11, 4147. [Google Scholar] [CrossRef]
- Zamani, S.; Mahfoud, F.; Stoiber, L.; Boehm, M.; Pieske, B.; Gebker, R.; Stawowy, P.; Kelle, S. Renal denervation improves diastolic dysfunction in patients with HFpEF—Initial results of a multicenter CMR study. Eur. Heart J. 2019, 40, 3165. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Murphy, S.P.; Ibrahim, N.E.; Januzzi, J.L. Heart Failure with Reduced Ejection Fraction: A Review. JAMA 2020, 324, 488–504. [Google Scholar] [CrossRef]
- Koufou, E.-E.; Arfaras-Melainis, A.; Rawal, S.; Kalogeropoulos, A.P. Treatment of Heart Failure with Mid-Range Ejection Fraction: What Is the Evidence. J. Clin. Med. 2021, 10, 203. [Google Scholar] [CrossRef]
- Borlaug, B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020, 17, 559–573. [Google Scholar] [CrossRef]
- Wintrich, J.; Kindermann, I.; Ukena, C.; Selejan, S.; Werner, C.; Maack, C.; Laufs, U.; Tschöpe, C.; Anker, S.; Lam, C.; et al. Therapeutic approaches in heart failure with preserved ejection fraction: Past, present, and future. Clin. Res. Cardiol. 2020, 109, 1079–1098. [Google Scholar] [CrossRef]
- Patel, H.C.; Hayward, C.; Di Mario, C. SYMPLICITY HTN 3: The death knell for renal denervation in hypertension? Glob. Cardiol. Sci. Pr. 2014, 2014, 94–98. [Google Scholar] [CrossRef][Green Version]
- Barbato, E.; Azizi, M.; Schmieder, R.; Lauder, L.; Böhm, M.; Brouwers, S.; Bruno, R.M.; Dudek, D.; Kahan, T.; Kandzari, D.; et al. Renal Denervation in the Management of Hypertension in Adults. A Clinical Consensus Statement of the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Available online: https://eurointervention.pcronline.com/article/renal-denervation-in-the-management-of-hypertension-in-adults-a-clinical-consensus-statement-of-the-esc-council-on-hypertension-and-the-european-association-of-percutaneous-cardiovascular-interventions-eapci (accessed on 4 March 2025).
| Author Name | Study Type | Cohort | Endpoints | Follow-Up Duration | Results |
|---|---|---|---|---|---|
| Patel et al., 2016 [40] | Randomized Controlled Trial | 25 HFpEF patients (2:1 ratio RDN vs. OMT) | MLHFQ score, peak VO2, BNP, E/e′, LA volume index, LVMI, macro- and micro-vascular function, renal blood flow | 12 months | No sustained improvement in primary endpoints; improvements in VO2 peak and E/e′ at 3 months in the RDT group, but this was not sustained at 12 months. |
| Kresoja et al., 2021 [41] | Observational Study | 164 patients who underwent RDN (99 with HFpEF and 64 with uncontrolled AHT) | SVI, aortic distensibility, LV systolic/diastolic stiffness, NT-proBNP, E/e′, BPV, CPOI, PWV, NYHA class | 6 months | Improvement in LV systolic and diastolic stiffness and reduced SBP, DBP, and NT-proBNP levels. |
| Rommel et al., 2023 [42] | Observational Study | 60 patients with HTN who underwent RDN (30 HFpEF patients vs. 30 controls) | Pulsatile afterload, LV diastolic stiffness, SBP, DBP, BPV, NT-proBNP, E/E′, aortic distensibility, PWV | 3 months | Improved NYHA class, decreased NT-proBNP levels, pulsatile left ventricular load, and arterial stiffness post-RDN. |
| Zamani et al., 2024 [51] | Observational study | 22 patients with resistant HTN and HFpEF (16 underwent RDN vs. six received OMT | LVMI, GLS | 6 months | Improvement in GLS at 6 months. |
| Vogt et al., 2024 [48] | Observational Study | 70 patients who underwent RDN (22 patients with HFpEF) | E/E′, LA volume index, LVMI, HFA-PEFF score, BNP, SBP, DBP | 9 years | Significant long-term reduction in LVMI and BNP/NT-proBNP in addition to reduced HFA-PEFF score. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamil, D.; Mojaddedi, S.; Kollman, P.; Bangash, N.; Abdelhai, O.S.; Aburuman, Y.; Lotfi, A.S. The Role of Renal Denervation in HFpEF. J. Clin. Med. 2025, 14, 4115. https://doi.org/10.3390/jcm14124115
Jamil D, Mojaddedi S, Kollman P, Bangash N, Abdelhai OS, Aburuman Y, Lotfi AS. The Role of Renal Denervation in HFpEF. Journal of Clinical Medicine. 2025; 14(12):4115. https://doi.org/10.3390/jcm14124115
Chicago/Turabian StyleJamil, Dawood, Sanaullah Mojaddedi, Patrick Kollman, Najeebullah Bangash, Omar Sami Abdelhai, Yazeed Aburuman, and Amir S. Lotfi. 2025. "The Role of Renal Denervation in HFpEF" Journal of Clinical Medicine 14, no. 12: 4115. https://doi.org/10.3390/jcm14124115
APA StyleJamil, D., Mojaddedi, S., Kollman, P., Bangash, N., Abdelhai, O. S., Aburuman, Y., & Lotfi, A. S. (2025). The Role of Renal Denervation in HFpEF. Journal of Clinical Medicine, 14(12), 4115. https://doi.org/10.3390/jcm14124115






